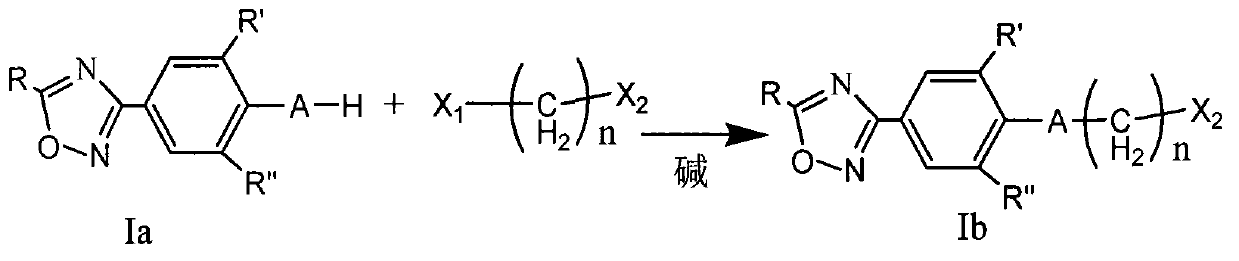
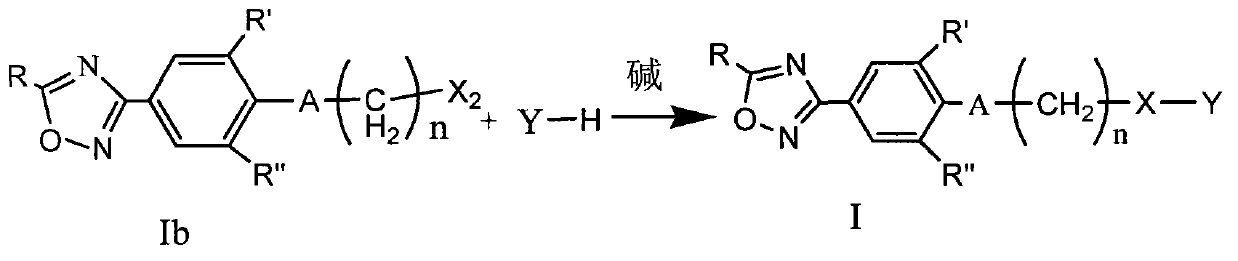
Oxadiazole compound and preparation method thereof, medicine composition and application thereof
A technology of oxadiazoles and compounds, applied in the field of synthesis of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Embodiment 1: Intermediate Ia of the present invention 1 Synthesis
[0213]
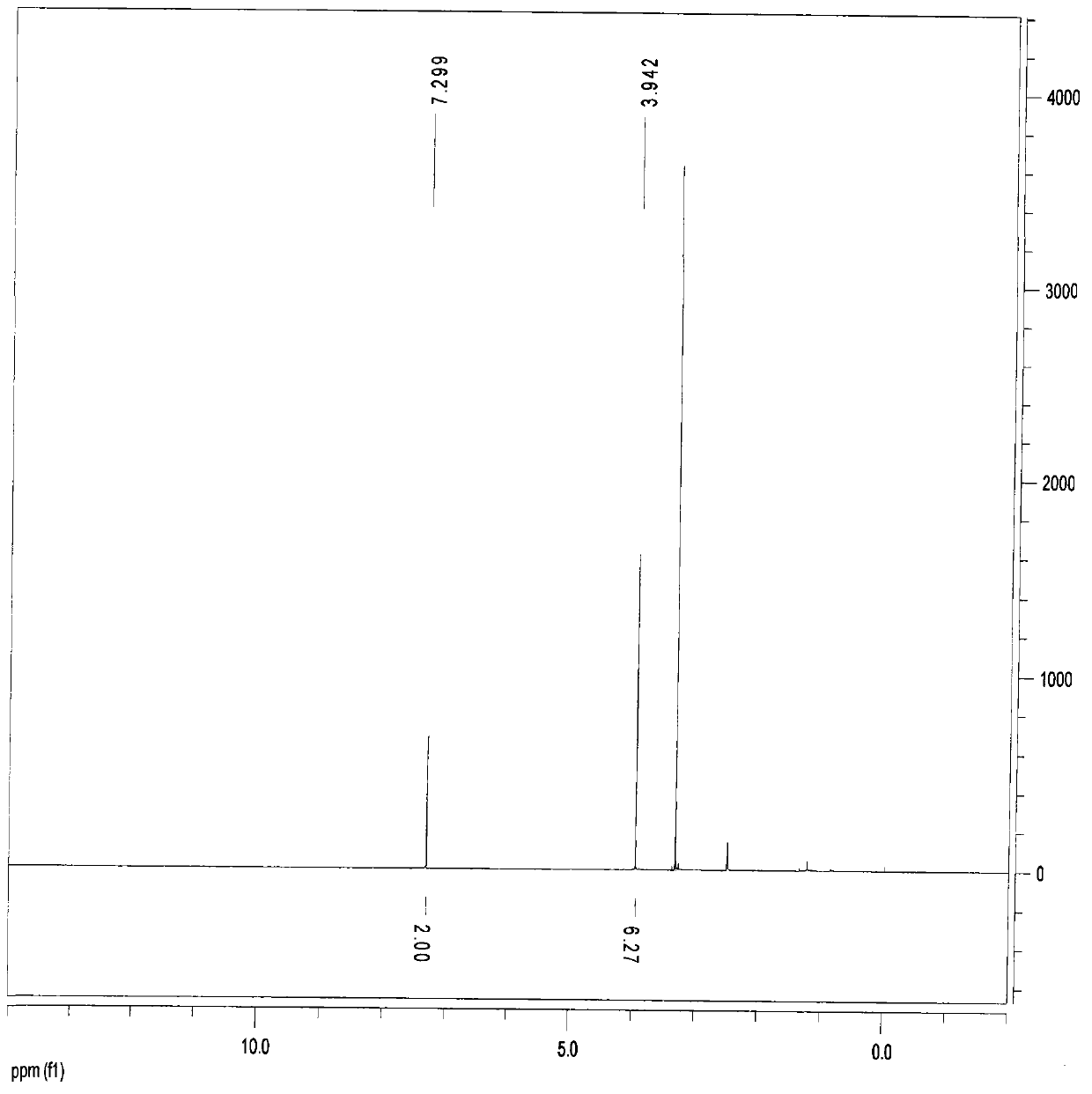
[0214] 50g of 3,5-dimethyl-4-hydroxybenzonitrile and 47g of hydroxylamine hydrochloride were added to 1000ml of ethanol, heated to reflux for 4 hours, detected by thin layer chromatography, 3,5-dimethyl-4-hydroxybenzonitrile The basic reaction is complete. After distilling off ethanol, add 1000ml of tetrahydrofuran, slowly add 286g of trifluoroacetic anhydride, heat and reflux for 4 hours, after cooling, filter to obtain 30g of compound Ia of the present invention 1 , namely 2,6-dimethyl-4-(5-trifluoromethyl-1,2,4-oxadiazol-3-yl-)phenol, which is a solid.
[0215] Ia 1 of 1 HNMR (DMSO, 400 MHz) δ: 3.942 (s, 6H), 7.299 (s, 2H).
Embodiment 2
[0216] Embodiment 2: Intermediate 2,6-dimethyl-4-(5-methyl-1,2,4-oxadiazol-3-yl-)phenol Ia of the present invention 2 Synthesis
