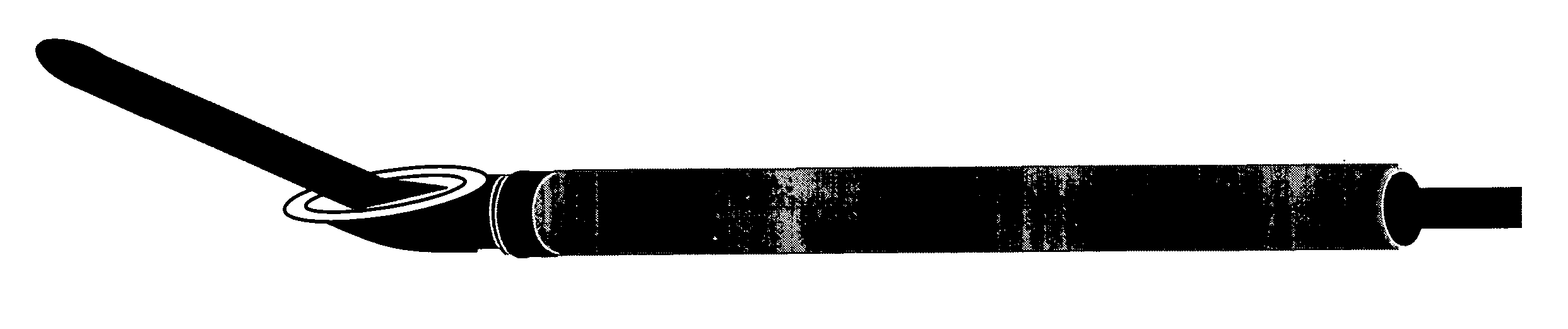Patents
Literature
32 results about "Neural foramen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
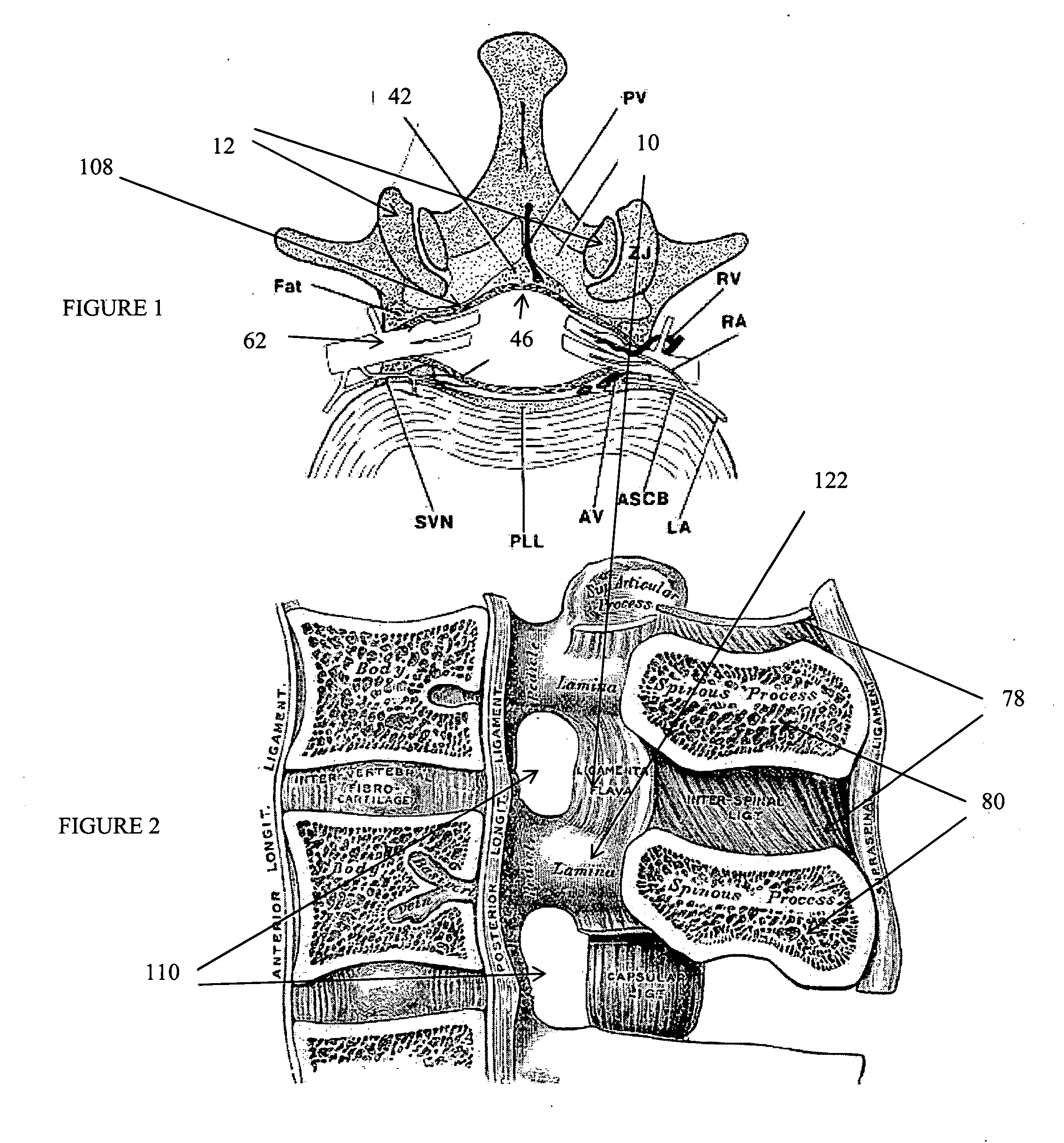
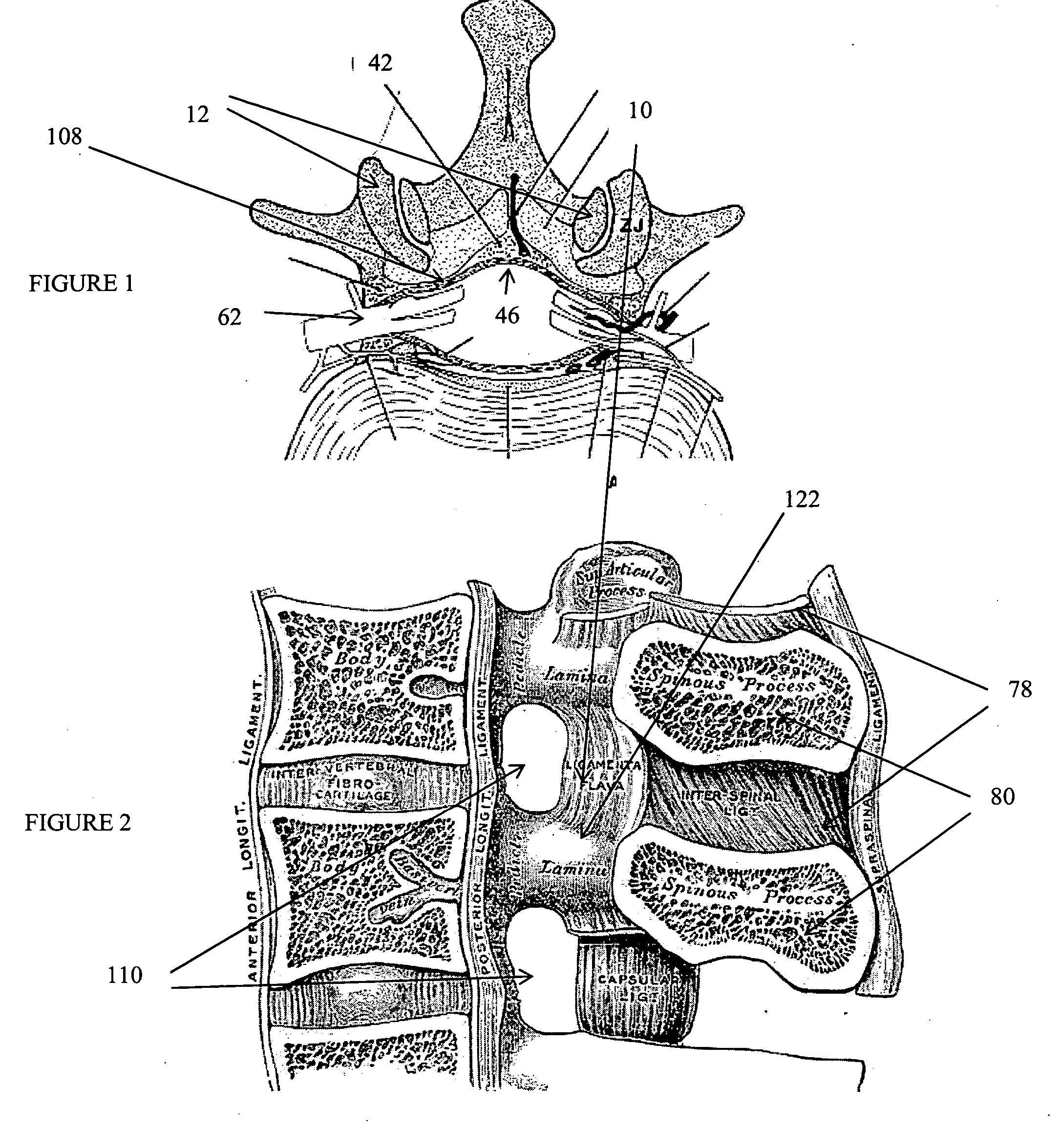
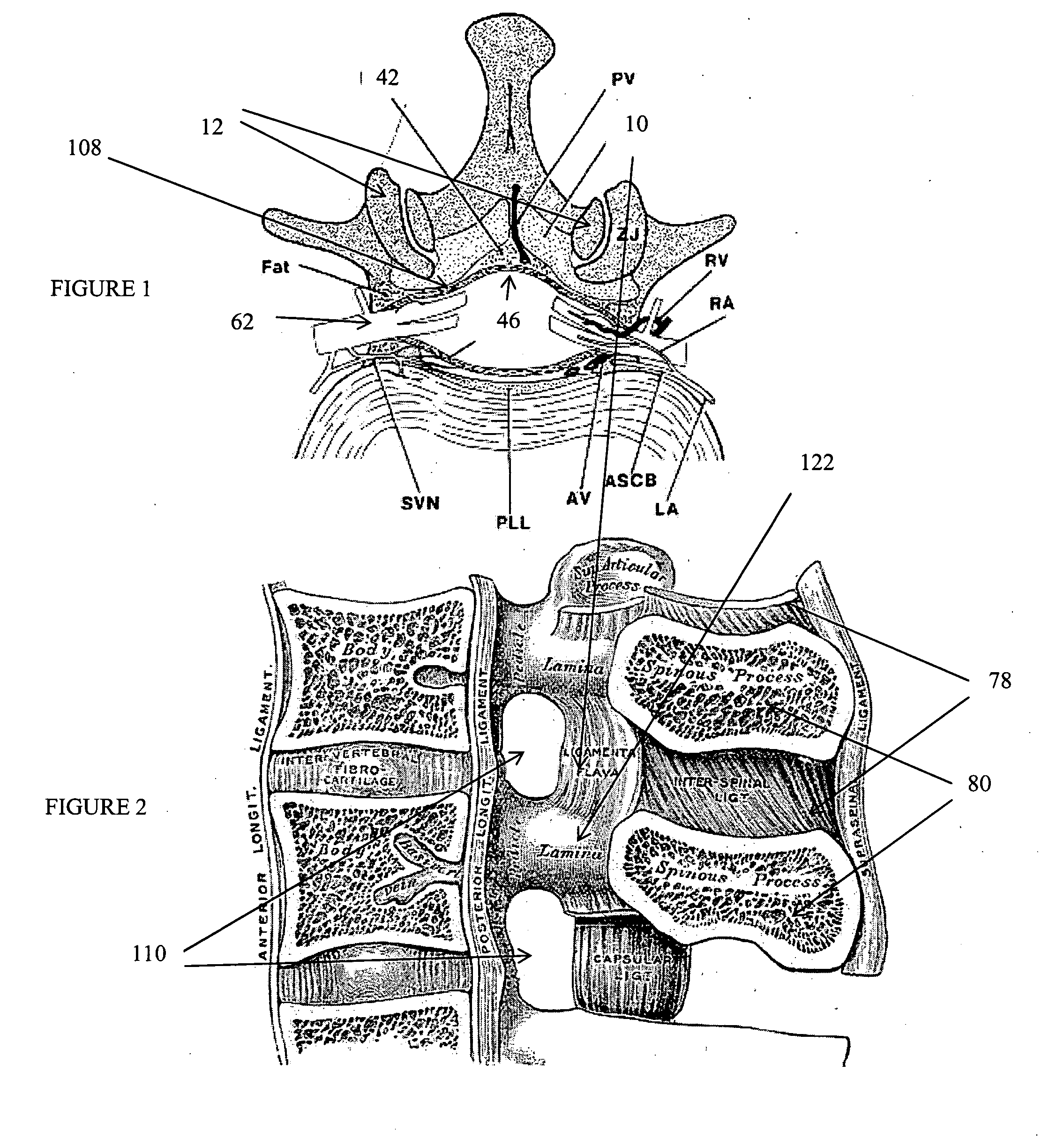
