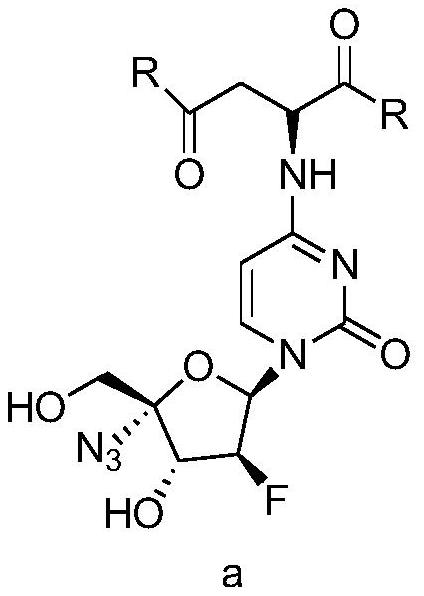
4-aspartic acid substituted cytosine nucleoside compound and medicinal application thereof
A technology of cytidine nucleoside derivatives and aspartic acid, which is applied in the fields of sugar derivatives, sugar derivatives, organic chemistry, etc., can solve problems such as 4-substituted-cytidine nucleoside compounds that have not been found, and achieve good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of compound 2:
[0029] Compound 1 (1.06g, 2mmol, 1.0eq) was dissolved in anhydrous dichloromethane (200ml), DMAP (0.05g, 0.40mmol) and N-ethyldiisopropylammonia (1.95g, 15mmol) were added, and the reaction After the mixture was stirred at room temperature under nitrogen protection for 1 hour, TPSCl (1014 g, 3.8 mmol) was added, and the stirring reaction was continued for 22 hours, and water (100 ml) was added to quench the reaction, and the organic phase was separated, extracted twice with dichloromethane, and combined The organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off under reduced pressure, then L-dimethyl aspartic acid dimethyl ester hydrochloride (0.47g, 2.4mmol, 1.2eq) and DMAP (0.12g, 1mmol, 1eq ), under nitrogen protection, anhydrous acetonitrile (15ml) was added, and diisopropylethylamine (0.78g, 6mmol, 3eq) was added. The reaction was stirred overnight at room temperature to obtai...
Embodiment 2
[0030] Embodiment 2: the synthesis of compound 3:
[0031] After compound 2 (0.90g, 1.3mmol, 1.0eq), tetrahydrofuran (8ml) and methanol (2.5ml) mixture was cooled to zero, potassium carbonate (0.37g, 2.6mmol) was added, and the reaction was continued for 1 hour under ice-bath conditions , after the reaction was complete, filtered, washed with methanol and concentrated under reduced pressure, column chromatography (dichloromethane:methanol=10:1) gave white powder compound 3 (0.55g, 95%). 1 HNMR (400Hz, DMSO-d 6 ):8.33(1H,d,J=8.0Hz),7.62(1H,d,J=7.2Hz),6.41-6.37(2H,m),5.95(1H,d,J=7.6Hz),5.76-5.66 (1H,m),5.27-5.11(1H,ddd,J=4.8,4.2,44.0Hz),5.01-4.94(1H,m),4.47-4.39(1H,ddd,J=4.8,5.2,12.0Hz) ,3.77-3.72(2H,m),3.66(3H,s),3.63(3H,s),2.93-2.82(3H,m); 13 C NMR (100Hz, DMSO-d 6 ): 171.0, 170.4, 163.1, 154.2, 141.4, 96.9 (J FC =8.4Hz), 95.5(J FC =191.0Hz), 94.6, 82.3 (J FC =15.9Hz), 74.9(J FC =24.3Hz), 62.3, 54.9, 51.8, 49.2, 35.5.m / z (ESI) 453 (M + +Na,100%)[found: M + +Na,453.11...
Embodiment 3
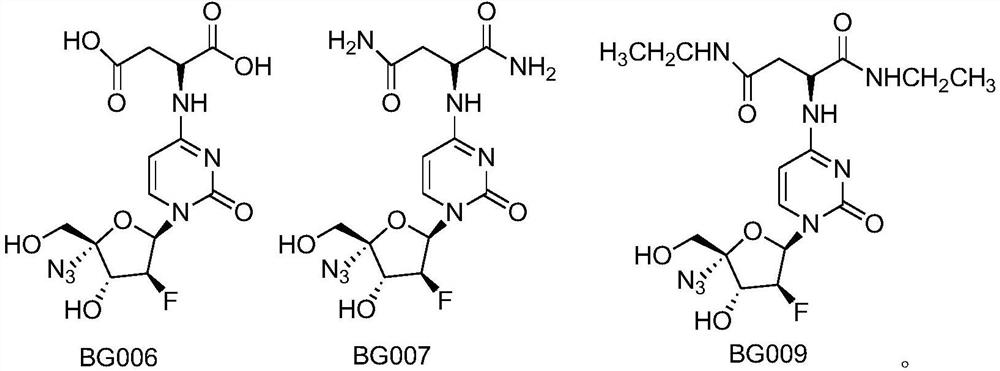
[0032] Embodiment 3: the synthesis of compound BG006:
[0033] Compound 3 (43mg, 0.1mmol) was dissolved in methanol (4ml), sodium hydroxide solution (0.5ml, 0.5mmol, 1M) was added, stirred at room temperature for 6 hours, acetic acid (35ml, 0.6mmol) was added, and separated by climbing a large plate ( Dichloromethane:methanol:acetic acid=10:1:1) to obtain white powder BG006 (38mg, 95%). 1 H NMR (400Hz, CD3OD): 7.10 (1H, d, J = 6.8Hz), 6.56-6.52 (1H, dd, J = 4.8, 10.8Hz), 6.05 (1H, d, J = 6.8Hz), 5.15 ( 1H,d,J=54.0Hz),4.75(1H,brs),4.59-4.53(1H,dd,J=4.0,22.8Hz),3.89(2H,s),3.21-3.15(1H,dd,J= 6.8,14.4Hz),2.80(2H,m); 13 C NMR (100Hz, CD3OD): 164.4, 158.3, 141.5, 98.34, 98.00, 97.50 (J FC =192.0Hz), 94.97, 84.43 (J FC =8.4Hz), 75.97 (J FC =15.9Hz), 63.02, 54.25, 47.32, 40.18.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com