Human parainfluenza virus distinguishing and quantitative detection regent kit
A detection kit and the technology of the kit, which are used in the determination/inspection of microorganisms, material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of inability to diagnose parainfluenza virus and limitations of clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
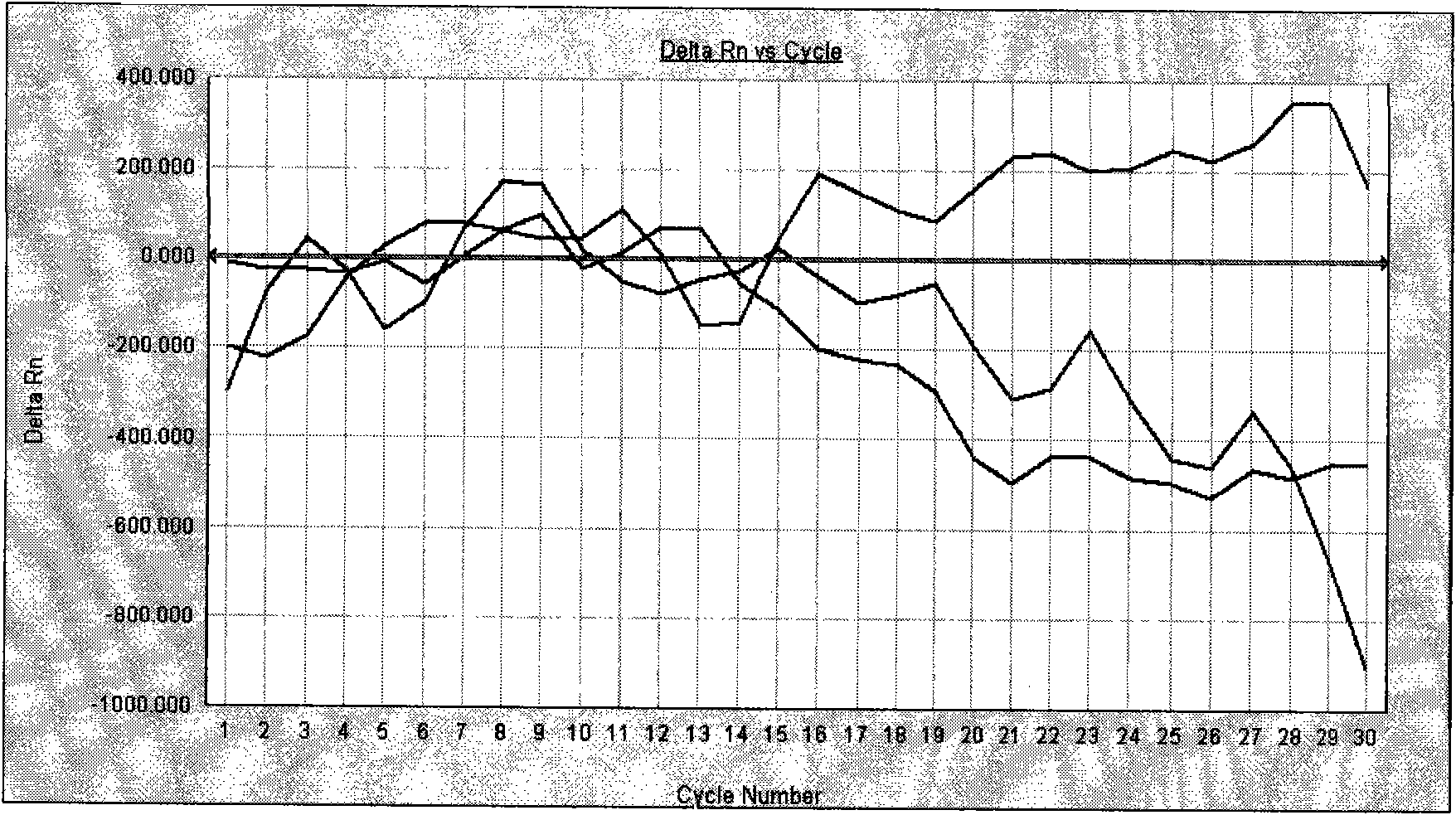
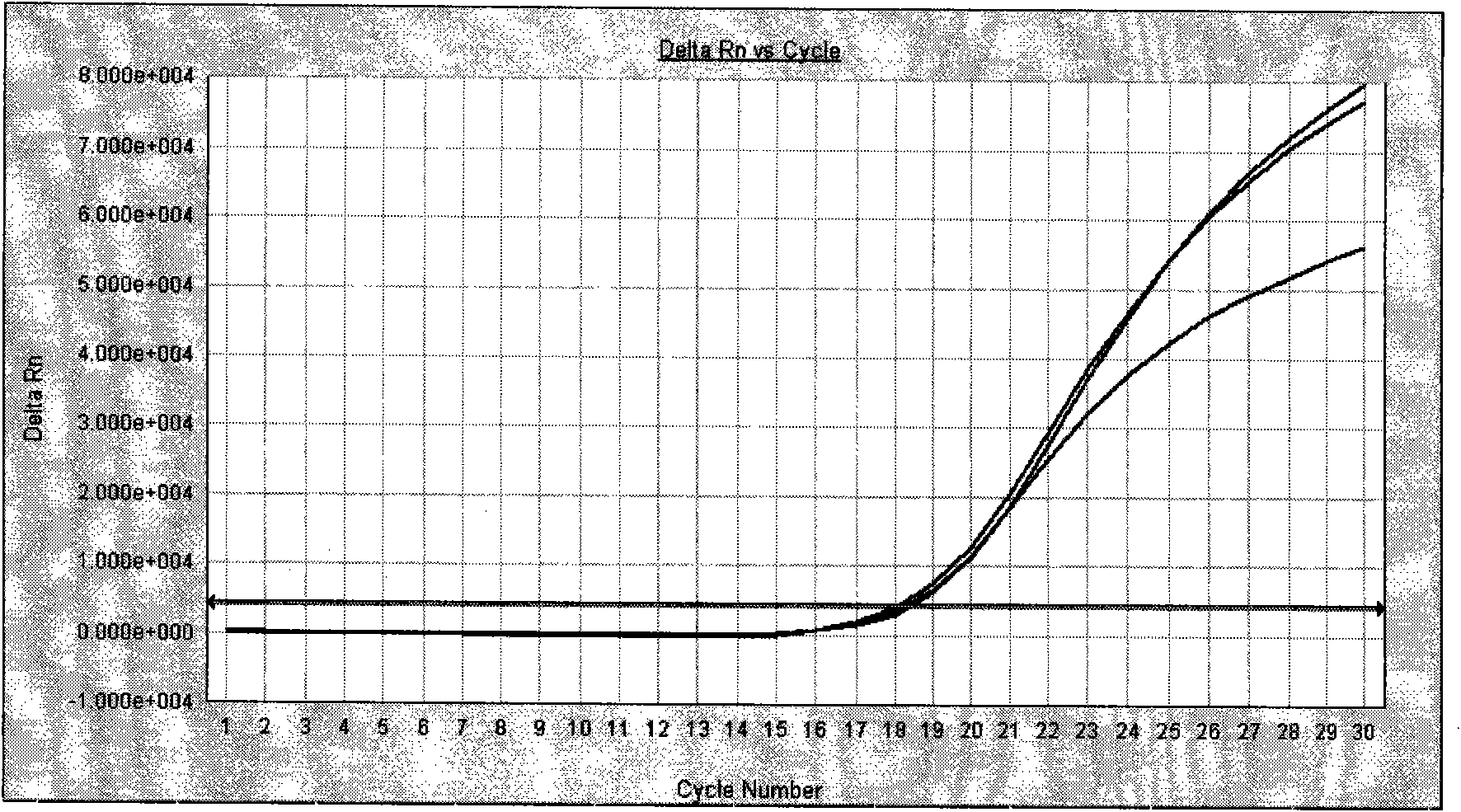
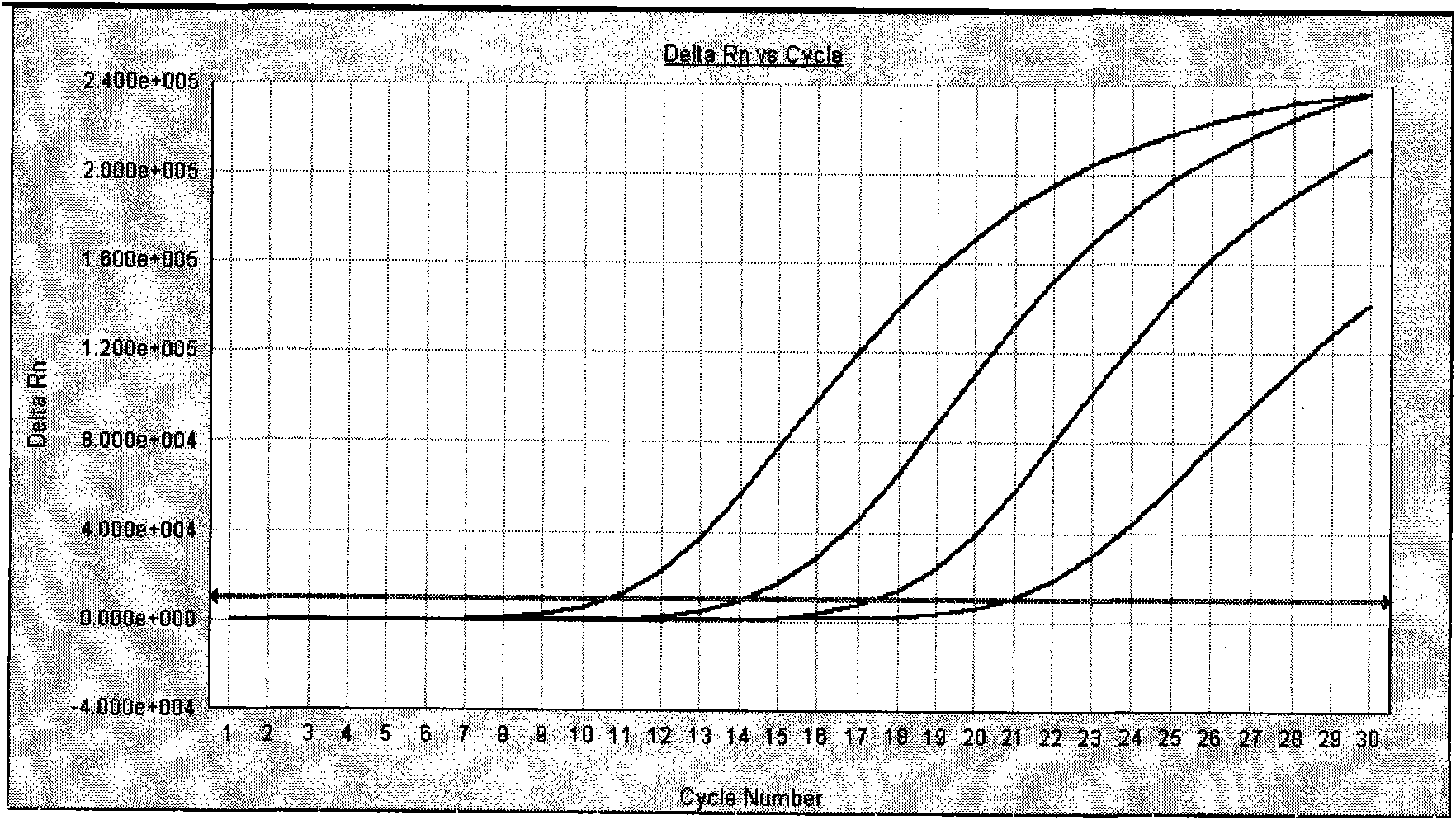
Image
Examples
Embodiment 1
[0059] Embodiment 1. The composition of human parainfluenza virus RT-PCR one-step typing, quantitative detection kit
[0060] 1.1 Extraction reagent: DEPC.H 2 O; RNA extraction solution A (for DEPC.H 2 O diluted, and autoclaved after 10% SiO 2 );
[0061] Trizol; Chloroform.
[0062] 1.2 RT-PCR reaction reagents
[0063] Including HPIV1 reaction tube, HPIV2 reaction tube, HPIV3 reaction tube
