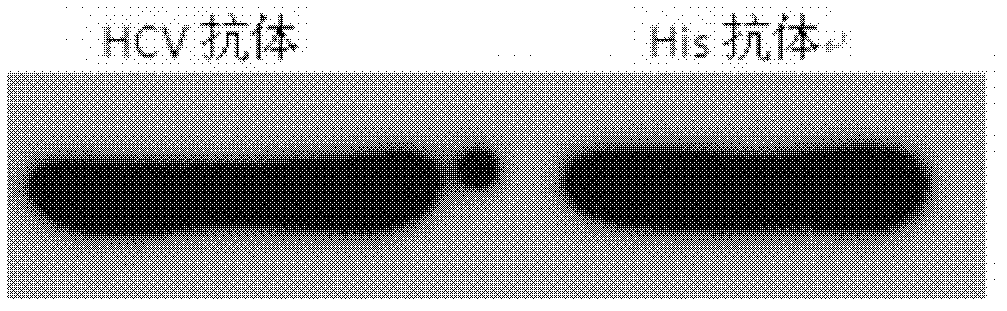
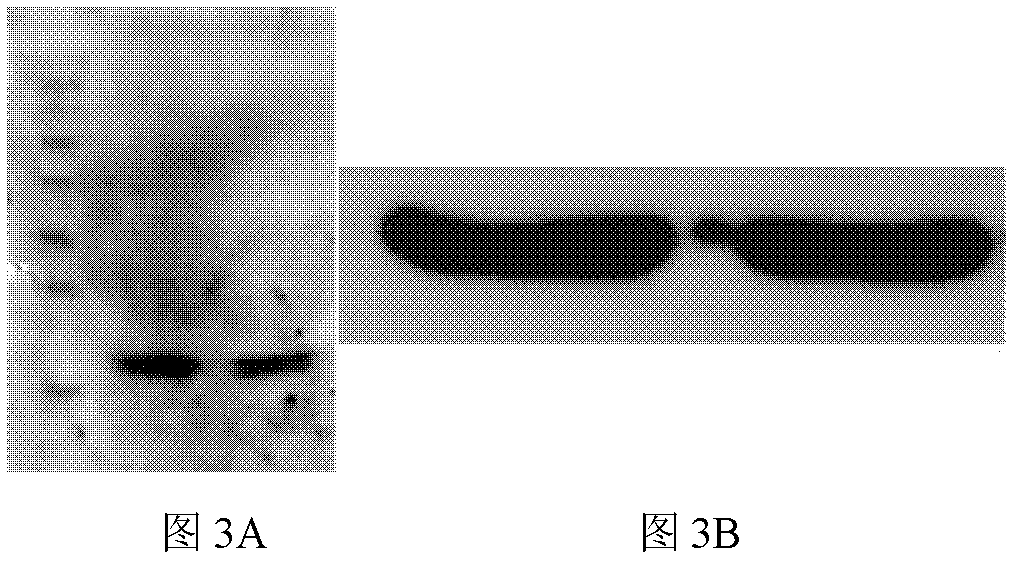
HCV (hepatitis C virus) core antigen and antibody thereof as well as hybridoma cell lines secreting antibody
A technology of hepatitis C virus and hybridoma cell lines, applied in the direction of viral peptides, antiviral immunoglobulins, analytical materials, etc., can solve the problems of difficult large-scale screening, complicated detection operations, expensive prices, etc., and achieve reliable Detection method, high purity, and high-efficiency secretion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment one immunogen
[0031] 1. Design and synthesize the primer sequence of the HCV core region gene fragment (synthesized by Shanghai Boshang Biotechnology Co., Ltd.), as shown in SEQ ID NO.3 and 4.
[0032] 2. Extract HCV infected patients (20 HCV serum samples used for HCV RNA extraction are from HCV-RNA positive but HCV antibody-negative patients collected by Qilu Hospital of Shandong University, including 16 males and 4 females, with an average age of 45 years old. The genotyping test results were 13 cases of Ib subtype, 7 cases of 2a subtype) serum RNA, reverse transcription PCR, and the reaction system was as follows:
[0033]
[0034] The PCR reaction was carried out according to the following conditions: 94°C for 30s, 55°C for 30s, 72°C for 30s, after 35 cycles, 72°C for 10min.
