A real-time fluorescent RT-PCR kit for detecting human astrovirus and its application
A technology of RT-PCR and astrovirus, which is applied in the direction of fluorescence/phosphorescence, measurement/inspection of microorganisms, DNA/RNA fragments, etc., can solve the problems of inability to cover HAstV serotypes, lack of cross-reactive antigens, application limitations, etc., to achieve Avoid plateau effects, avoid false positives and environmental pollution, change the effect of defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Development of one-step real-time fluorescent quantitative PCR reagent for human astrovirus
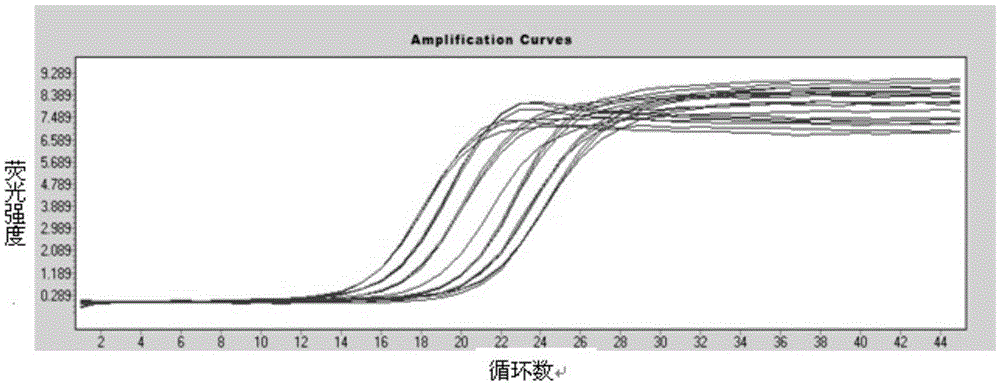
[0035] 1. Design of primers and probes: by using DNAman software to perform sequence alignment analysis on the existing human astrovirus nucleic acid sequences in the Genebank database, the conserved fragment of the ORF1-ORF2 junction region of the human astrovirus genome was used as the amplification target According to the basic principles of primer-probe design, multiple pairs of primers and probes are manually designed using software.
[0036] 2. Selection of samples: According to relevant literature reports at home and abroad, samples such as stool and anal swab can be selected.
[0037] 3. Establishment and optimization of the reaction system
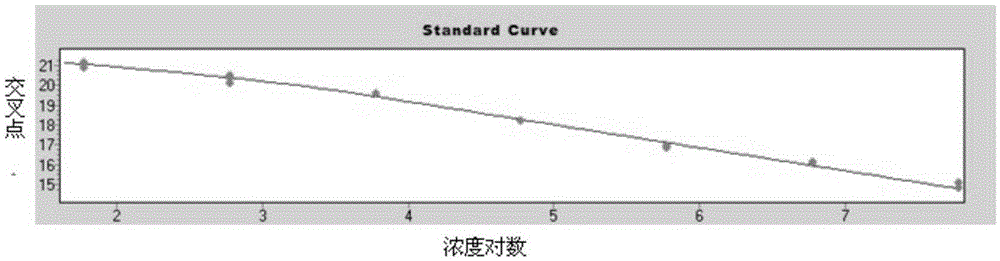
[0038] Sample preparation: 10 samples that were identified as positive for human astrovirus were used as HAstV positive reference products, namely HAstV-1, HAstV-2, HAstV-3, HAstV-4, HAstV-5, HAstV-6, HAstV- 7. HAstV-8...
Embodiment 2
[0047] Example 2: Human astrovirus one-step fluorescent real-time quantitative RT-PCR detection kit and its use
[0048] 1. Prepare a kit including the following components: RNA extraction solution, RT-PCR amplification reaction solution, negative quality control substance, positive quality control substance, quantitative reference substance, and DEPC-treated water.
[0049] 2. Collection, transportation and storage of specimens
[0050] 2.1 Applicable specimen types: stool, anal swab, etc.
[0051] 2.2 Specimen collection and pretreatment (pay attention to aseptic operation)
[0052] 2.2.1 Stool specimen collection: Stool collection should be carried out by specially trained personnel. The specimen collector collects 5g (5ml) of stool and puts it in a sterile stool sampling cup (without adding any reagents).
[0053] 2.2.2 Anal swab specimen collection: soak cotton swab in normal saline, insert it into the anus 2-3cm, wipe it from the folds around the anus, or gently rotate...
Embodiment 3
[0067] Example 3: Human astrovirus one-step fluorescence real-time quantitative RT-PCR detection kit clinical detection use
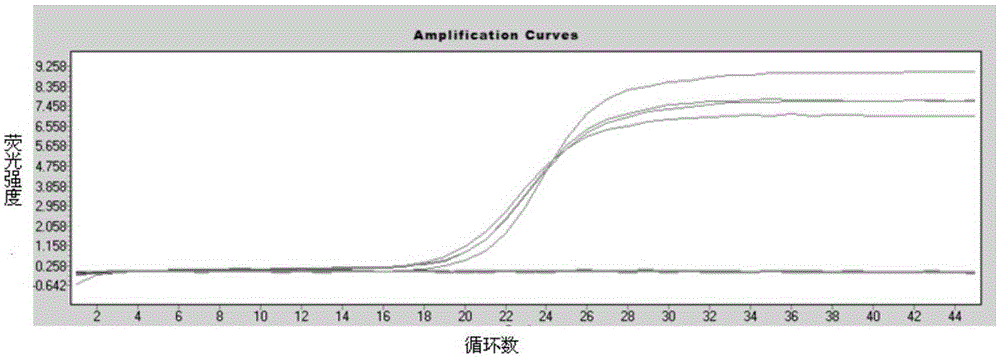
[0068] The above method was used to test 18 stool samples of other patients suspected of human astrovirus infection, among which 4 cases were positive for HAstV, and the virus fluorescence quantitative PCR amplification curve is shown in image 3 According to the Ct values of these 4 cases of positive results combined with the amplification curve, the virus concentrations of these 4 cases of HAstV positive samples were automatically analyzed by RocheLightCycler480 analysis software. The specific results are shown in Table 1.
[0069] Table 14 Cases of HAstV positive specimen virus concentration
[0070] Sample serial number Ct value HAstv virus concentration (copy number / μl) sample 1 23.14 2.45X10 2 sample 2 21.89 2.2g×10 2 sample 3 21.46 2.58×10 3 Sample 4 21.86 2.48×10 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com