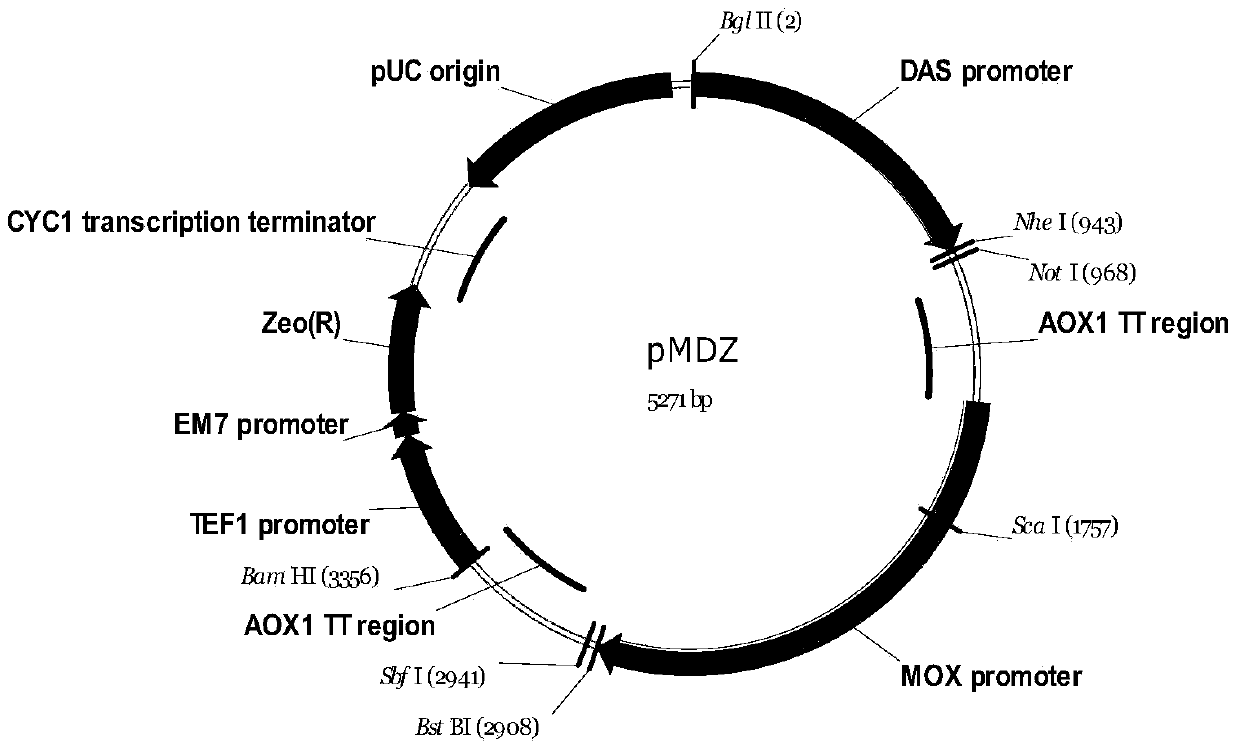
Method for preparing recombinant coxsackievirus A16 type virus-like particles
A coxsackie virus, virus-like technology, applied in the field of immunity, can solve the problems of lack of viral nucleic acid and non-infectivity, and achieve the effect of good immunogenicity, uniform shape and stable character
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 virus particle
[0054] 1. Selection of expressed genes and optimal design of codons
[0055] The DNA sequence encoding CA16-P1 protein and CA16-3C protein refers to Chinese strain SZ / HK08-7 (GenBank: GQ279371.1), and the amino acid sequence of SZ / HK08-7 strain CA16-P1 protein is shown in SEQ ID NO:1 , the amino acid sequence of the CA16-3C protein is shown in SEQ ID NO:2.
[0056] Transform the wild-type DNA sequence encoding the CA16-P1 protein shown in SEQ ID NO:1 and the CA16-3C protein shown in SEQ ID NO:2 in the SZ / HK08-7 strain, and use the codons used in Hansenula as much as possible Higher frequency codons, while avoiding transcription factor binding regions, repetitive sequences, and RNA high-level structures that may affect expression. After codon optimization, the sequence of the recombinant gene encoding the CA16-P1 protein is shown in SEQ ID NO:3, and the sequence of the recombinant gene encoding the CA16-3C protein is sho...
Embodiment 2
[0090] Antigen performance detection of embodiment 2 virus particles
[0091] 1. VLP Vaccine Preparation
[0092] The purified CA16 virus-like particle protein is adsorbed with aluminum adjuvant to prepare the immunogenic CA16 vaccine.
[0093] 2. Determination of the immunogenicity of CA16 virus-like particles
[0094] SPF grade BALB / c mice aged 6-8 weeks were selected and divided into 4 groups with 6 mice in each group. One group of mice was immunized with buffer solution containing aluminum adjuvant (50mM PB pH7.0) (as a negative control group), and the other three groups were immunized with 1μg / mouse, 0.1μg / mouse, and 0.01μg / mouse , were immunized subcutaneously on the 0th and 14th day respectively, and were immunized twice in total, and blood was collected two weeks after the second immunization. Place the collected blood at 4°C overnight, centrifuge at 5000g for 10 minutes, and absorb the supernatant to obtain the mouse polyantiserum, store it at -20°C, and detect the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com