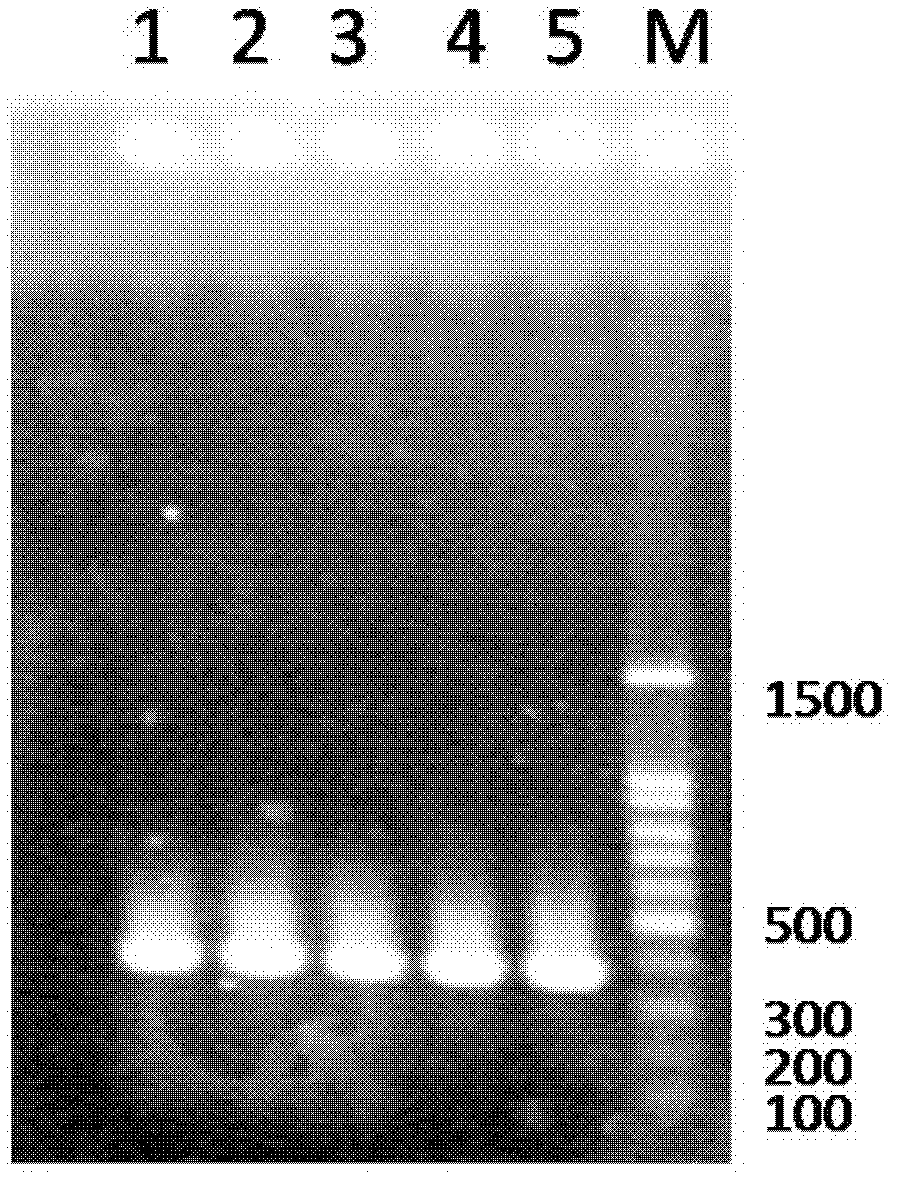
[0035] 3. After agarose gel electrophoresis, the target PCR product was recovered from the gel, connected to the pMD18-T vector, and transformed into DH5α ...
Embodiment 2
[0046] Embodiment 2 hybridoma preparation monoclonal antibody
[0047] 1. Animal immunity:
[0048] Select 6-week-old Balb / c female mice, subcutaneously inject the immunogen at multiple points, use Freund's complete adjuvant, mix at a ratio of 1:1, and inject subcutaneously with incomplete Freund's adjuvant 10 days after the initial immunization, once a week, three times in a row. After the last immunization, blood was collected by docking the tail, and the mouse antibody titer was initially identified by indirect ELISA method. After 10 days, a booster immunization was given.
[0049] 2. Cell fusion:
[0050] Before fusion, mouse myeloma cells SP2 / 0 were resuscitated and cultured with 8-azaguanine to ensure that the cells were in the best growth state during fusion. On the day of fusion, mouse peritoneal macrophages were taken to prepare feeder cells, and 10ml HAT medium was added to seed them in 96-well plates.
[0051] Three days after the booster immunization of the im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com