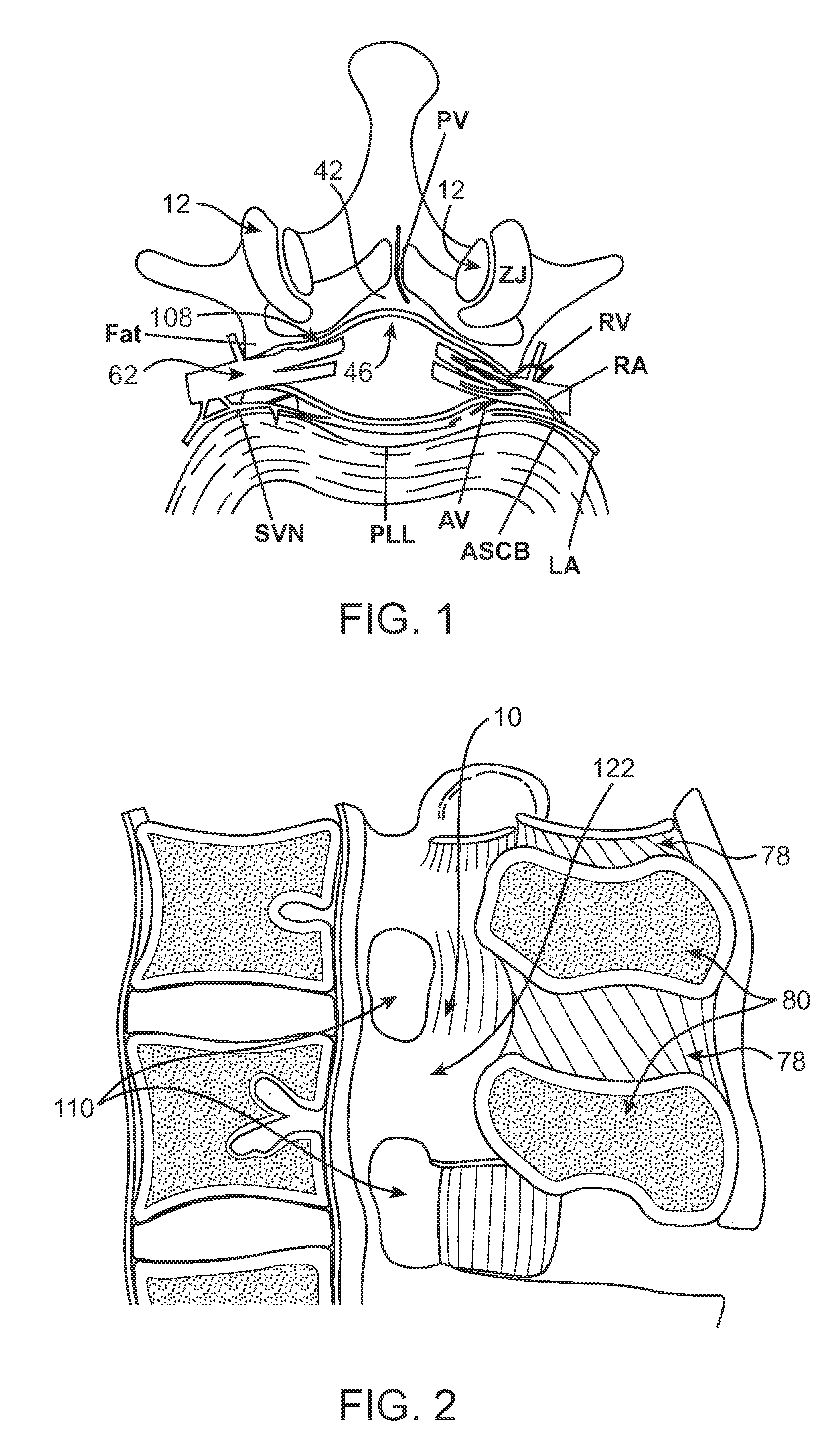
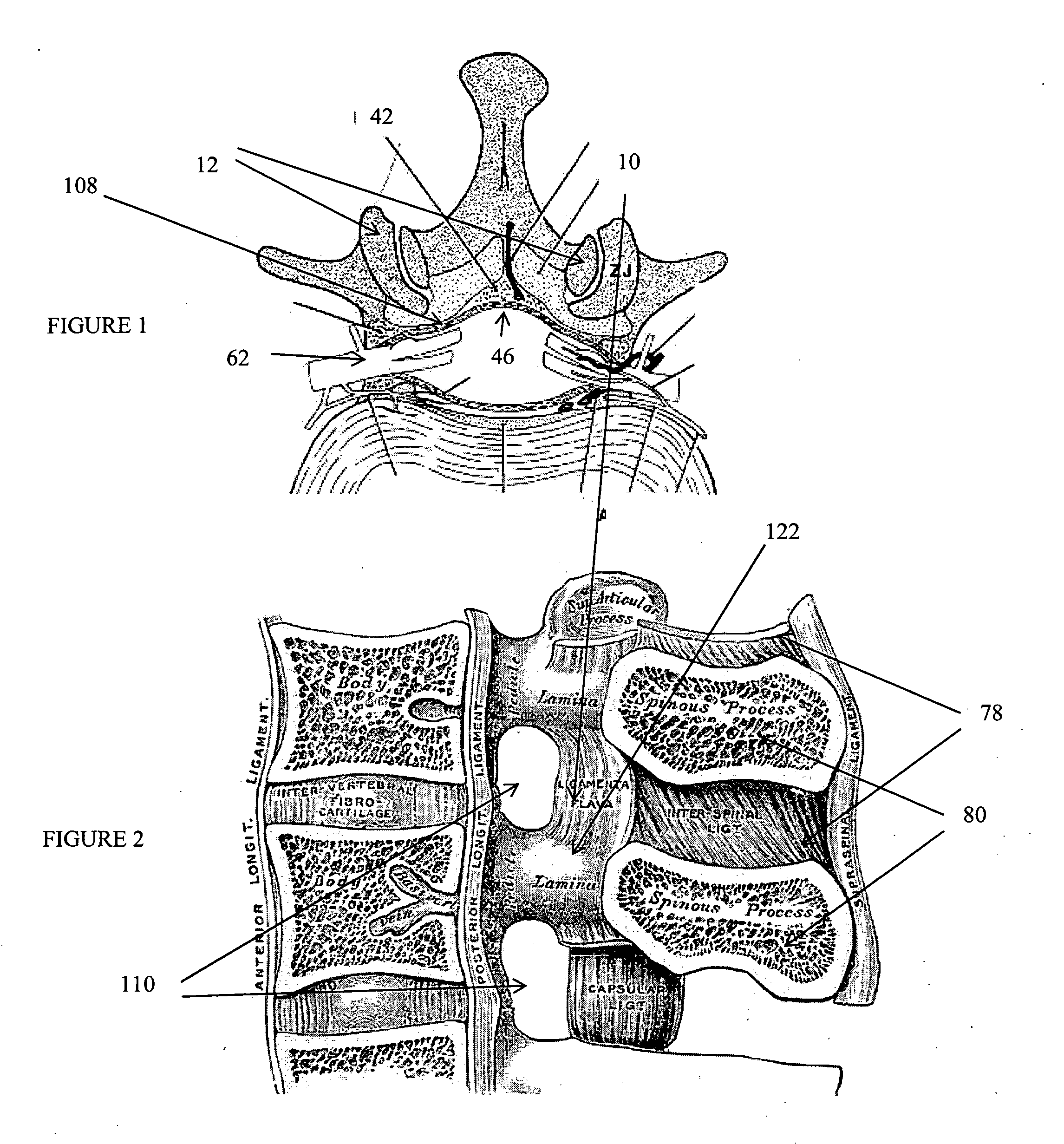
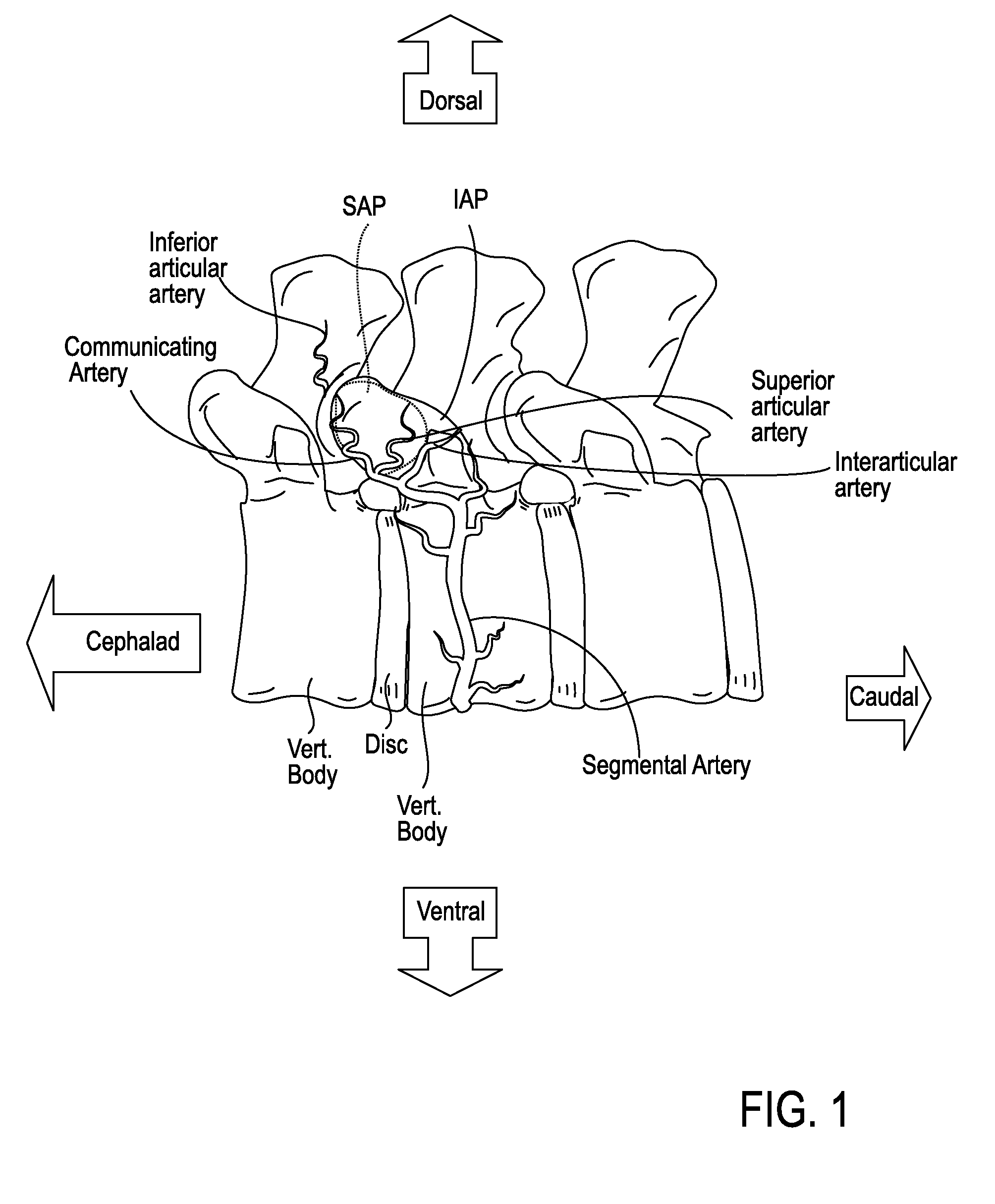
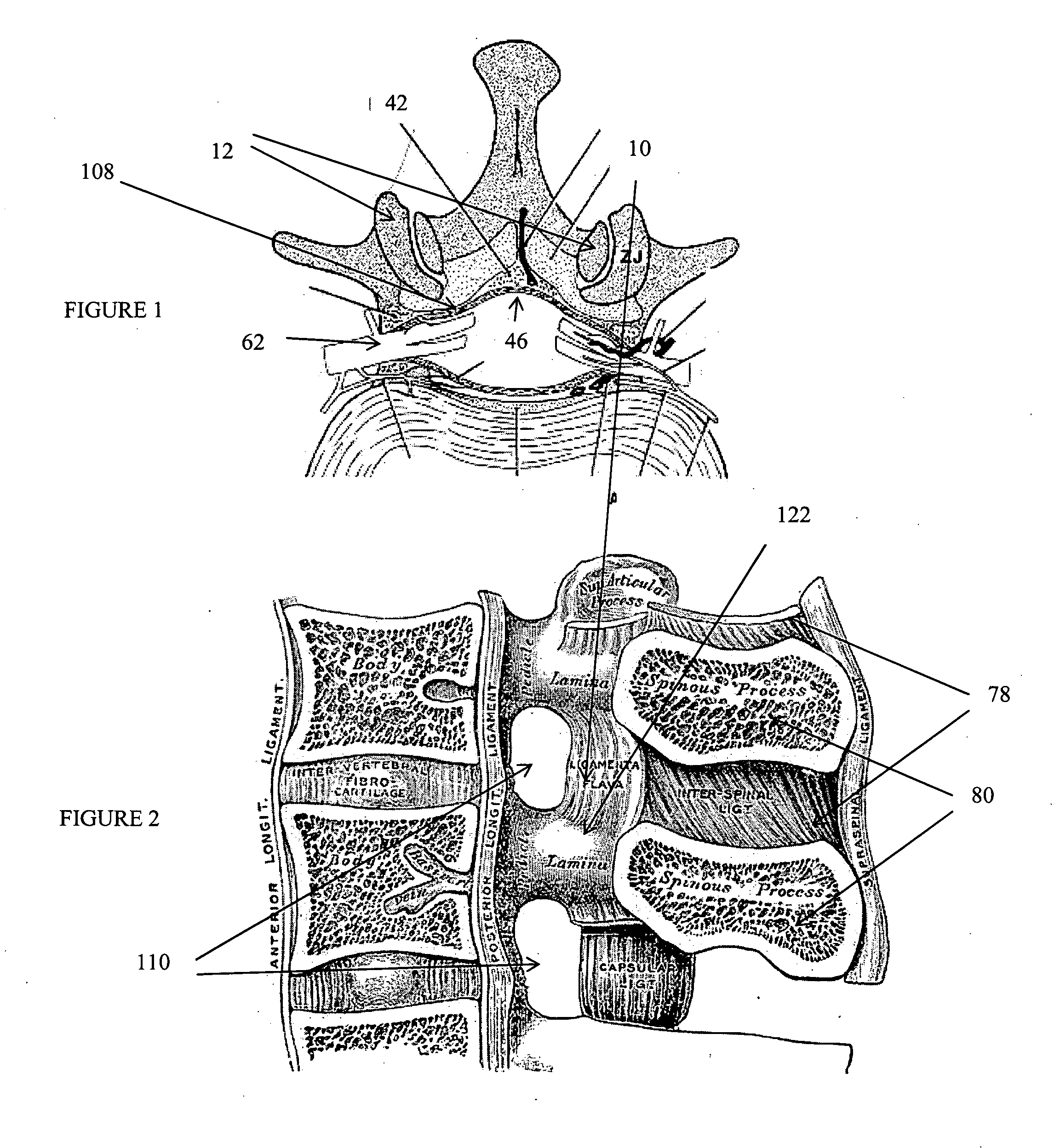
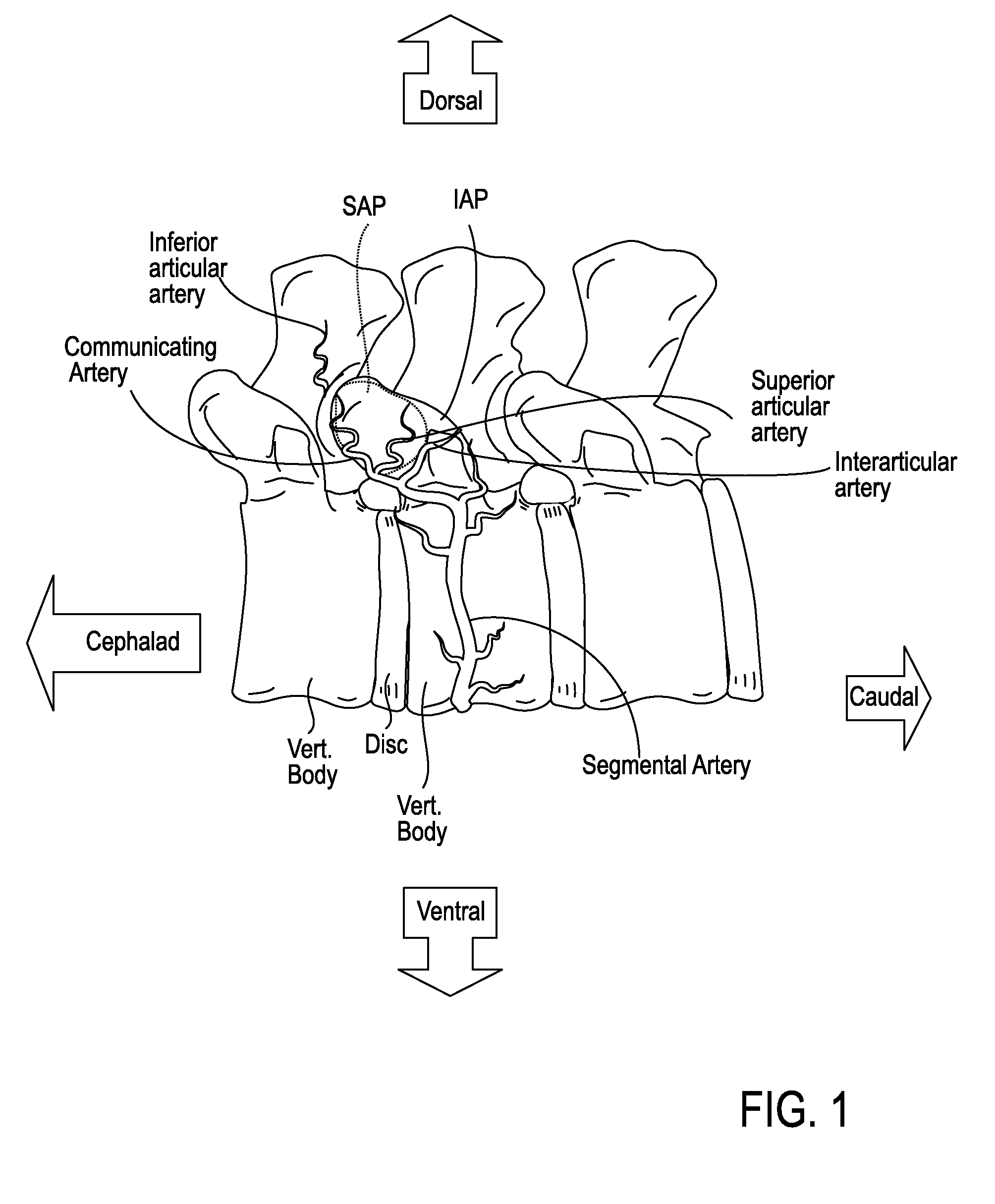
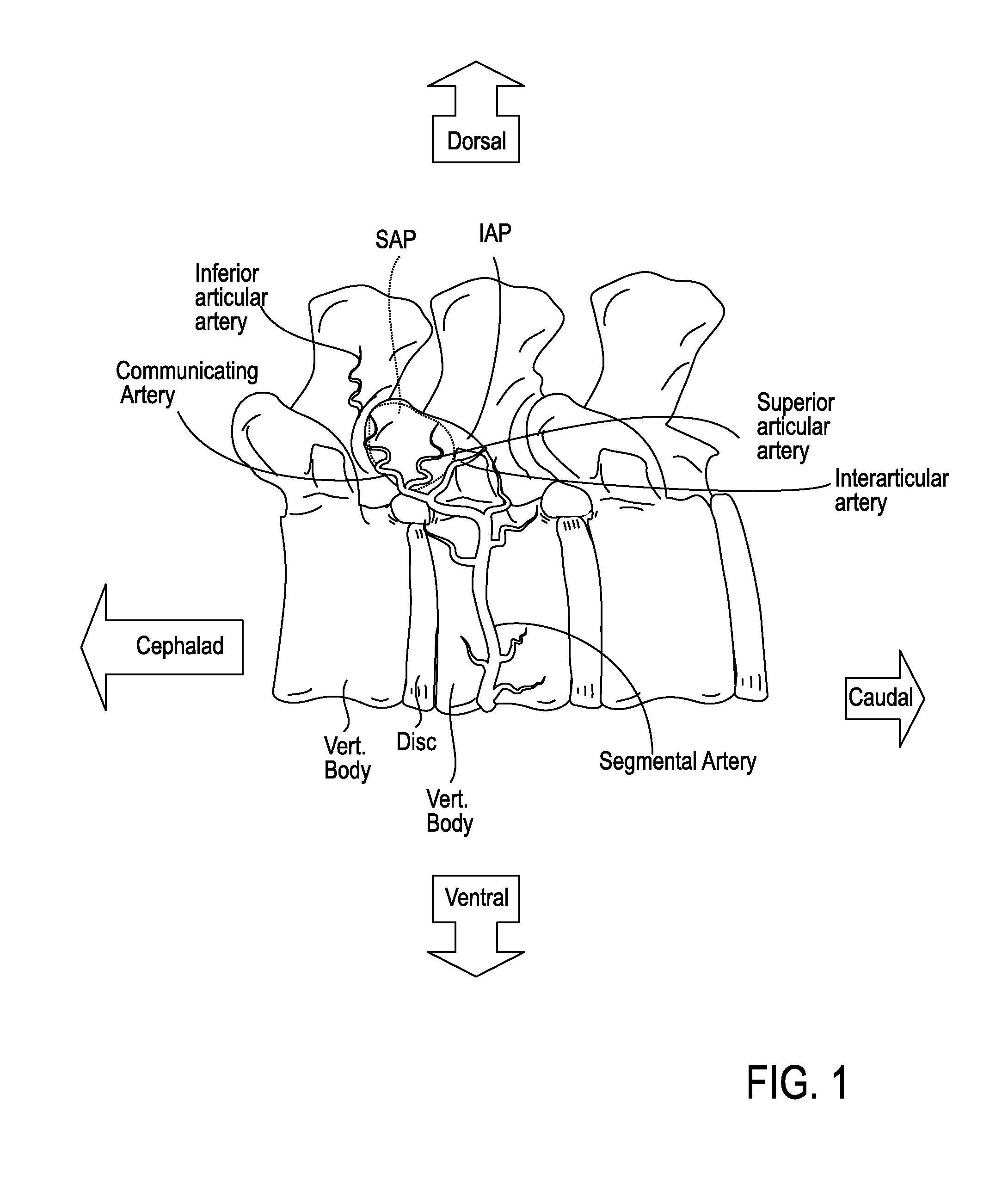
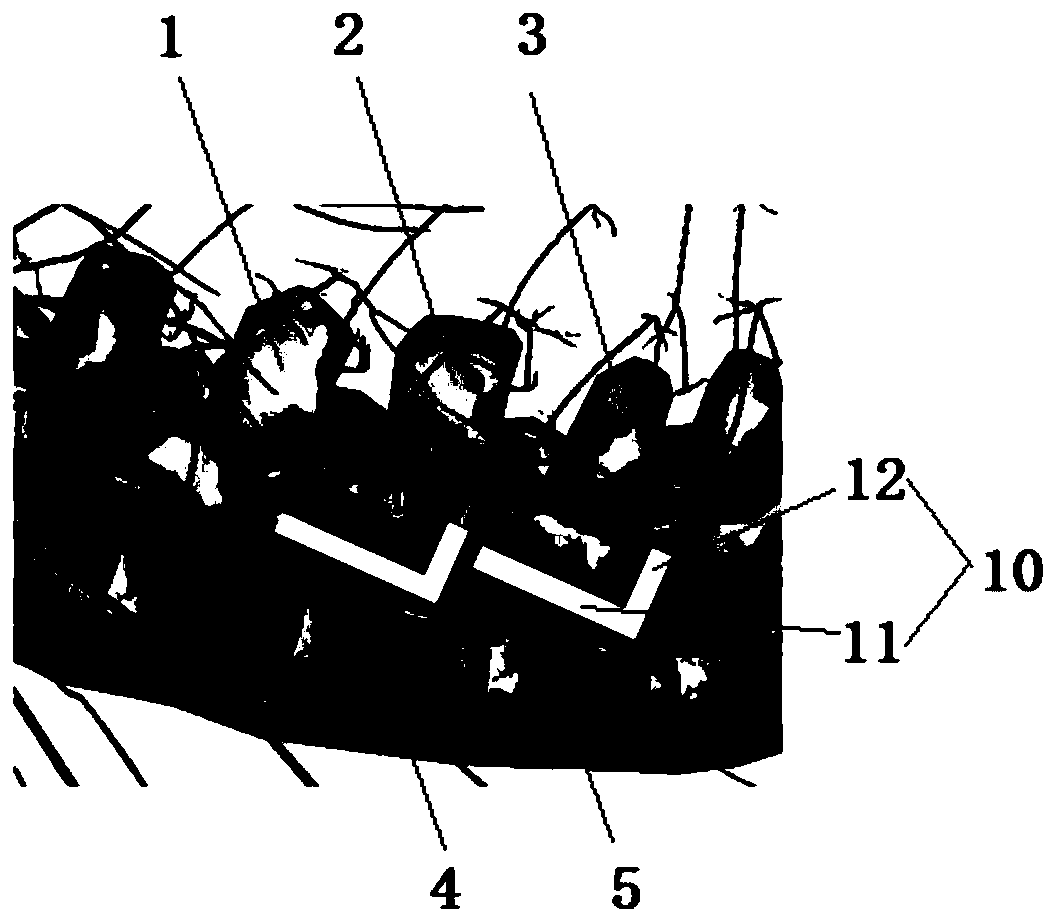