[0217] Adopt the preparation method of embodiment 1, replace trifluoroacetic anhydride with acetic anhydride, obtain 27g intermediate Ia 2 , namely 2,6-dimethyl-4-(5-methyl-1,2,4-oxadiazol-3-yl-)phenol.
[0218] Ia 2 of 1 HNMR (DMSO, 400 MHz) δ: 3.942 (s, 6H), 3.845 (s, 3H), 7.299 (s, 2H).
Embodiment 3
[0219] Embodiment 3: the synthesis of compound 1 of the present invention
[0220] 20g compound Ia 1 , 1,2 dibromoethane 102g, potassium carbonate 5.6g (40.8mmol), was added to 500ml of acetonitrile, heated to reflux, reacted overnight, cooled to room temperature. Filtration, the filter cake was washed with methanol, and the filtrate was concentrated to obtain 3-[3,5-dimethyl-4-(2-bromoethoxy)phenyl]-5-(trifluoromethyl)-1,2 , 22g of 4-oxadiazole white solid compound.
[0221] 5g of 3-[3,5-dimethyl-4-(2-bromoethoxy)phenyl]-5-(trifluoromethyl)-1,2,4-oxadiazole, 3-hydroxy- 2.3 g of 5-methylisoxazole and 5.5 g of potassium carbonate were added to 100 ml of acetonitrile, heated to reflux, monitored by thin-layer chromatography, and cooled to room temperature after the disappearance of raw materials. After filtration, the filtrate was concentrated to obtain 4.9 g of compound 1 of the present invention, namely 3-[3,5-dimethyl-4-[2-(5-methylisoxazole-3-oxyl)ethoxy] Phenyl]-5-(trif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com