Hepatitis B virus surface L protein related peptide
A protein and virus technology, applied in viral peptides, antiviral agents, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
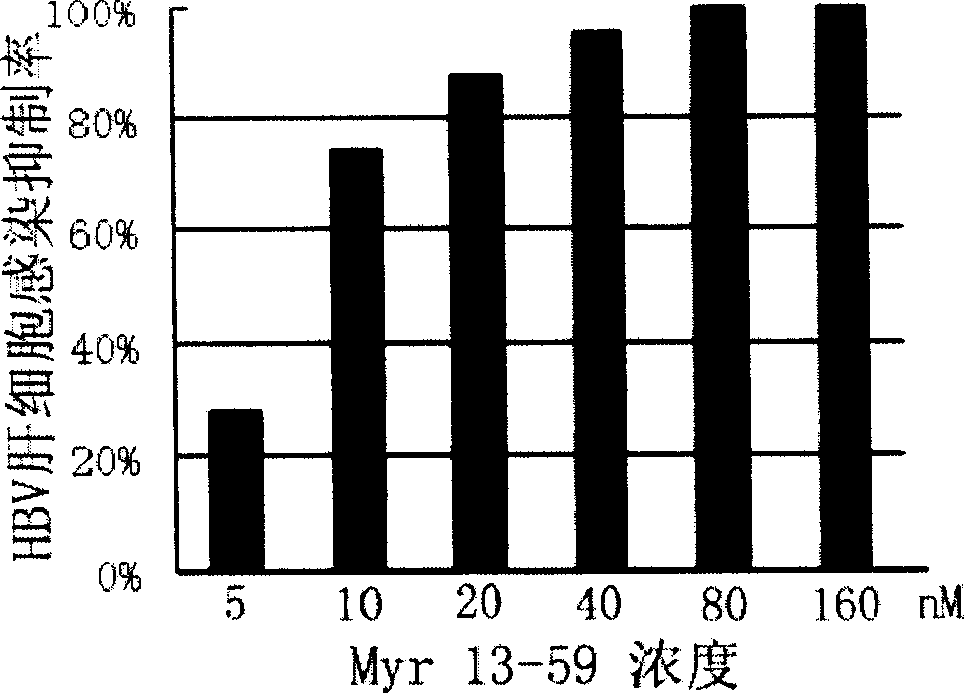
[0084] Example 1: Inhibition of HBV hepatocyte infection by peptides derived from HBV surface L protein of B genotype adw serotype and C genotype adr serotype
[0085] 1. Preparation of peptides derived from surface L protein of HBV serotype B genotype adw serotype and C genotype adr serotype
[0086] N-terminal myristoyl-modified HBV surface L protein-derived peptides of B genotype adw serotype and C genotype adr serotype (SEQ ID NO: 3 amino acid glycine modified with myristoyl, expressed as Myr 13-59) The AB 431A peptide synthesizer follows the standard Fmoc protocol and uses 0.25mM HMP as the starting resin to extend the synthesis residue by residue from the carboxyl end to the amino end. To enhance the stability of the polypeptide, the C-terminus of the polypeptide was further amidated. After peptide synthesis, cut with cutting solution, filter out resin with G6 glass sand funnel, and vacuum dry the filtrate. The peptide cleavage product was dissolved in ion-free water, ...
Embodiment 2
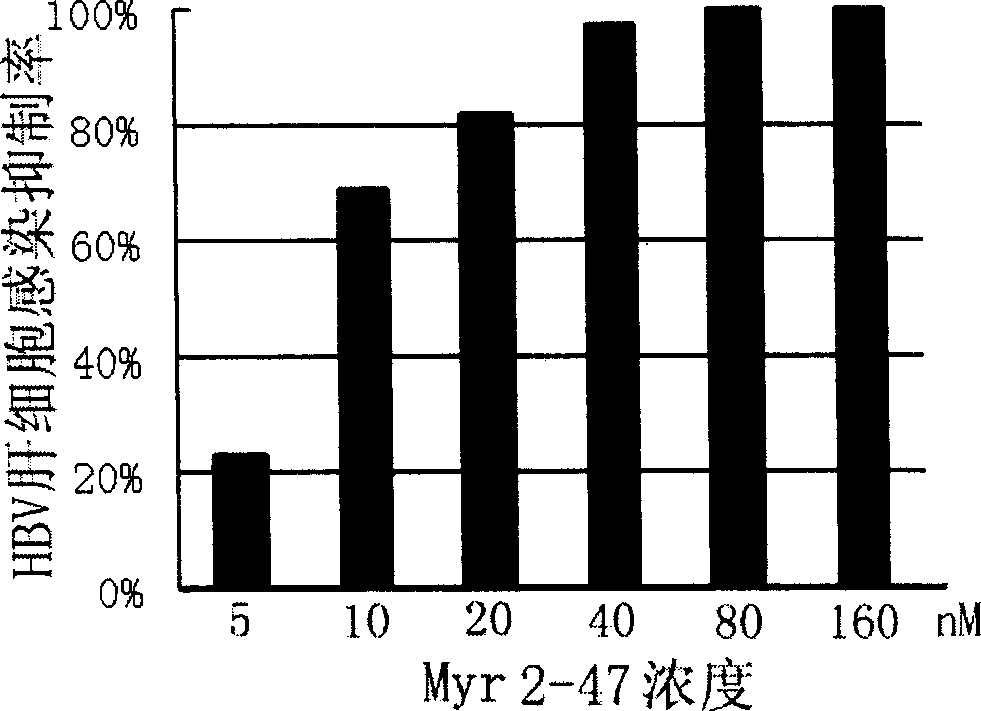
[0096] Example Two: Inhibition of HBV Hepatocyte Infection by Peptides Derived from Surface L Protein of D Genotype Ayw Serotype HBV Virus
[0097] 1. The synthesis of Myr 2-47 (SEQ ID NO: 9) derived from surface L protein of HBV serotype D ayw serotype D with myristoyl modification (SEQ ID NO: 9) and the culture of primary human hepatocytes are the same as in Example 1.