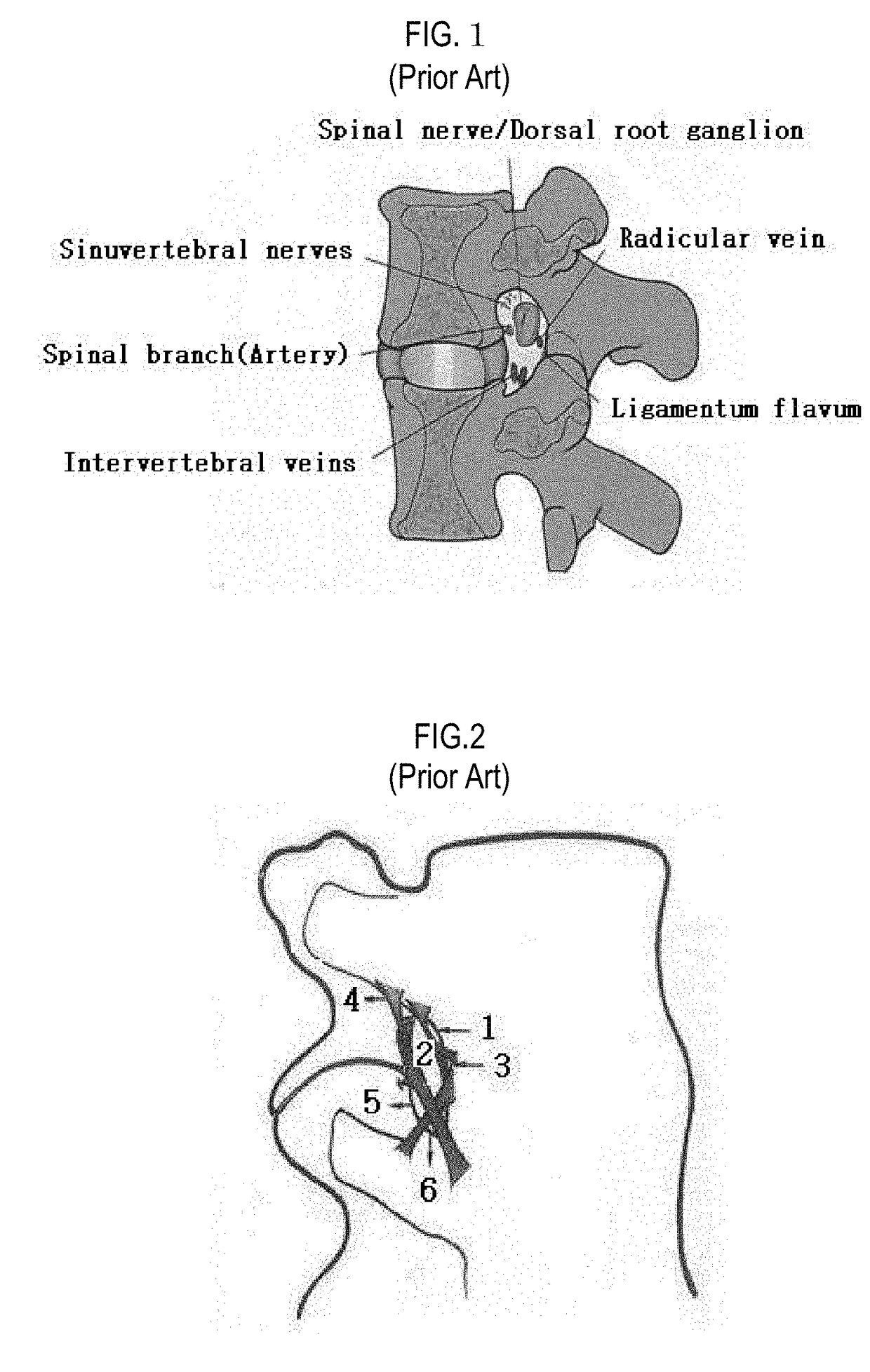
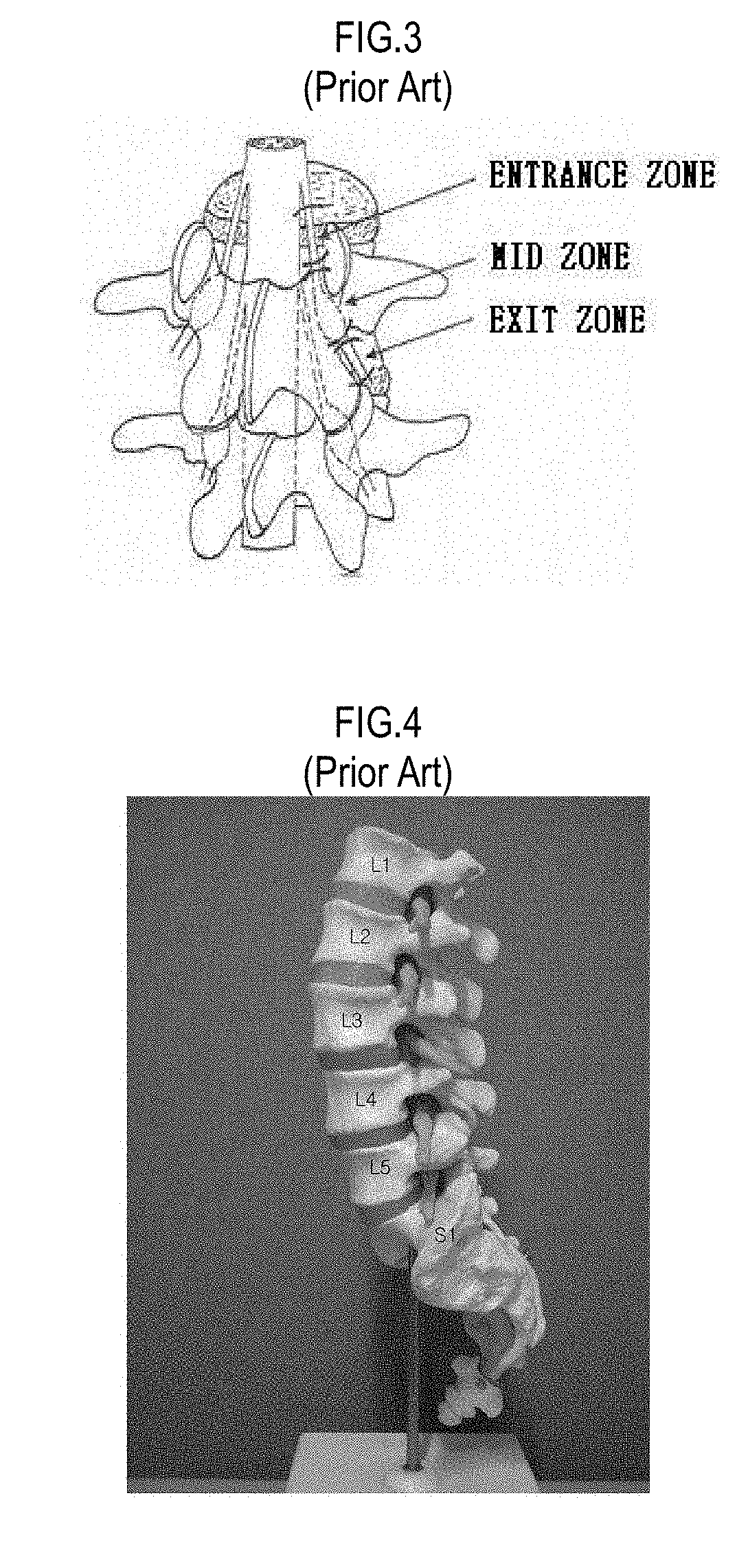
The intervertebral foramen (also called neural foramen, and often abbreviated as IV foramen or IVF), is a foramen between two spinal vertebrae. Cervical, thoracic, and lumbar vertebrae all have intervertebral foramina.