[0064] 1.3 Quality control: positive quality control-P (HPIV1, HPIV2 and HPIV3 in vitro transcription RNA mixture); negative quality control-N (normal saline); quantitative reference-S1~S4 (10 4 copies / ml~10 7 copies / ml HPIV1 or HPIV2 or HPIV3 in vitro transcribed RNA)
Embodiment 2
[0065] Embodiment 2. Use human parainfluenza virus RT-PCR one-step typing, quantitative detection kit to detect the infection of human parainfluenza virus in the sample to be tested
[0066] 2.1 Collection, preservation and transportation of specimens: Detectable specimens include sputum, throat swab and bronchoalveolar lavage fluid. The collection methods are as follows: ① Sputum: suck sputum under negative pressure, seal it and send it for inspection; ③Bronchoalveolar lavage fluid: About 1ml of bronchoalveolar lavage fluid was collected by clinicians, sealed and sent for examination. Specimens can be used for testing immediately, or can be stored at -70°C for testing, and the storage period is 6 months. Specimens were transported using 0°C ice cubes.
[0067]2.2 Nucleic acid extraction: ①Pipe 100μl of sputum sample into a 1.5ml sterilized centrifuge tube; centrifuge throat swab sample liquid or bronchoalveolar lavage fluid at 12,000rpm for 5 minutes, discard the supernatan...
Embodiment 3
[0077] Embodiment 3. The composition of human parainfluenza virus RT-PCR two-step method typing, quantitative detection kit
[0078] 3.1 Extraction reagent: same as 2.1 in Example 2
[0079] 3.2 RT-PCR Reagents
[0080] Including mixed reverse transcription reaction tube (containing HPIV1, HPIV2, HPIV3 primers), reverse transcriptase system, HPIV1 reaction tube, HPIV2 reaction tube, HPIV3 reaction tube
[0081] 3.3 quality control product: the same as 2.3 in Example 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com