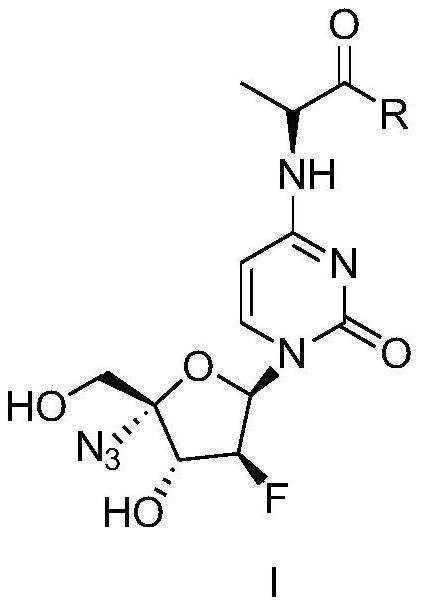
4-Alanine substituted cytosine nucleosides and their medicinal uses
A technology of cytidine nucleoside derivatives and alanine, which is applied in the direction of drug combination, organic chemistry, and pharmaceutical formulations, and achieves the effects of low toxicity, good application prospects, and simple and feasible preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
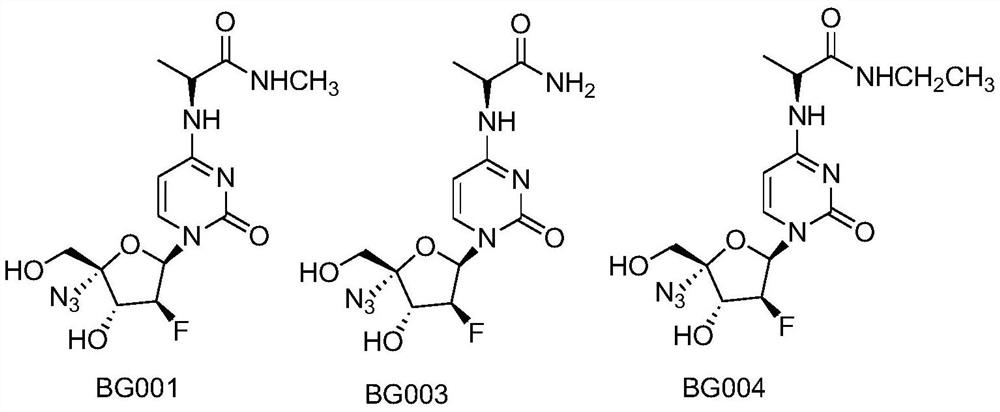
[0027] Embodiment 1: the synthesis of compound BG001:
[0028] Compound 1 ((50mg, 0.12mmol) was added to methylamine aqueous solution (5mL, mass percent concentration 40%) at room temperature, and then continued to stir for 2 hours. After the reaction was detected by TLC, the solvent was distilled off under reduced pressure, and quickly Column separation (dichloromethane:methanol=5:1) gave white powdery solid compound BG001
[0029] (48 mg, 96%). 1 H NMR (400Hz, CD 3 OD):7.73-7.71(1H,dd,J=1.2,7.6 Hz),6.50-6.46(1H,dd,J=7.2,12.0Hz),5.98-5.96(1H,d,J=7.6 Hz),5.26 -5.12(1H,m),4.61-4.60(1H,m),4.50-4.44(1H,dd,J=4.4, 21.6Hz),3.83(2H,s),2.74(3H,m),1.40-1.38 (3H,m); 13 C NMR (100Hz, CD 3 OD): 175.5, 165.2, 158.2, 142.0, 98.6 (J FC =6.7Hz), 97.1, 97.1, 95.2 (J FC =189.7Hz), 84.9(J FC =17.5Hz), 76.5(J FC =24.3Hz), 63.5, 51.1, 18.3.
Embodiment 2
[0030] Embodiment 2: the synthesis of compound BG003:
[0031] Compound 1 (50mg, 0.12mmol) was added to concentrated ammonia water (10ml, mass percentage concentration 17%) at room temperature, and then continued to stir for 2 hours. After the reaction was detected by TLC, the solvent was distilled off under reduced pressure, and the column was separated quickly. (dichloromethane:methanol=3:1) to obtain white powdery solid compound BG003 (46 mg, 96%). 1 H NMR (400Hz, CD 3 OD):7.72-7.70(1H,d,J=7.6Hz),6.50-6.46(1H,dd,J=7.2,12.4Hz),5.99-5.97(1H,d,J=8.0Hz),5.27-5.11 (1H,ddd, J=4.4,9.2,58.4Hz),4.69-4.63(1H,m),4.50-4.44(1H,dd,J=4.8, 21.6Hz),3.83(2H,s),1.42-1.41 (3H,m); 13 C NMR (100Hz, CD 3 OD): 177.6, 165.2, 158.2, 141.9, 98.6 (J FC =7.6Hz), 97.1, 95.2 (J FC =188.9Hz), 84.9(J FC =16.7Hz), 76.5(J FC =25.0Hz), 63.4, 18.4.
Embodiment 3
[0032] Embodiment 3: the synthesis of compound BG004:
[0033] Compound 1 (50mg, 0.12mmol) was added to ethylamine aqueous solution (4ml, mass percent concentration 60%) at room temperature, and then continued to stir for 2 hours. After the reaction was detected by TLC, the solvent was distilled off under reduced pressure, and the column was flashed. Isolation (dichloromethane:methanol=5:1) gave white powdery solid compound BG004 (50 mg, 97%). 1 H NMR (400Hz, CD 3 OD):7.72-7.70(1H,dd,J=1.2,7.6Hz),6.50-6.46 (1H,dd,J=7.2,12.0Hz),5.98-5.96(1H,d,J=7.6Hz),5.26 -5.11(1H, ddd, J=4.4,7.2,49.2Hz),4.63-4.57(1H,m),4.50-4.44(1H,dd,J=4.4, 21.6Hz),3.83(2H,s),3.24 -3.18(2H,m),1.41-1.39(3H,m),1.13-1.09(3H,m); 13 C NMR (100Hz, CD 3 OD): 174.6, 165.1, 158.1, 142.0, 131.0, 98.5 (J FC =6.7Hz), 97.1, 95.2 (J FC =191.0Hz), 84.8(J FC =17.5Hz), 76.4(JFC =21.6Hz), 63.4,51.1,35.4,18.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com