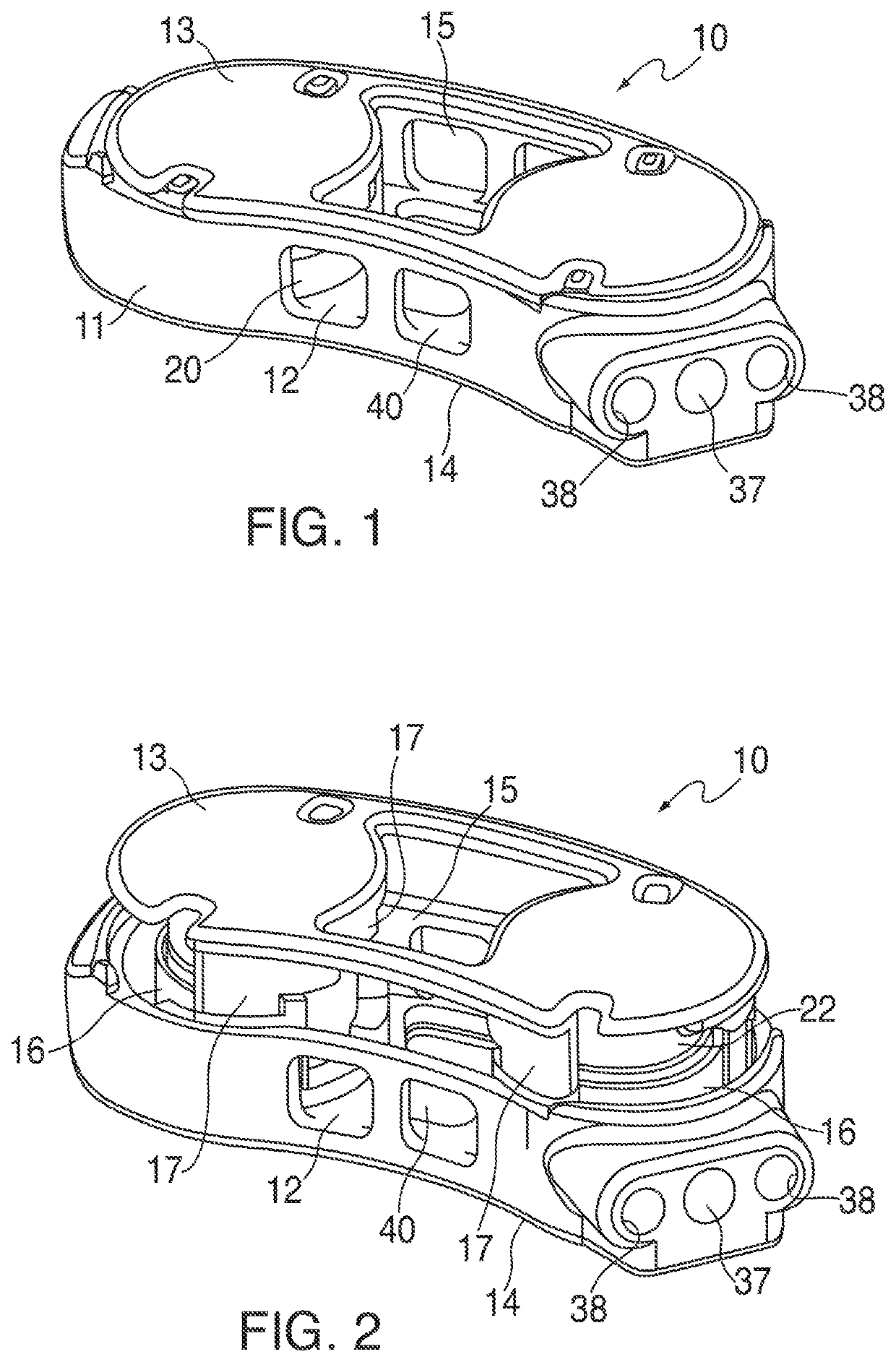
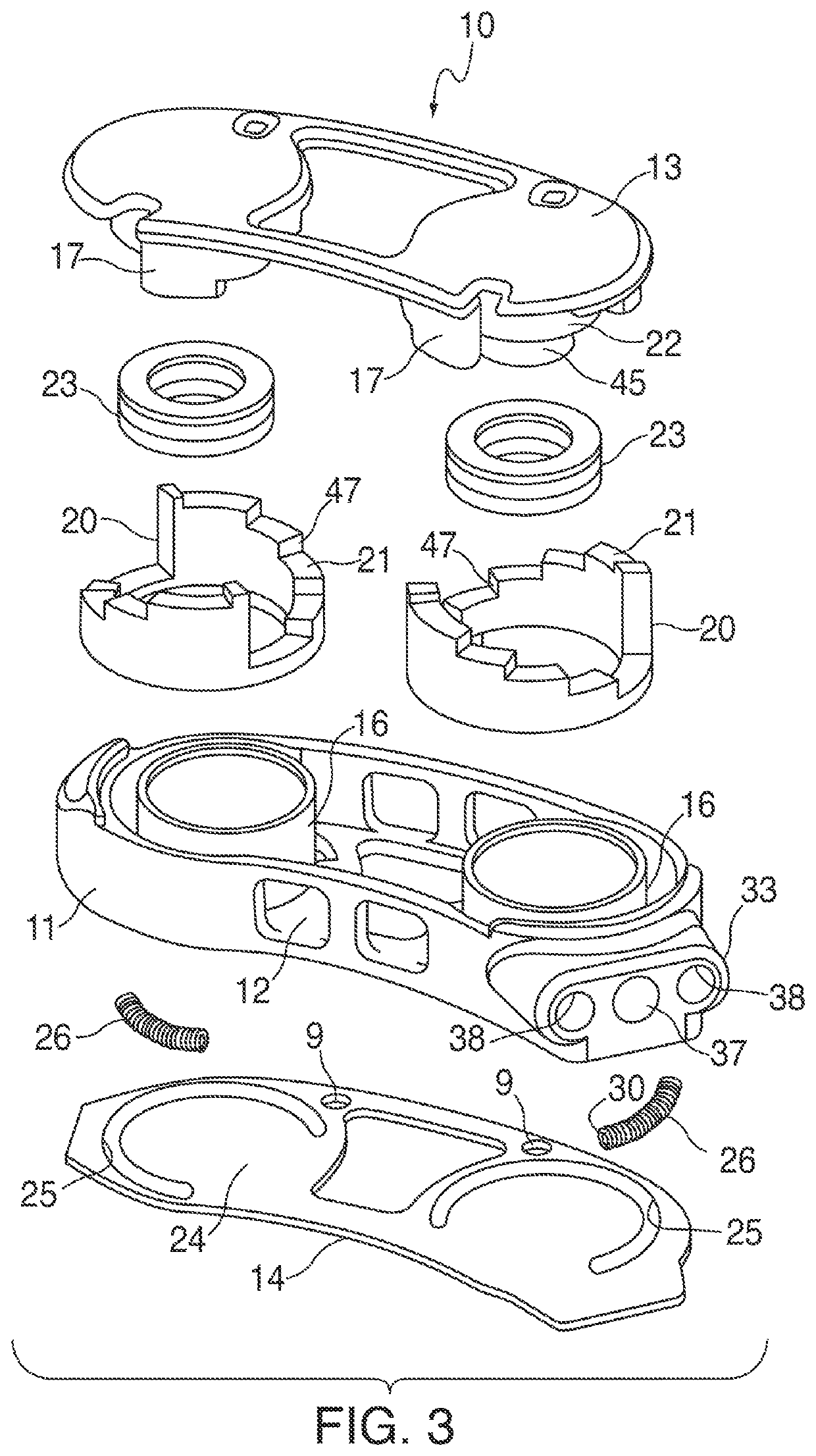
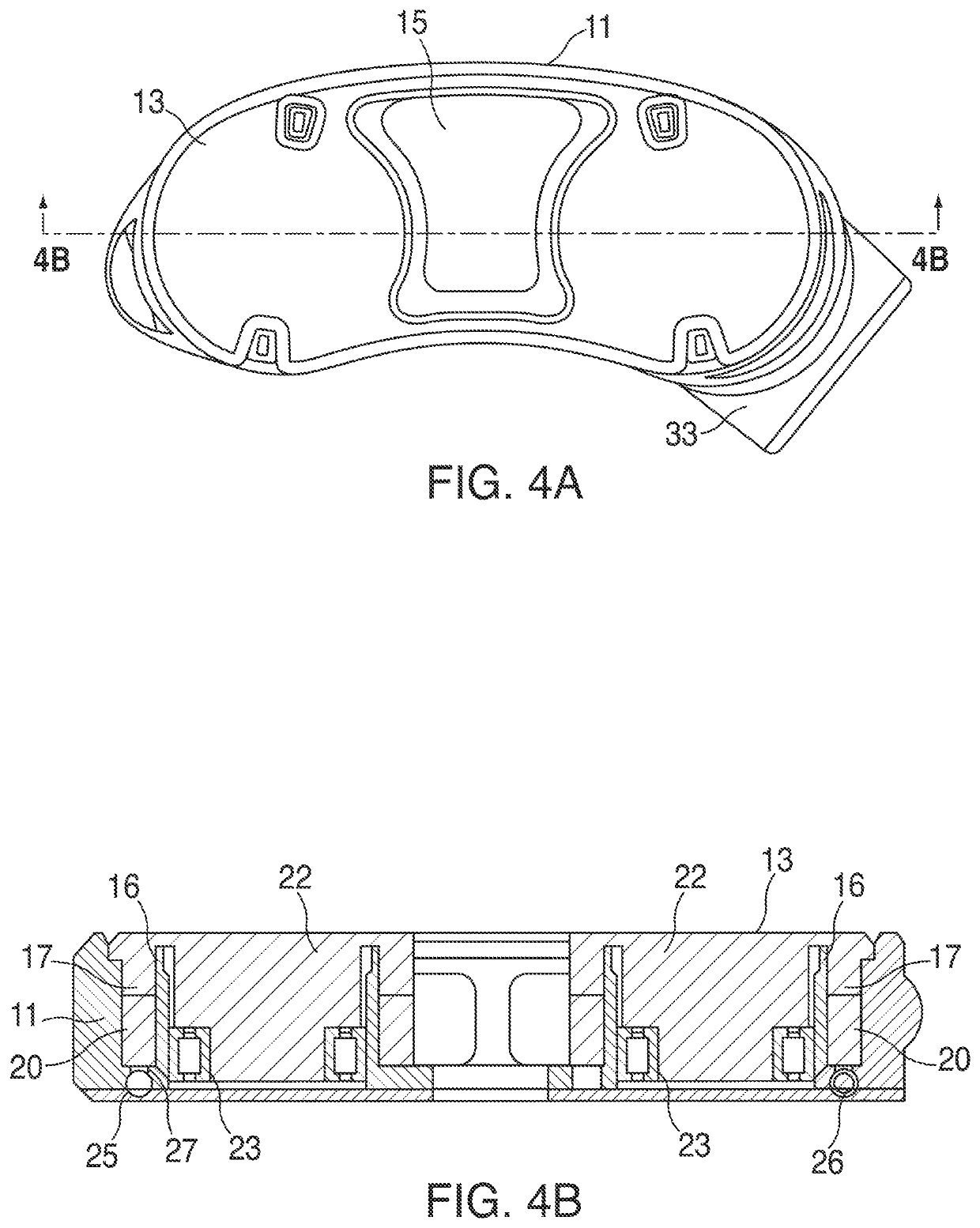
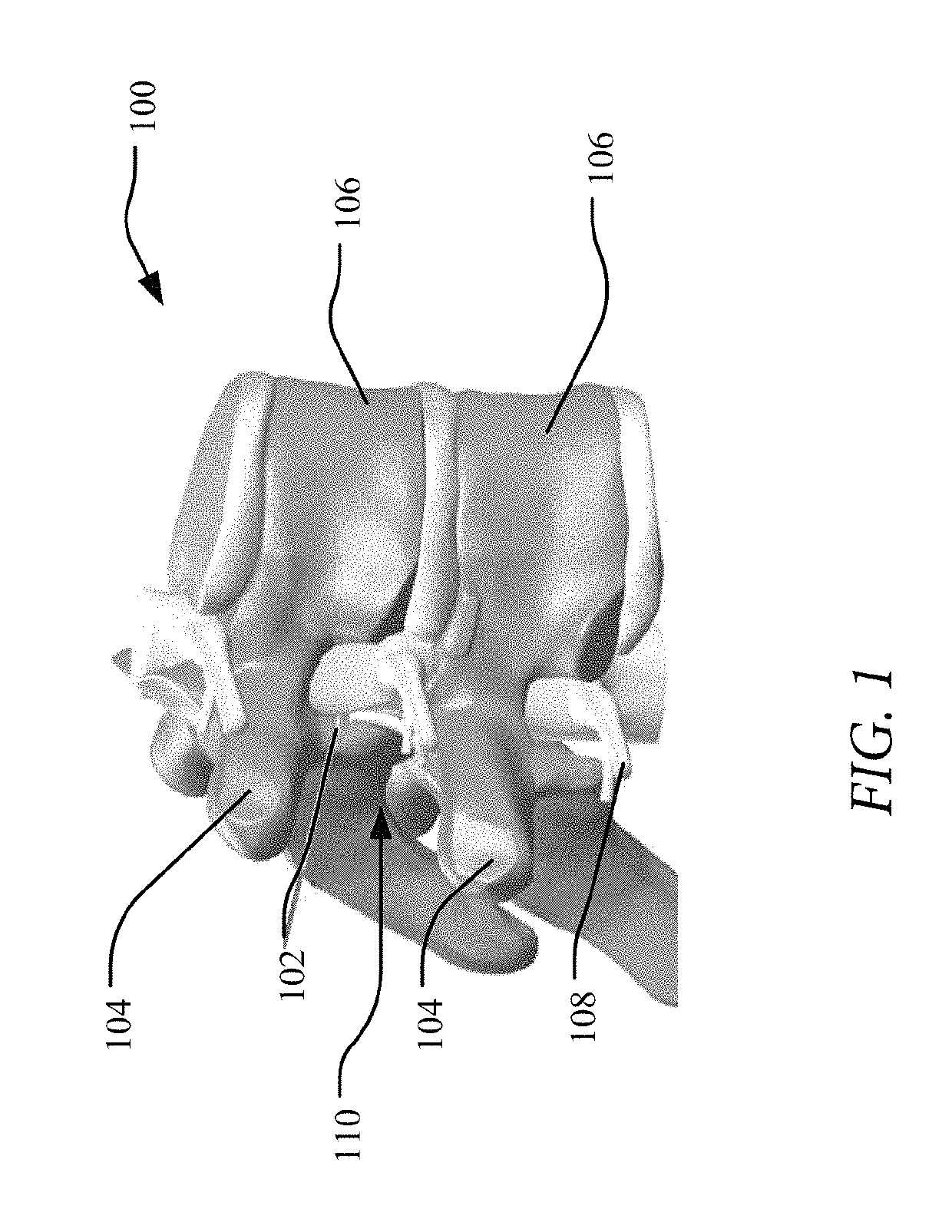
Devices and methods for tissue access
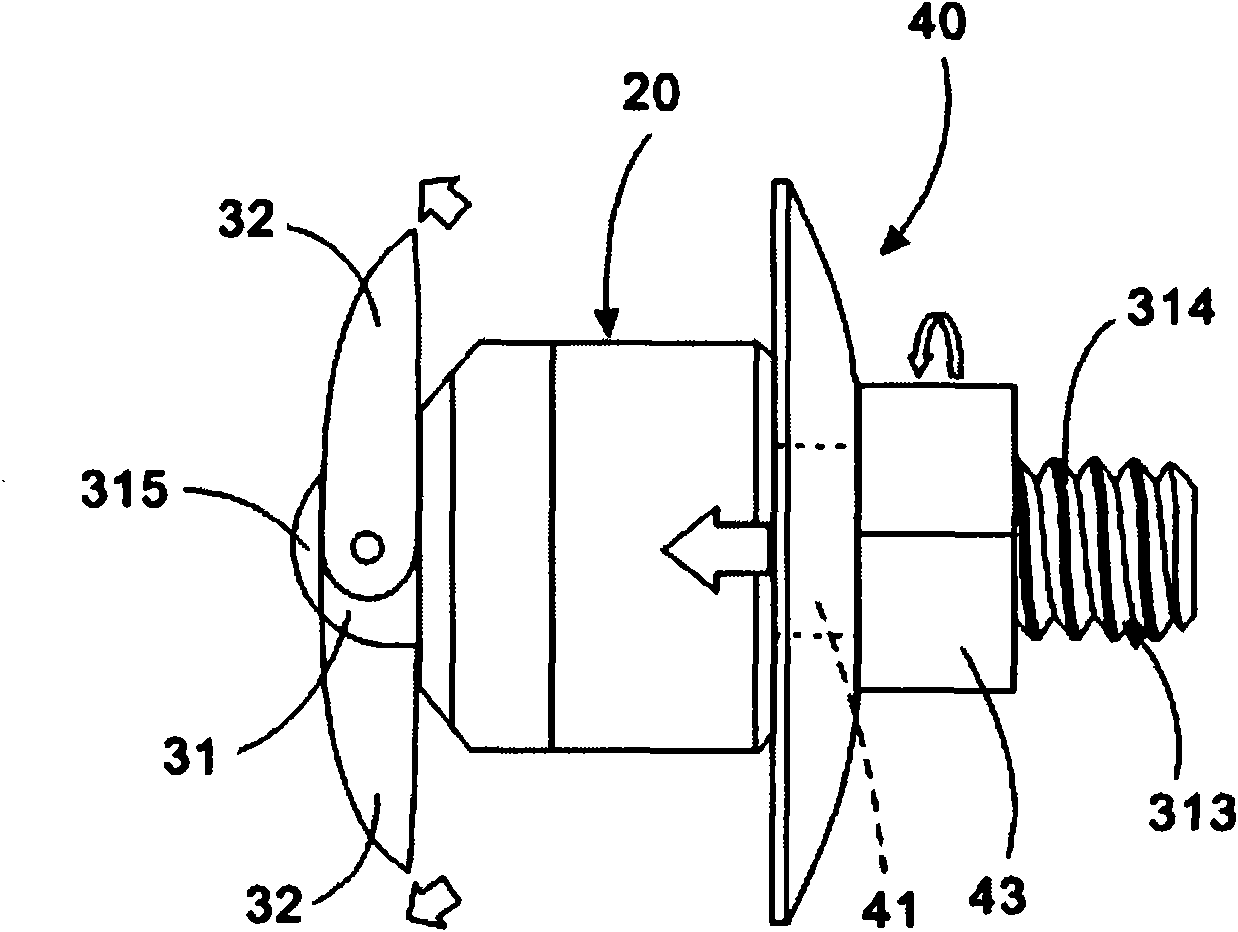
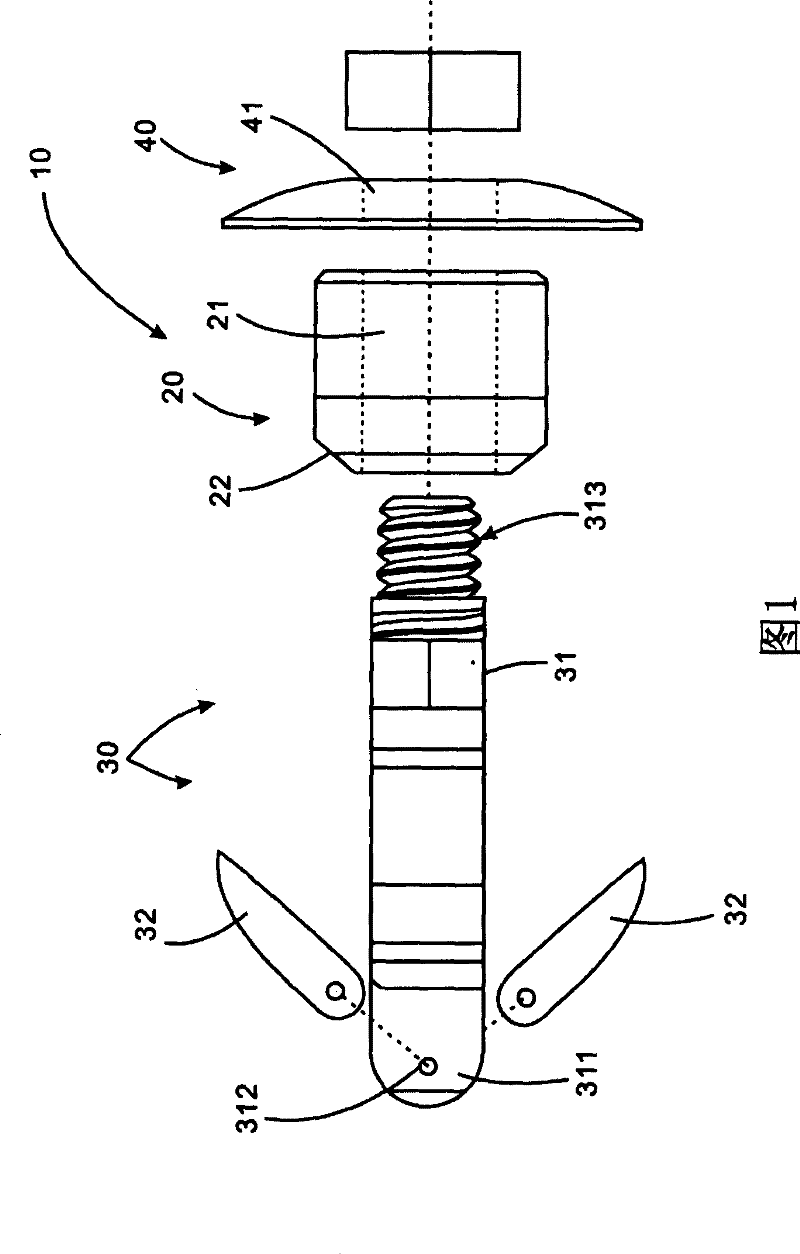
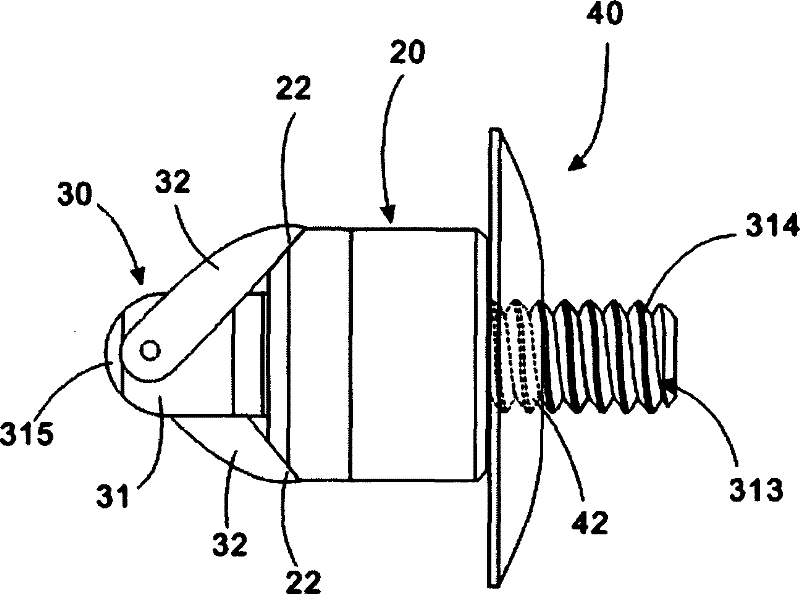
