PDGFRA gene mutation detection liquid-phase chip
A detection solution and chip technology, applied in the field of molecular biology, can solve the problems of high false positive rate, easy contamination of samples, high price, etc., and achieve the effects of avoiding cross-reaction, strong scalability, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 PDGFRA gene mutation detection liquid chip mainly includes:
[0018] 1. ASPE Primers
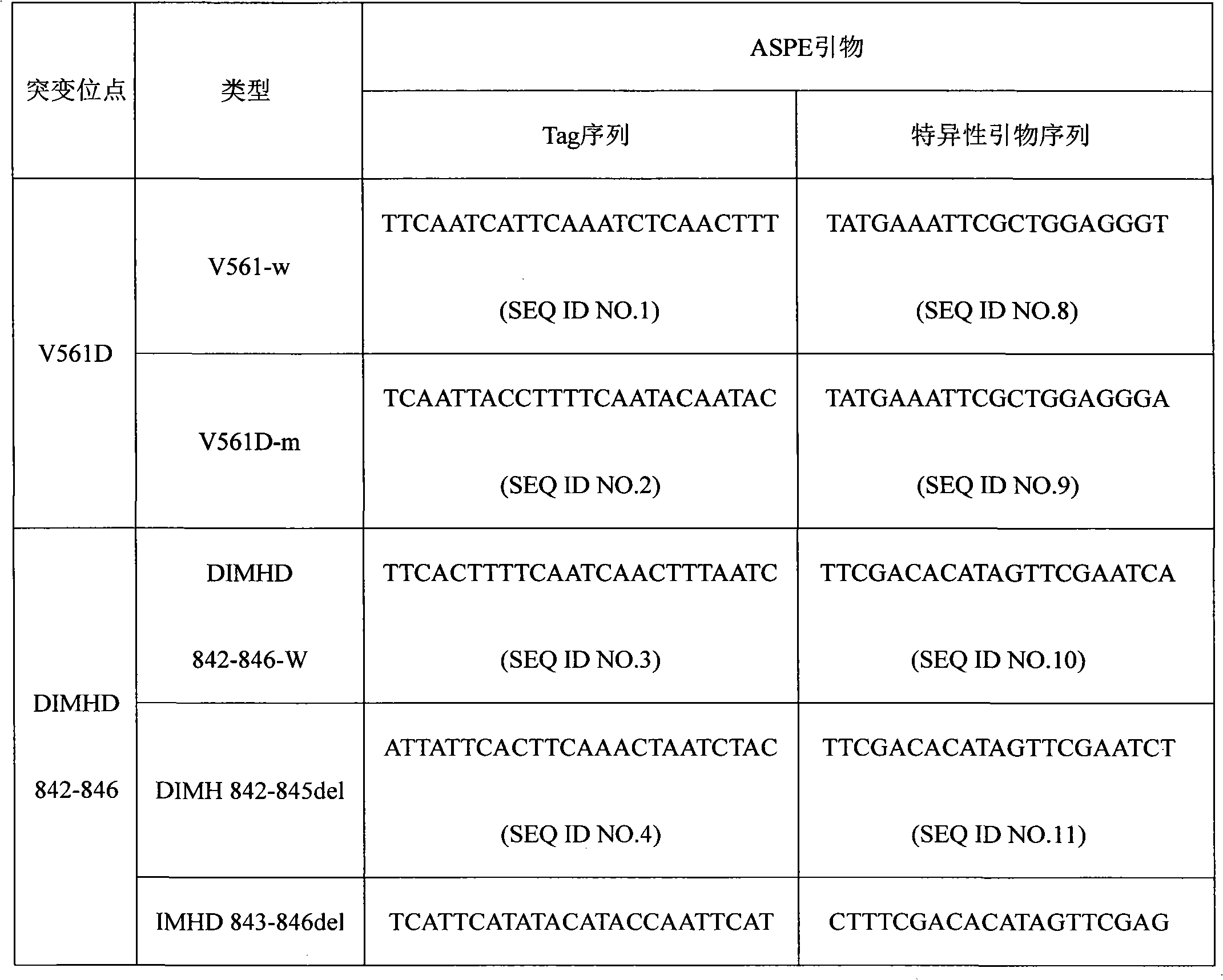
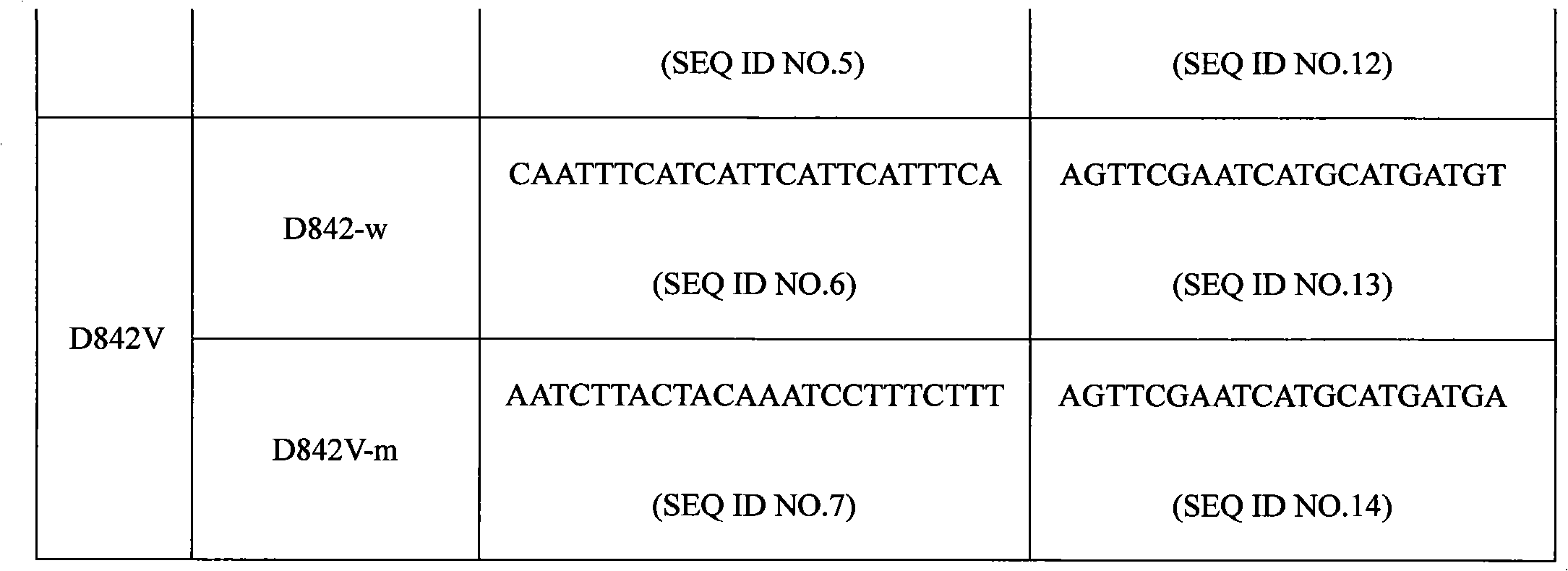
[0019] Specific primer sequences were designed for four common mutations V561D, DIMH 842-845 deletion mutation, IMHD 843-846 deletion mutation, and D842V of the PDGFRA gene. ASPE primers consist of "Tag sequence + specific primer sequence". ASPE primer sequences are shown in the table below:
[0020] Table 1 ASPE primer sequence (Tag sequence + specific primer sequence)
[0021]
[0022]
[0023] Each ASPE primer includes two parts, the 5' end is a specific tag sequence for the anti-tag sequence on the corresponding microsphere, and the 3' end is a mutant or wild-type specific primer fragment (as shown in the above table 1). All ASPE primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. Each synthesized primer was prepared into a 100pmol / mL stock solution with 10mmol / LTris Buffer.