[0098] 2. Cultivation of D genotype ayw serotype HBV virus
[0099] The 2.2.15 cell line, which can secrete complete D genotype ayw serotype HBV infected virus particles, was continuously cultured for 10 days. The culture supernatant was collected, centrifuged with 6% polyethylene glycol (PEG8000) to precipitate virus particles, the pellet was resuspended in phosphate buffer containing 25% fetal calf serum, and frozen at -80°C.
[0100] 3. Detection of HBsAg in hepatocyte culture supernatant after HBV infection (same as embodiment one)
[0101] 4. Inhibition of HBV hepatocyte infection by HBV surface L pro...
Embodiment 3
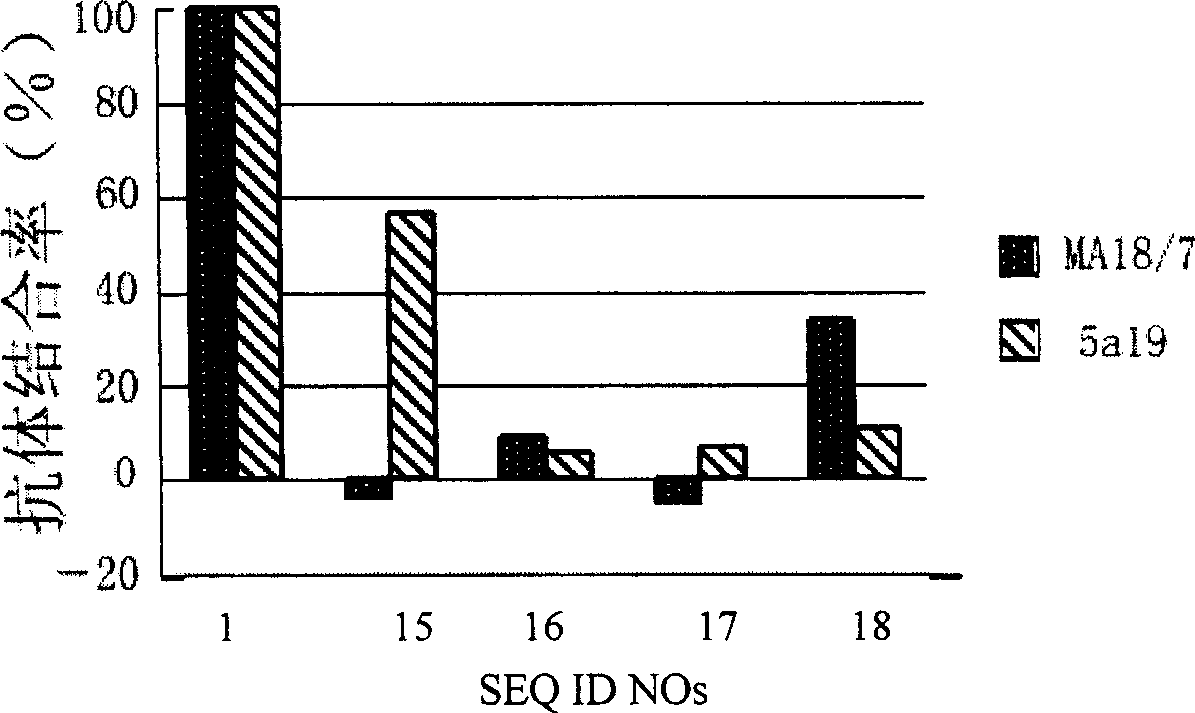
[0103] Example 3: Screening of HBV surface L protein derivatized peptides
[0104] 1, B genotype adw serotype and C genotype adr serotype HBV virus genotype serotype determination, virus culture, cultivation of human primary hepatocytes (same example 1).
[0105] 2. The construction of a recombinant plasmid carrying a complete HBV genome and having the complete replication ability of HBV.
[0106] Add 310 μl proteinase K lysate to 50 μl HBV virus culture suspension [1mg / ml proteinase K, 50mmol / L Tris-HCl (pH8.0), 200mmol / L NaCl, 10mmol / L EDTA, 2% SDS, 1μg / ml poly(A )]. After lysis at 60°C for 1 hour, extract with phenol / chloroform, precipitate with ethanol, and dissolve the precipitate in H 2 O is HBV DNA solution. Entire EN II replication element was amplified with upstream primer (Pst I) 5'ctgactgcagCACTGGATGGGGCTTGGCTATTGG (SEQ ID NO:21, 1202-1225) and downstream primer (EcoR I) 5'ttatggaattcCGACGCGGCGATTGAGACCTTC (2201-2180, SEQ ID NO:22). C genotype adr serotype HBV g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com