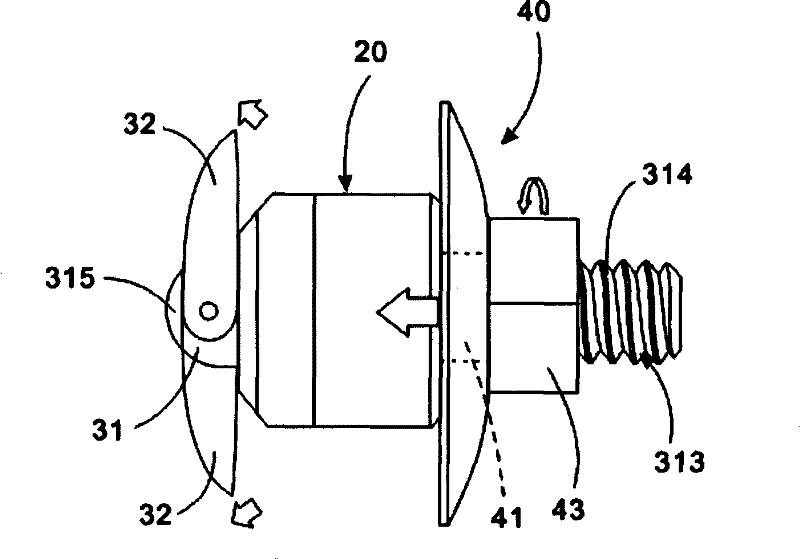
ActiveUS20060089633A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
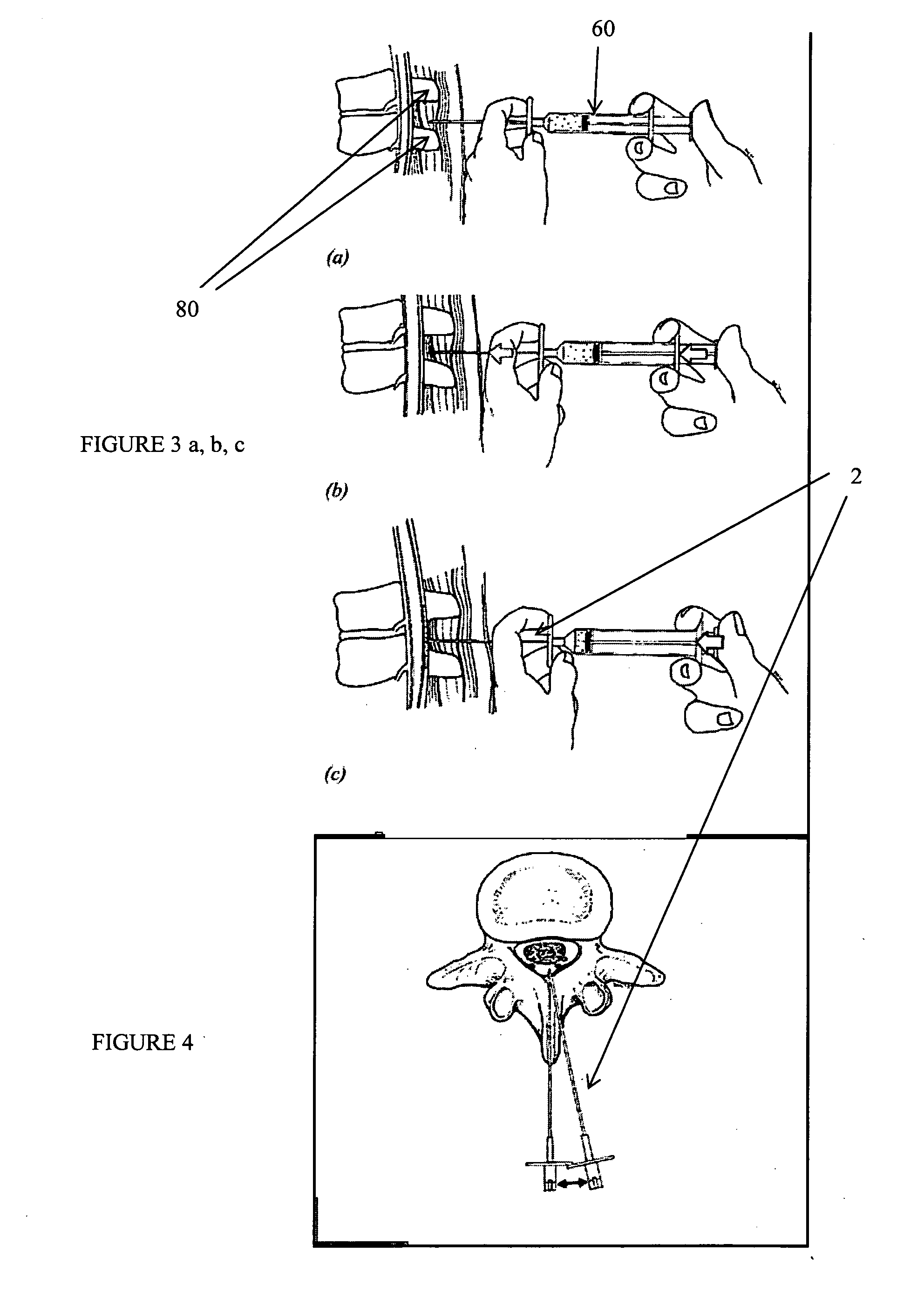
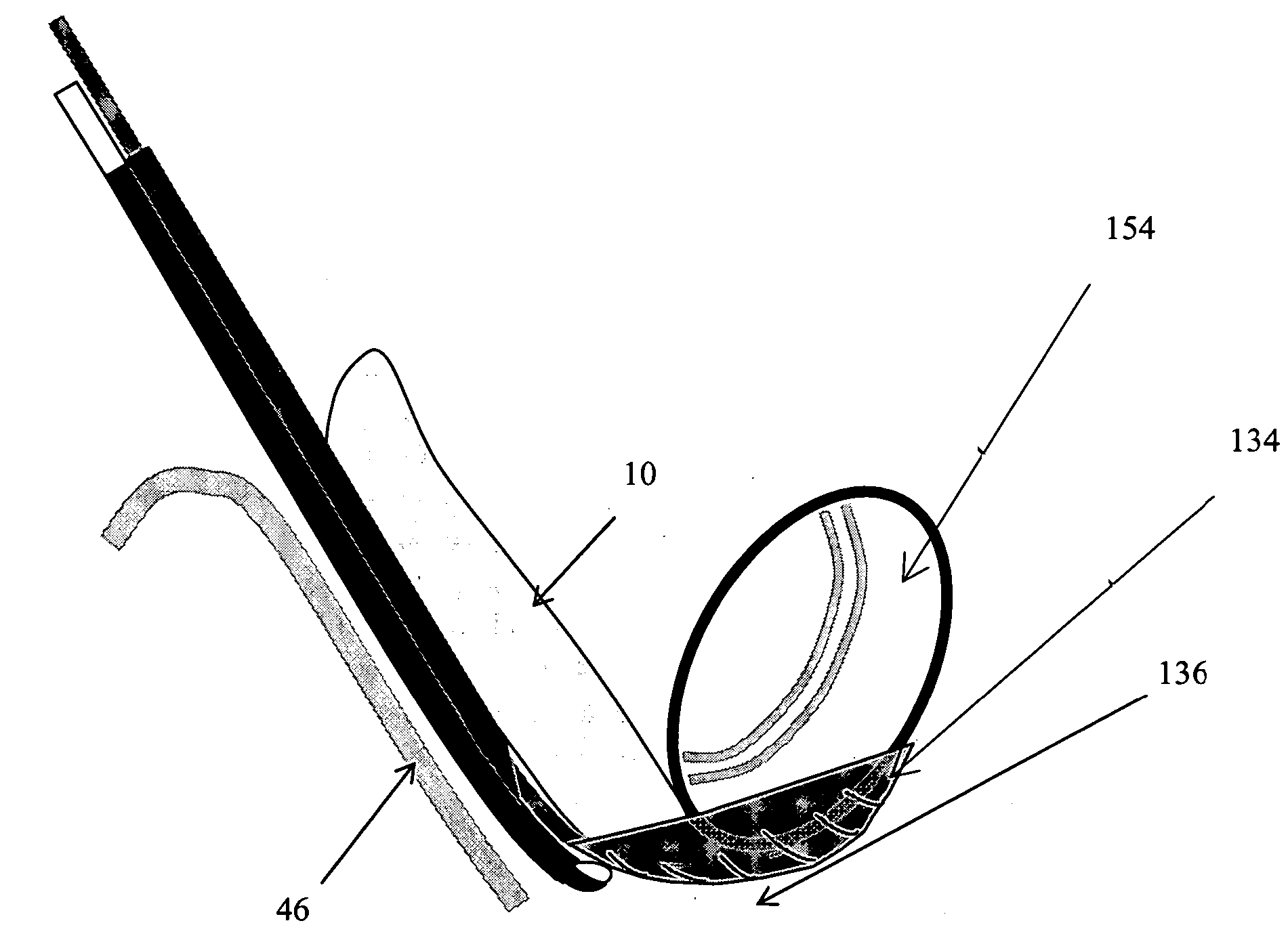
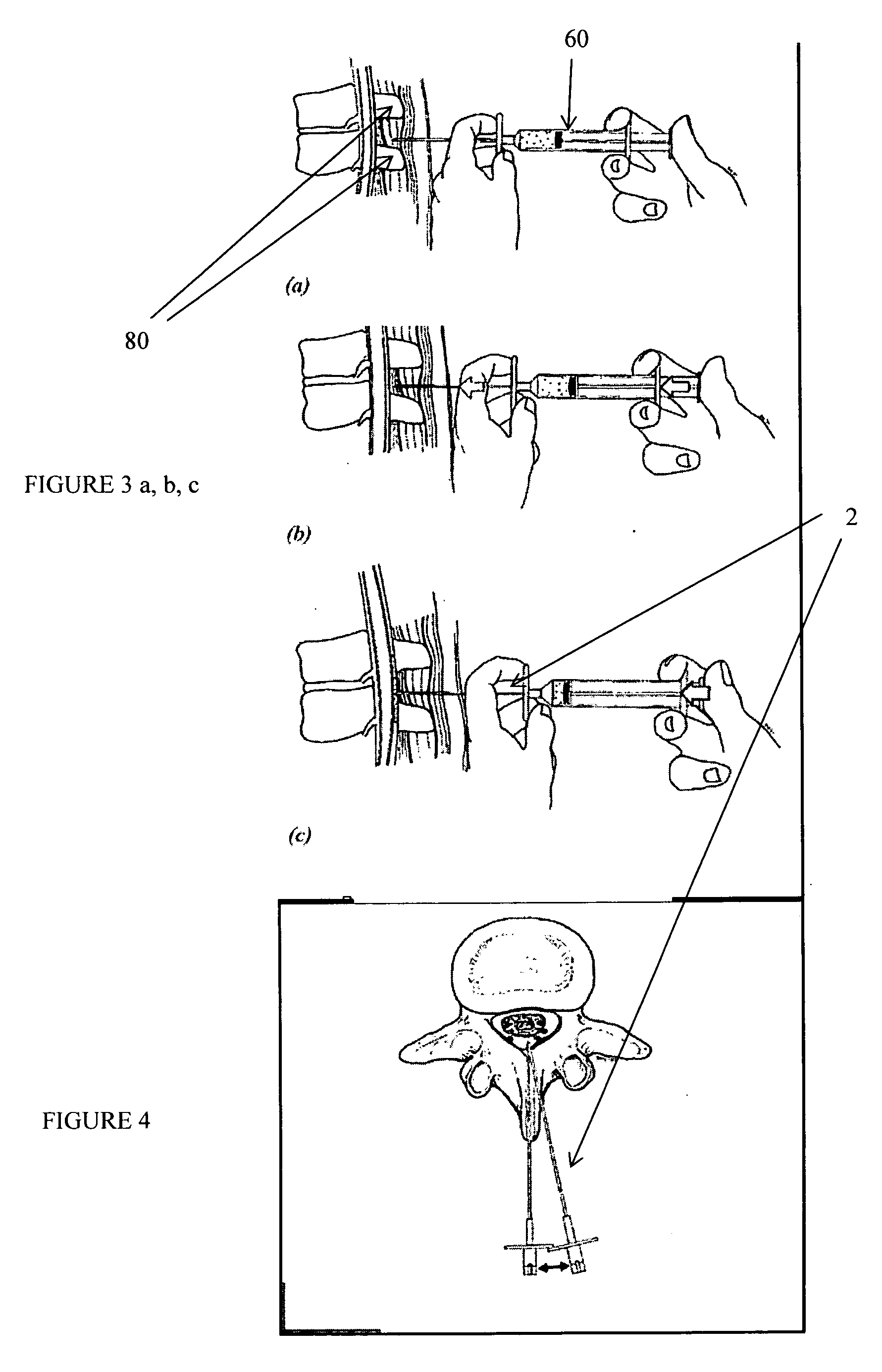
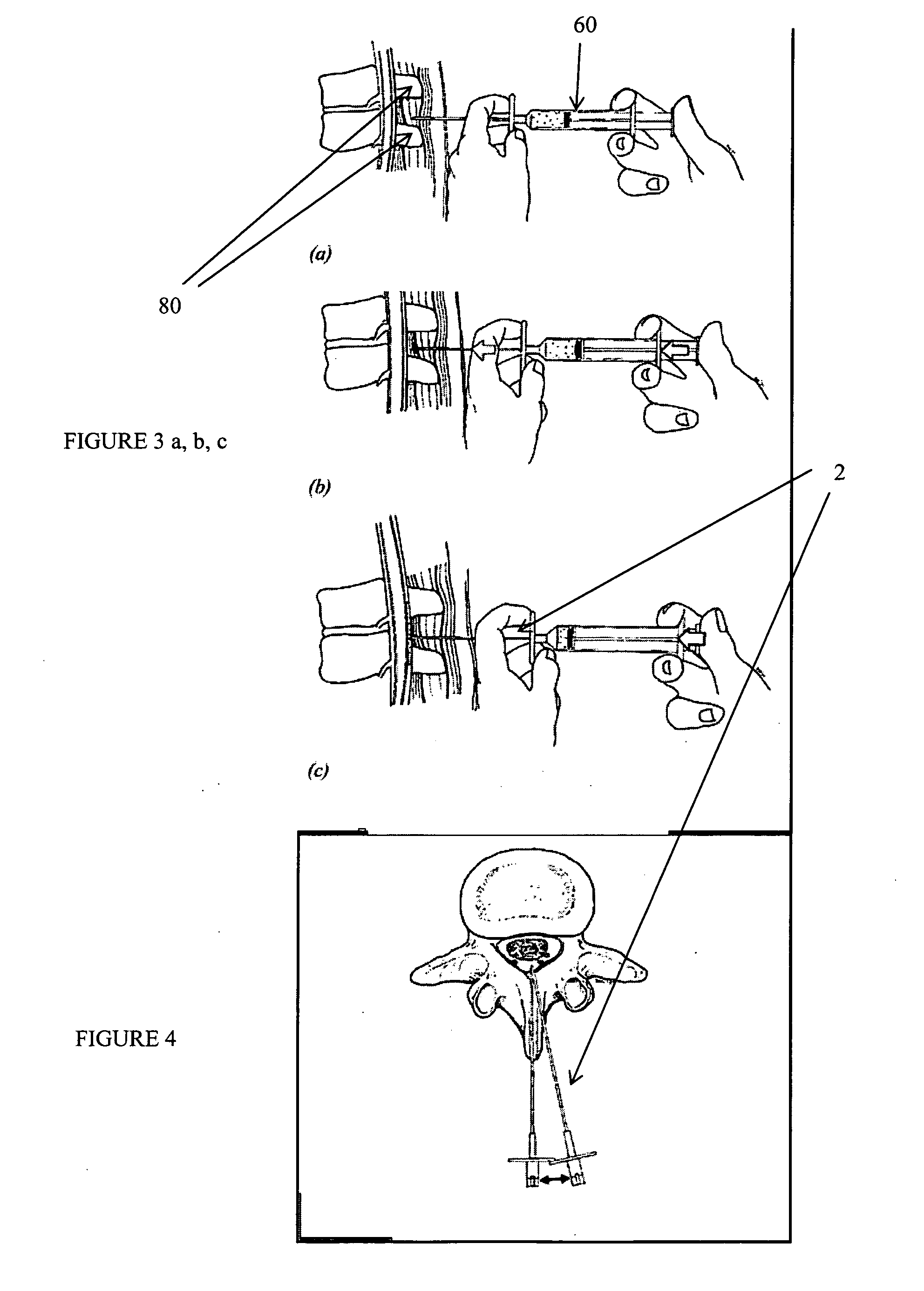
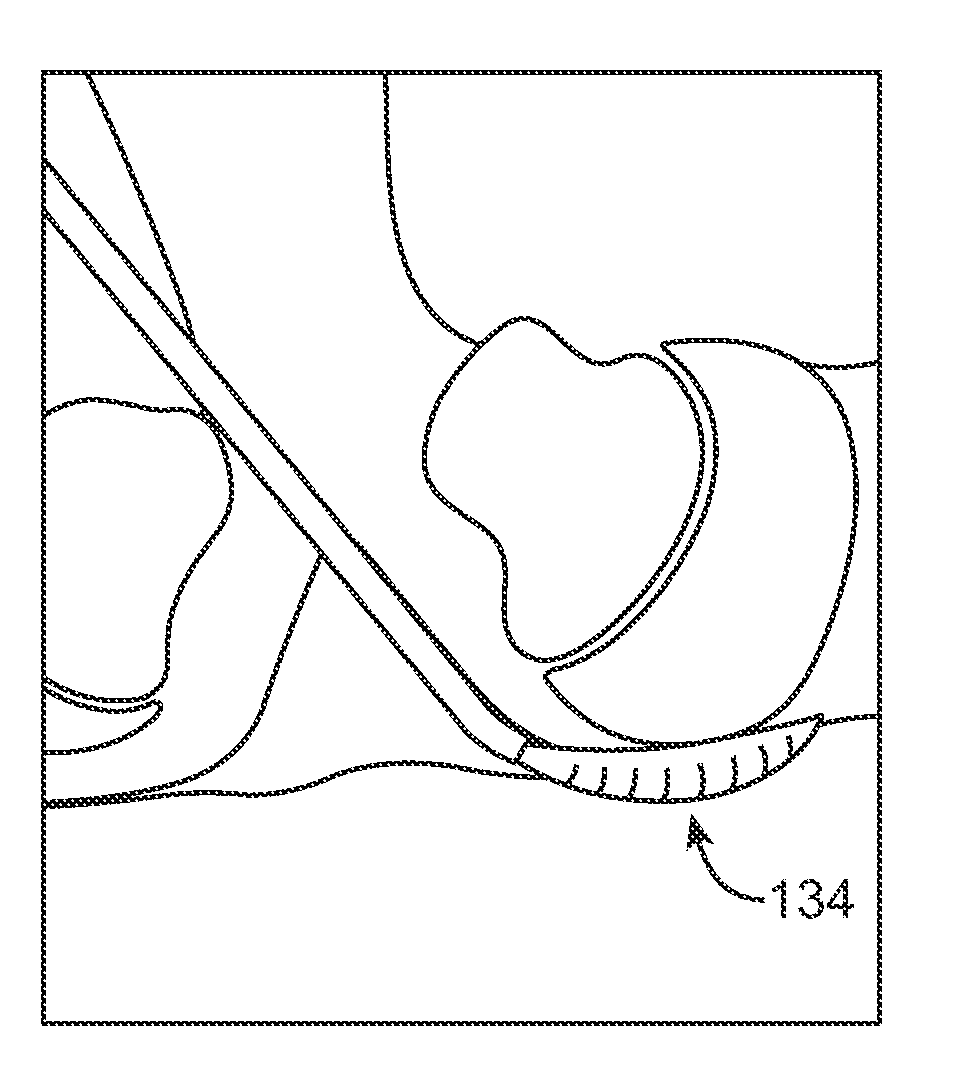
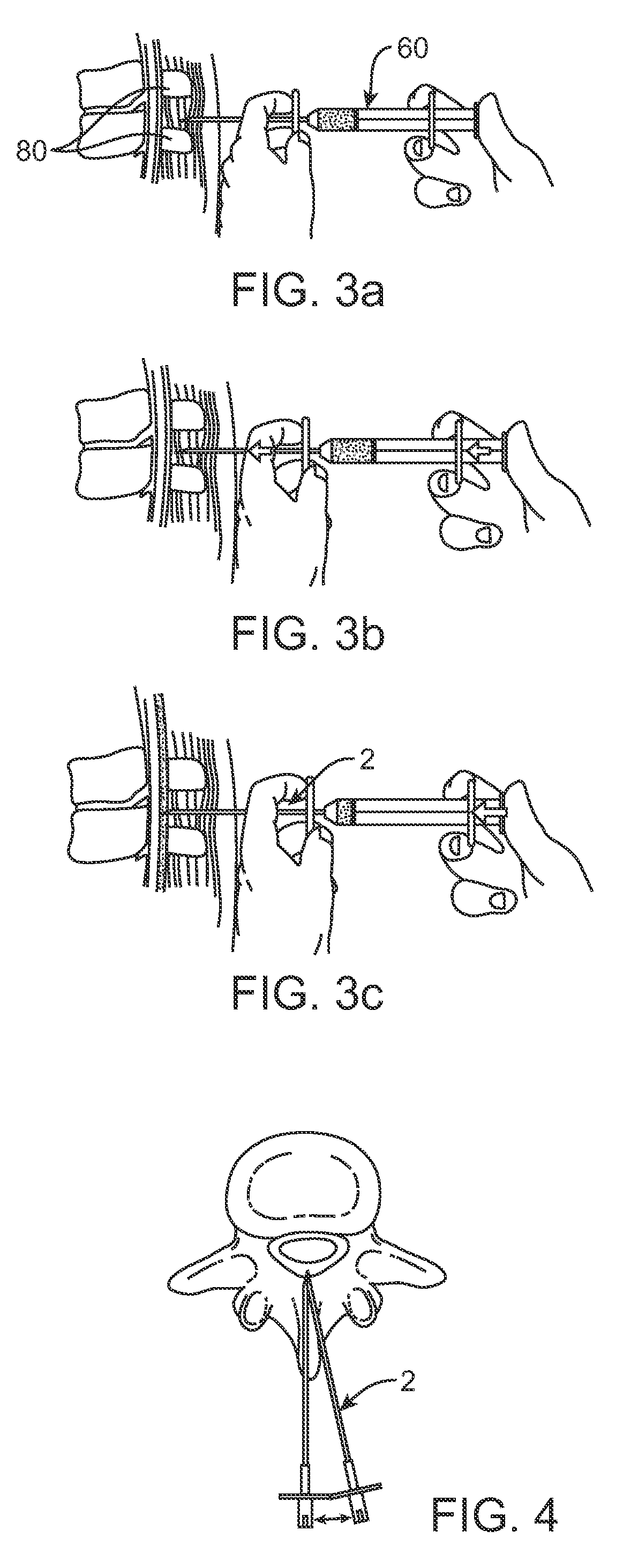