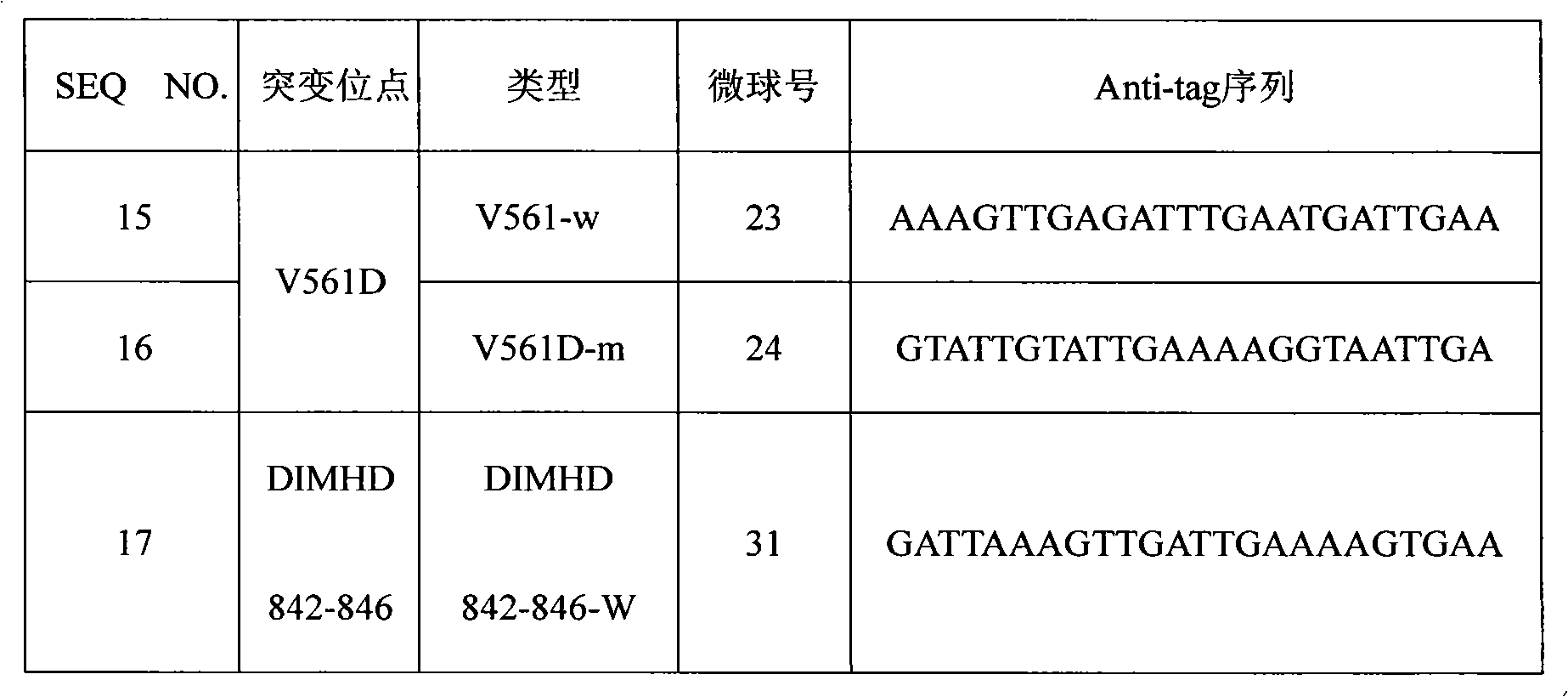
[0024] 2. Microspheres coated with anti-tag s...
Embodiment 3
[0102] The liquid phase chip of embodiment 3 different ASPE primers is to the detection of PDGFRA gene mutation site
[0103] 1. Design of liquid phase chip preparation (selection of Tag sequence and Anti-Tag sequence)
[0104] Taking the PDGFRA gene locus V561D mutation detection liquid chip as an example, the specific primer sequence of the 3' end of the ASPE primer was designed for the wild type and mutant type of V561D, and the Tag sequence at the 5' end of the ASPE primer was selected from SEQ ID NO.1 -SEQ ID NO.7, correspondingly, the anti-tag sequence coated on the microsphere and complementary to the corresponding tag sequence is selected from SEQ ID NO.15-SEQ NO.21. The specific design is shown in the following table (Table 7). The synthesis of ASPE primers, microspheres coated with anti-tag sequences, amplification primers, detection methods, etc. are as described in Example 1 and Example 2.
[0105] Table 7 Design of liquid phase chip preparation
[0106]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com