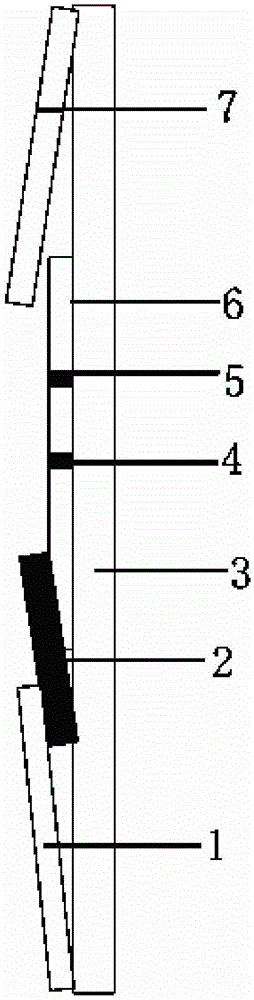
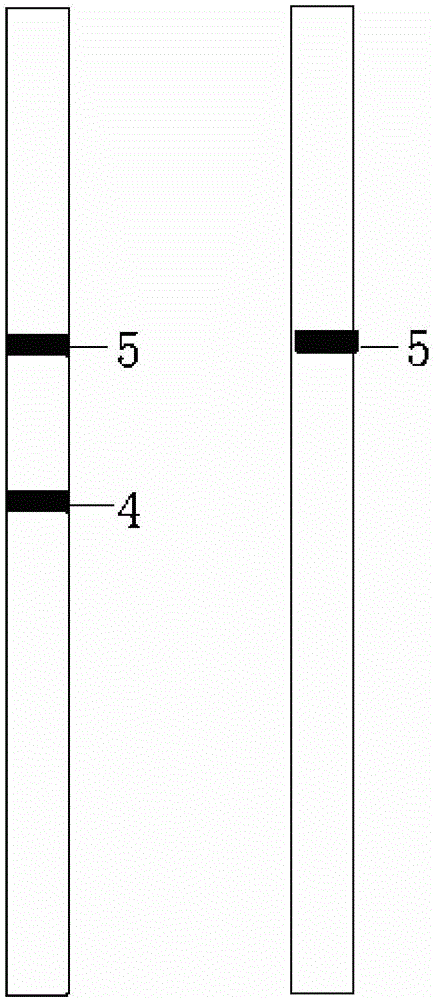
Florfenicol on-site test paper, and preparation and use methods thereof
A florfenicol, on-site detection technology, applied in the field of immunology, can solve problems such as weight reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
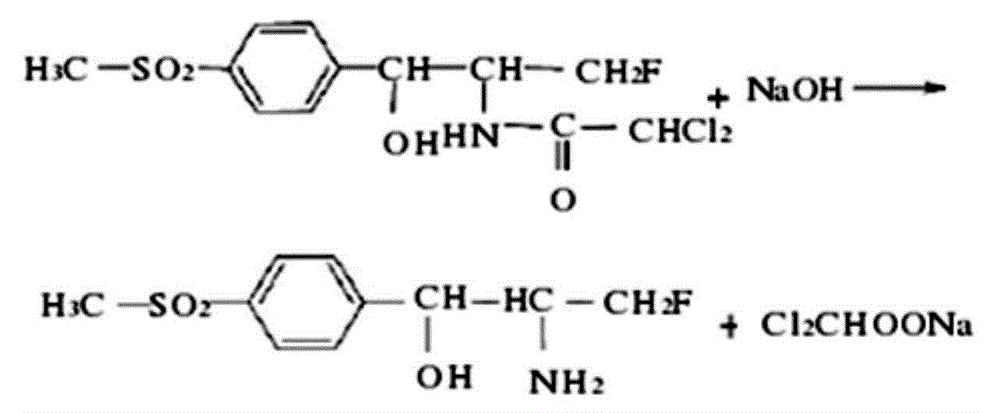
[0025] The synthesis of florfenicol amine: According to the molar ratio of 1:1, weigh FF10.74, add 2.56g of sodium hydroxide to 15ml of distilled water to dissolve, heat and stir until completely dissolved, at this time the solution is wine red and clear, then add 6.0 g of sodium chloride, stirred until completely dissolved, after cooling, use a thin-layer chromatography plate to chromatograph the mixed solution, fumigate with iodine for color development, and measure the Rf (the ratio of the distance from the origin to the center of the spot to the distance from the origin to the solvent front) value Unlike FFC, it is proven that FFA is generated. Leave the solution at room temperature overnight, filter with filter paper, and wash with distilled water until the effluent is colorless and dried FFA hapten. The synthetic route is as follows:
[0026]
Embodiment 2
[0028]Preparation of complete antigen: Weigh 25mg florfenicol amine and dissolve it in 2mL DMF; weigh 66mg BSA and dissolve it in 10ml pH7.4 PBS buffer; add florfenicol amine dropwise to the above BSA solution, at 4℃ After stirring, 100 μL of glutaraldehyde was added dropwise to the reaction liquid, and the reaction was stirred slowly for 24 h. The synthesized product was dialyzed with PBS for 72 hours, and the solution was changed every 8 hours to obtain the immunogen of florfenicol amine-bovine serum albumin (FFA-BSA), and the same method was used to prepare florfenicol amine-ovalbumin (FFA-OVA) ) is coated with the original, and the synthetic route is as follows:
[0029]
Embodiment 3
[0031] Preparation of anti-florfenicol amine monoclonal antibody: the hybridoma cells secreting anti-florfenicol amine monoclonal antibody of the present invention, its preparation and selection method are as follows:
[0032] (1) Preparation of Florfenicol amine (FFA) hapten by alkaline hydrolysis
[0033] (2) Synthesize the complete antigen of florfenicol amine with the glutaraldehyde method, including the immunogen FFA-BSA, and the former FFA-OVA of the coating;
[0034] (3) Immunizing Balb / c mice with the immunogen FFA-BSA;
[0035] (4) fusion of splenocytes and myeloma cells of immunized mice to obtain 120 strains of hybridoma cells;
[0036] (5) Screening positive hybridoma cell lines by indirect ELISA method coated with original FFA-OVA coated plates;
[0037] (6) Four of the positive hybridoma cells were cloned by the limiting dilution method;
[0038] (7) The antibody prepared by the secondary cloned cell line of the anti-florfenicol amine monoclonal antibody 2D6-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com