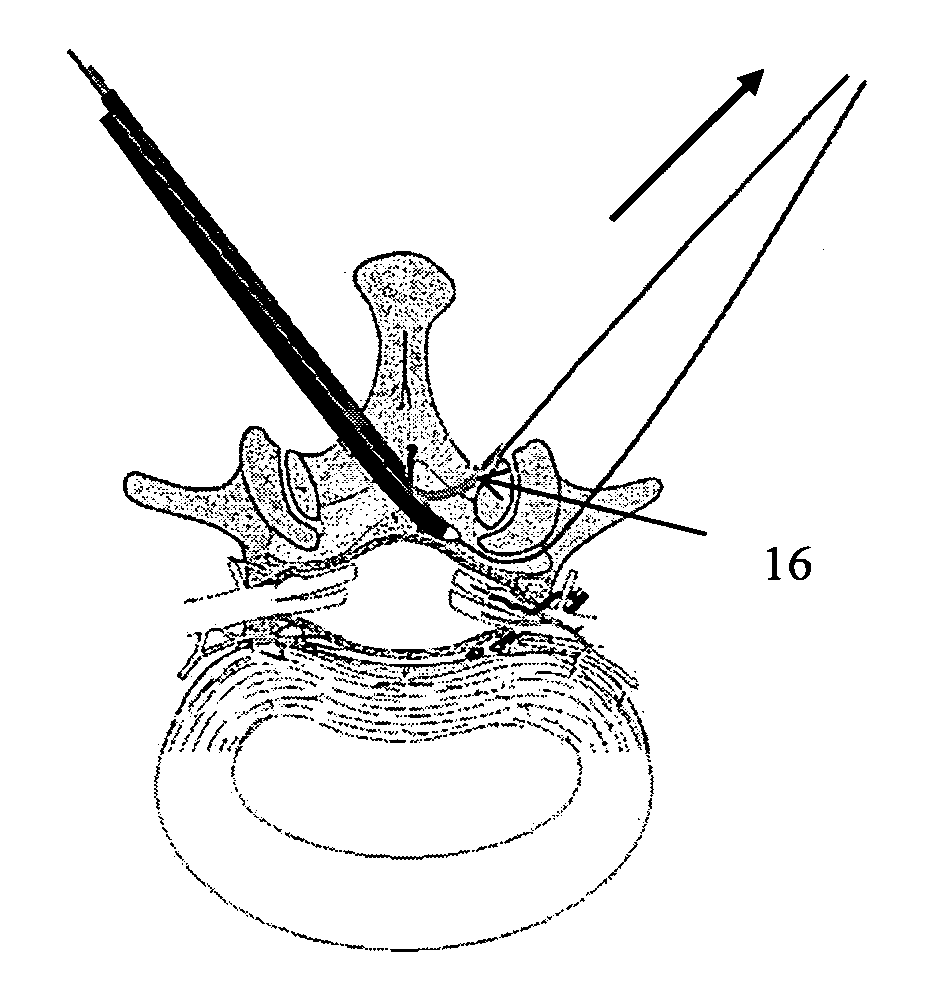
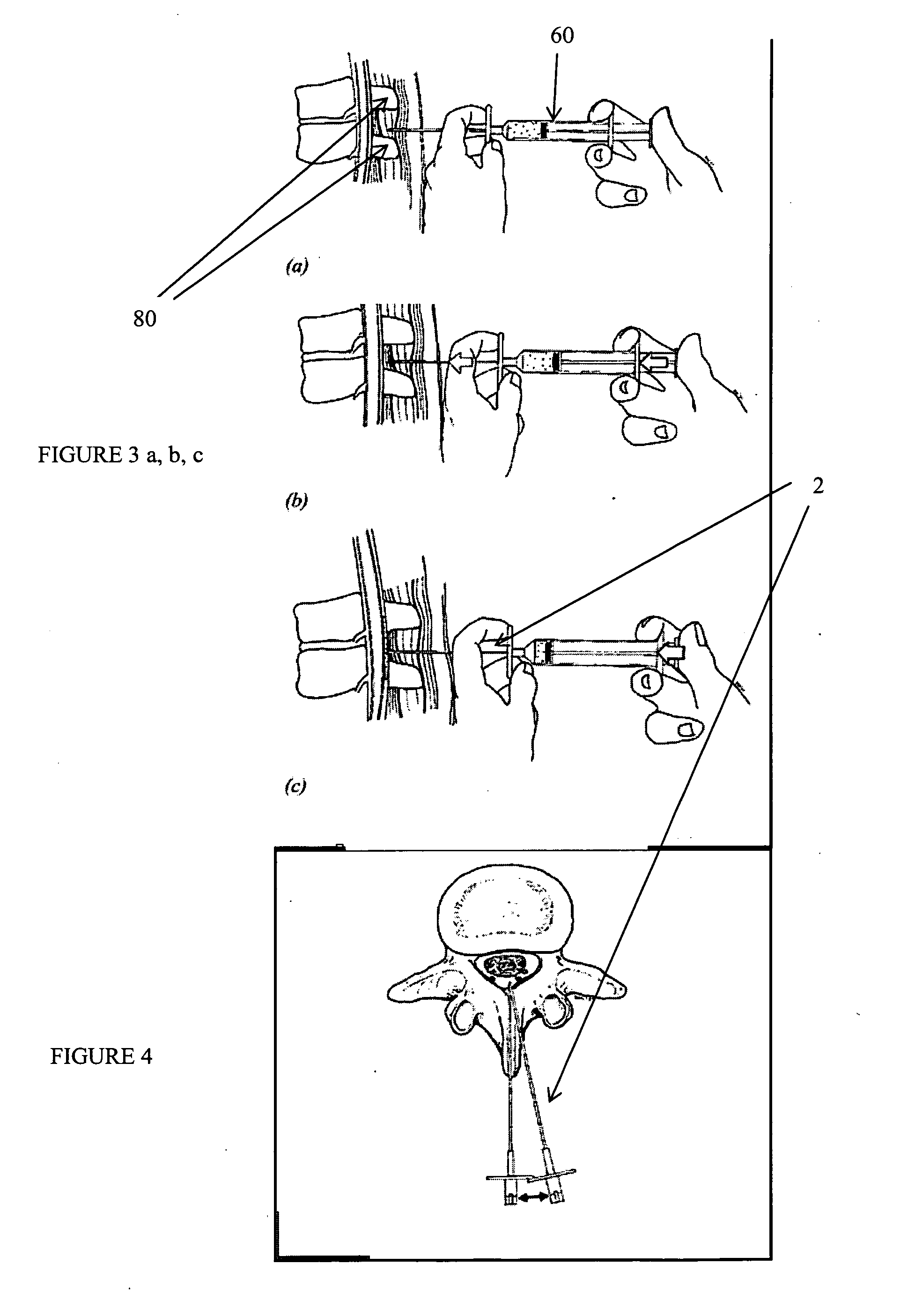
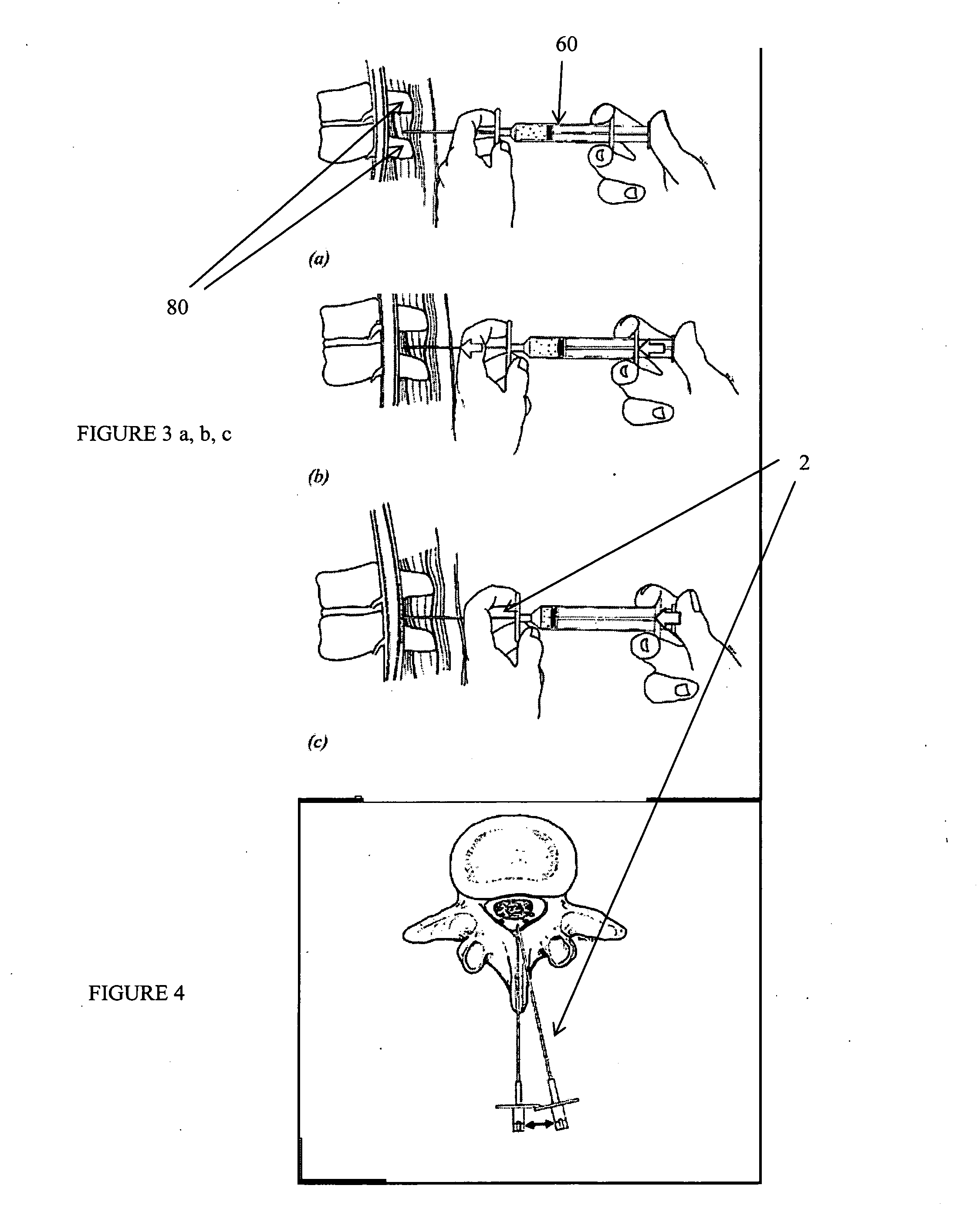
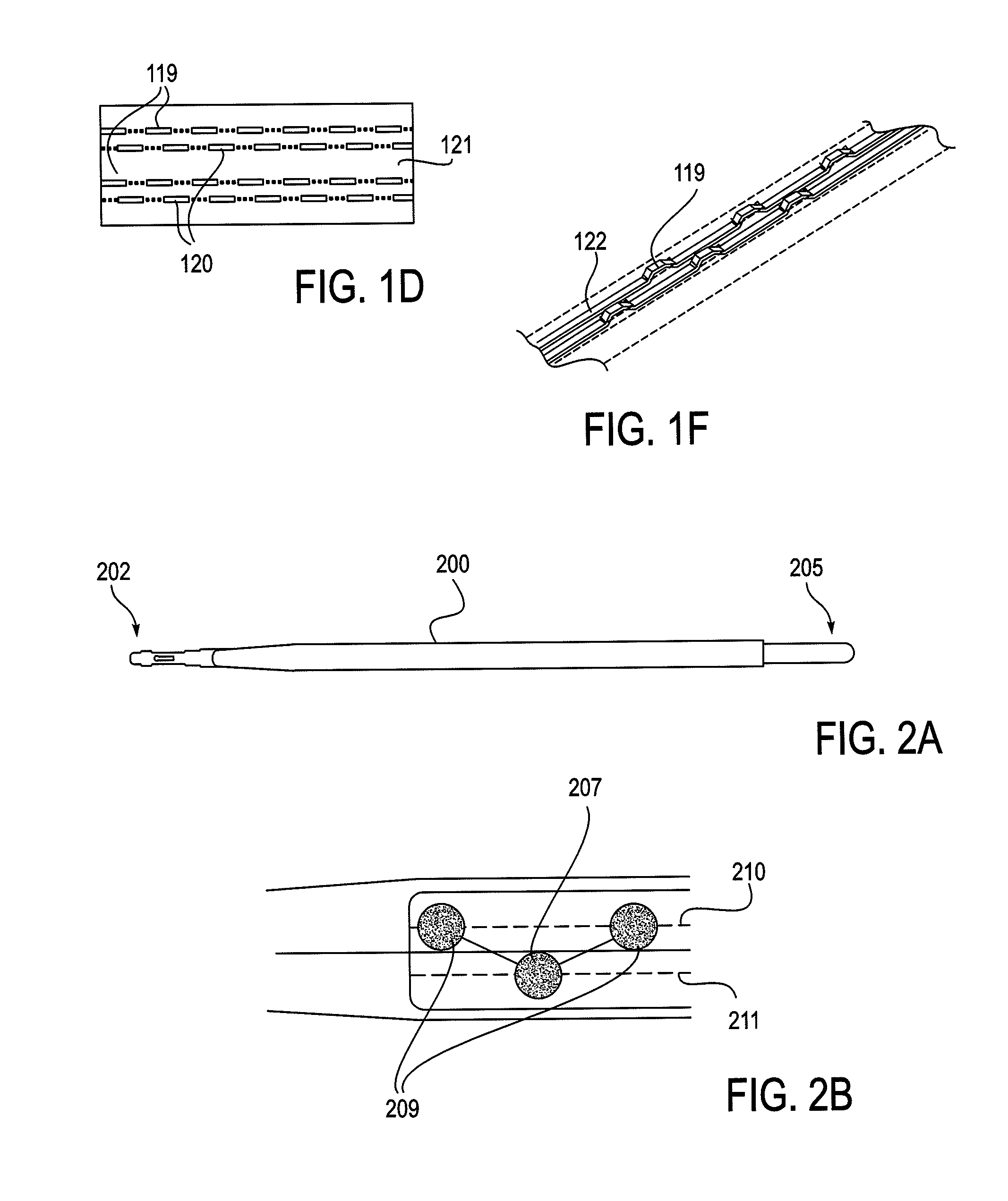
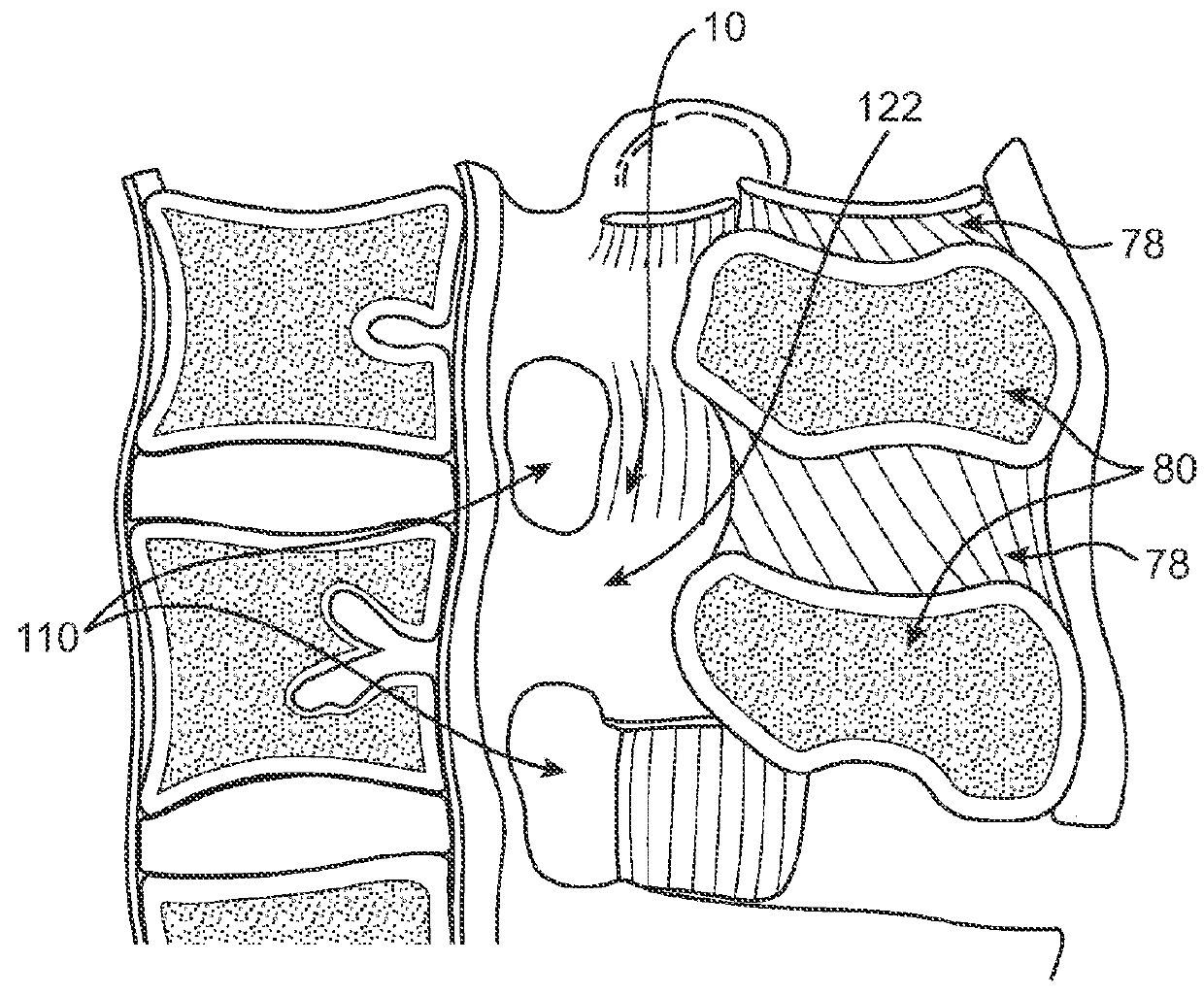
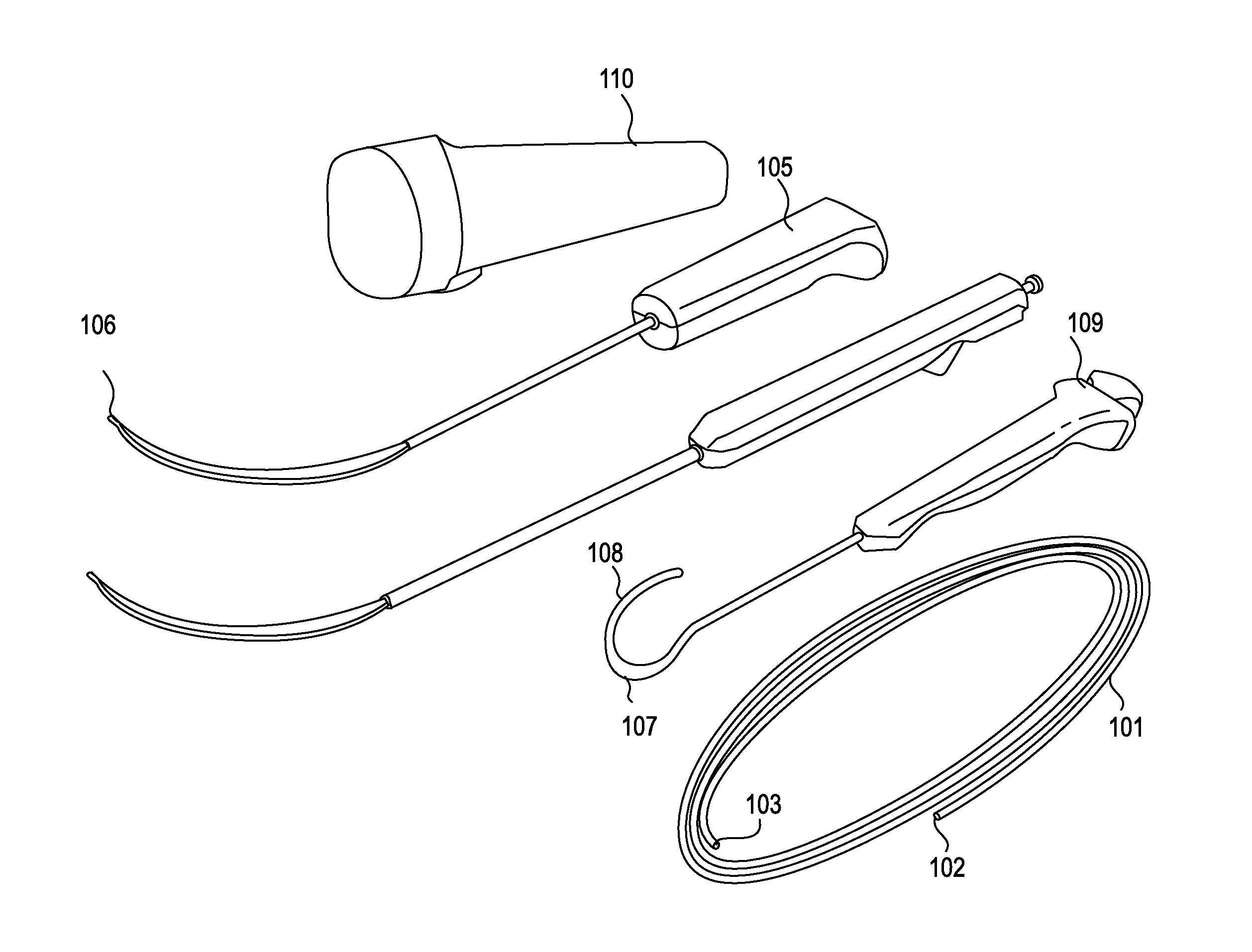
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue abrasion device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the abrasive surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
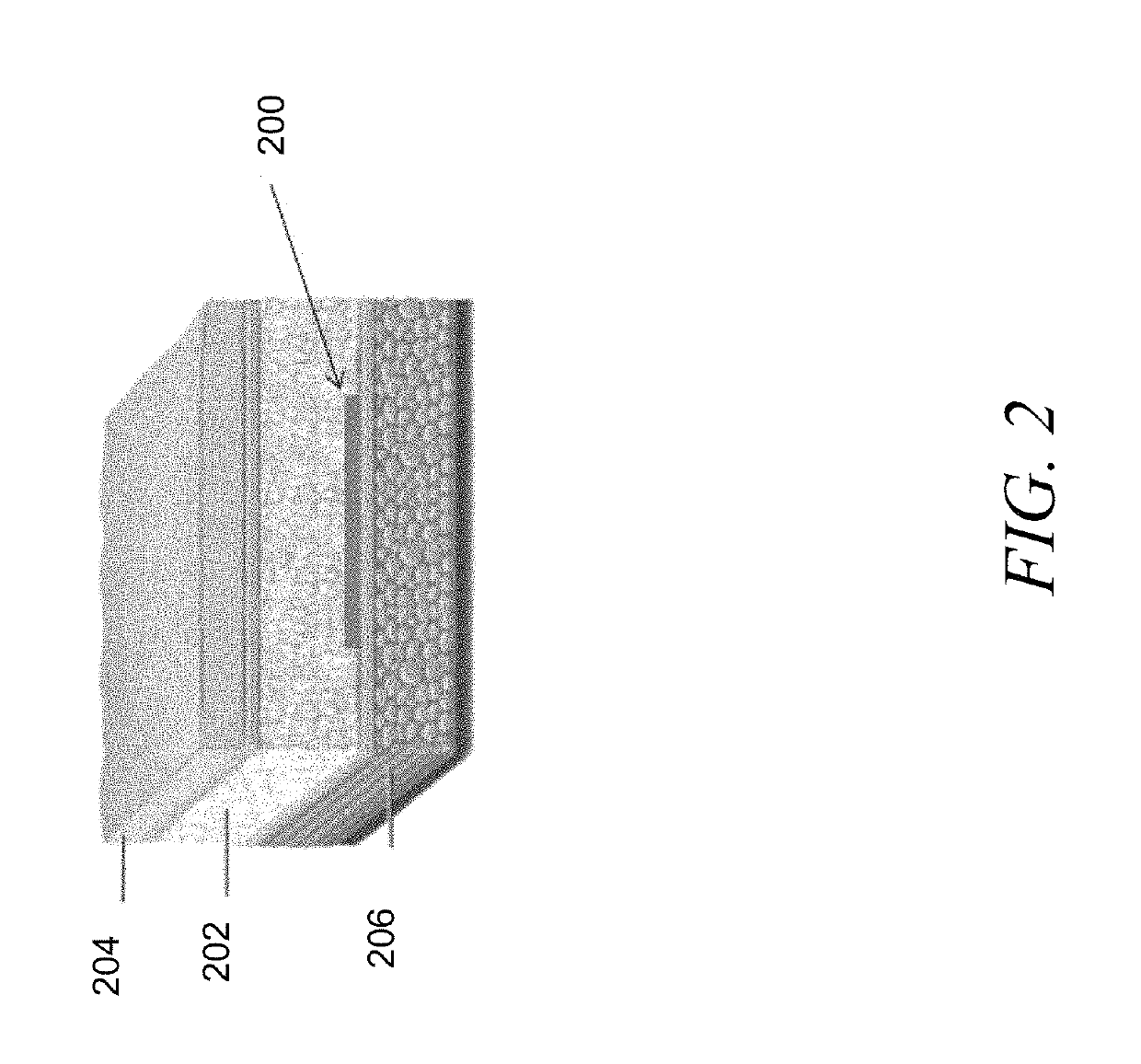
Devices and methods for tissue access
InactiveUS20060122458A1Enabling symptomatic reliefApproach can be quite invasiveCannulasDiagnosticsSurgical departmentNerve stimulation
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:BAXANO
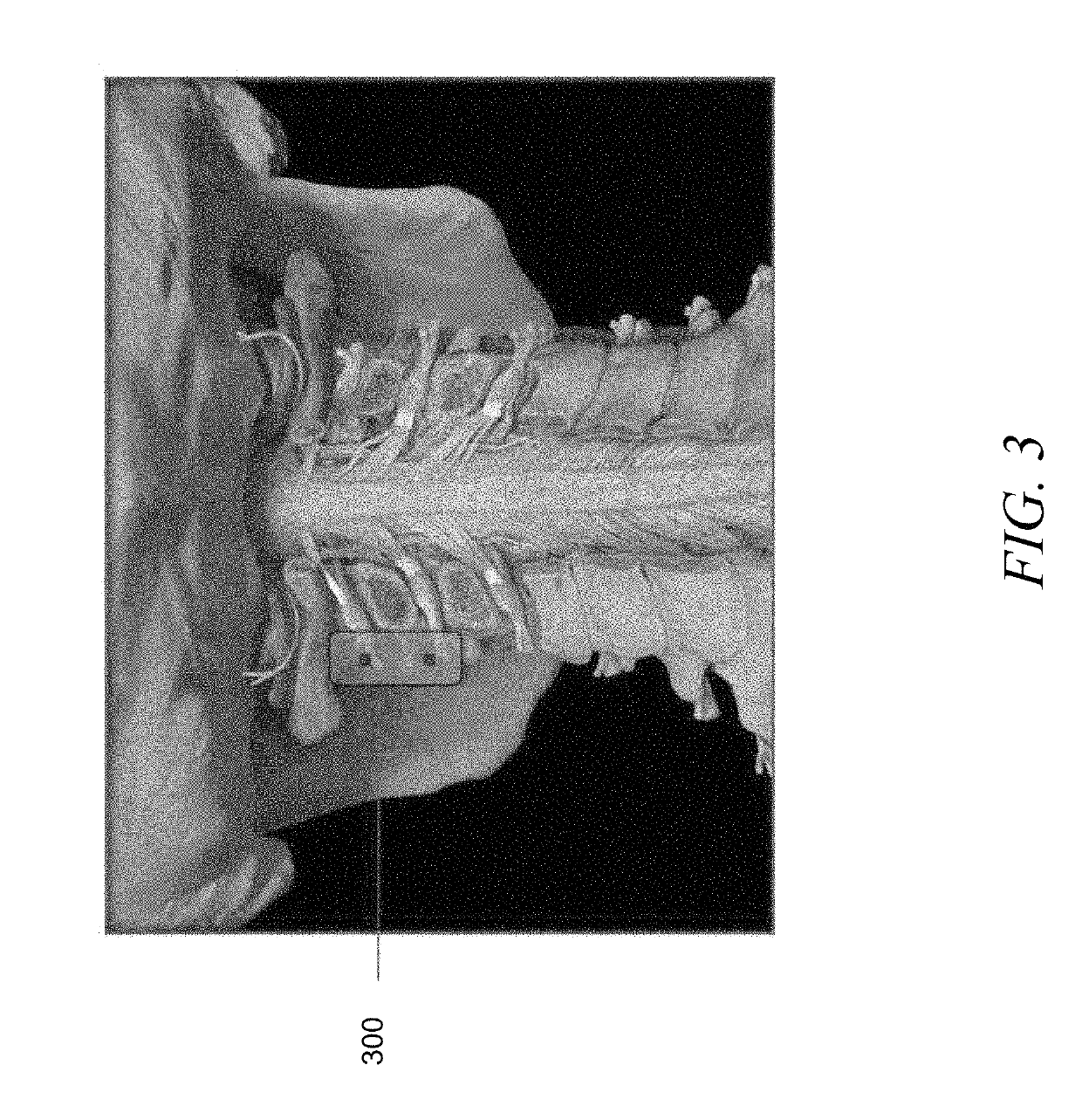
Devices and methods for tissue modification
ActiveUS20060095059A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
Owner:MIS IP HLDG LLC +1
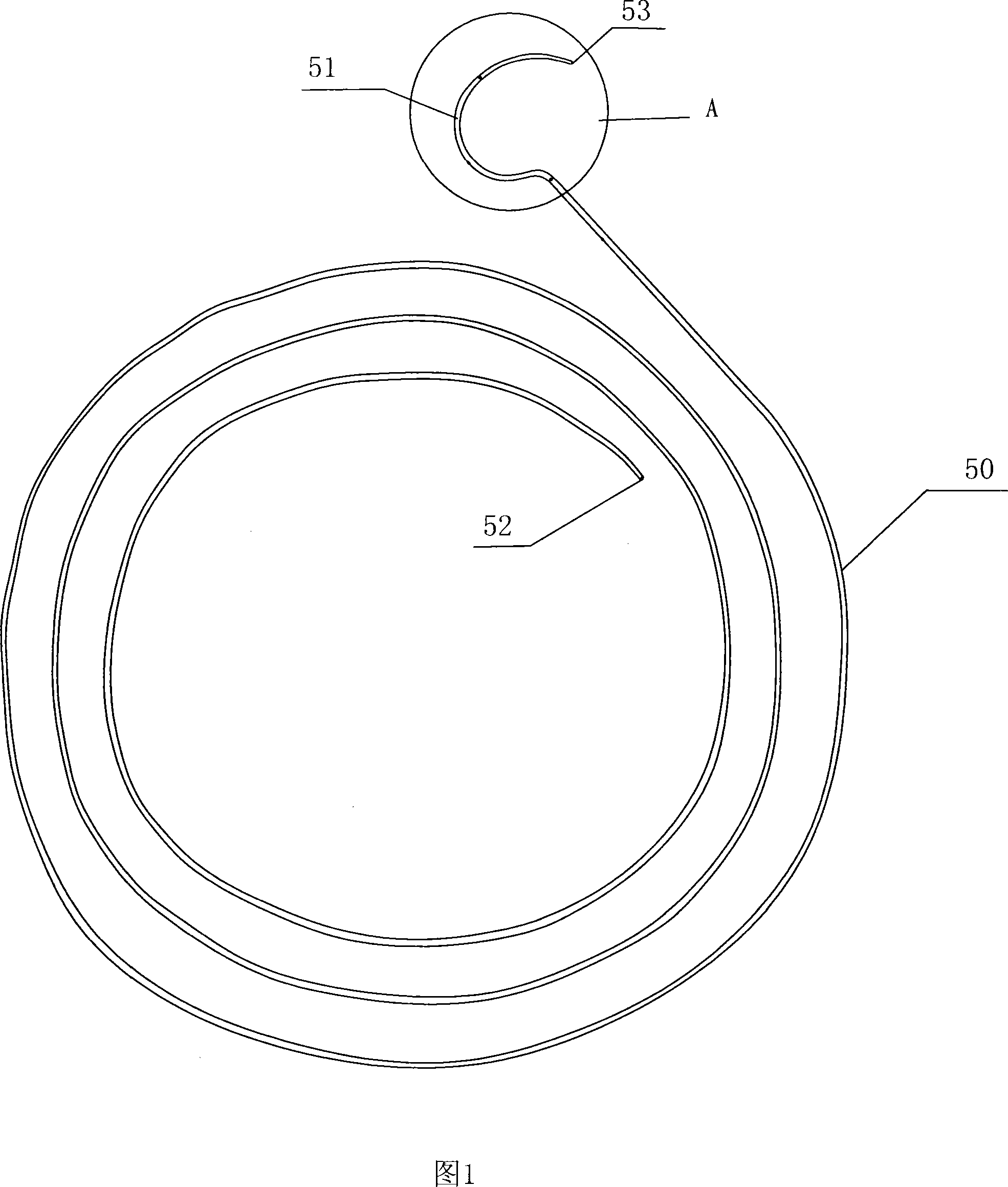
Flexible Neural Localization Devices and Methods
InactiveUS20110004207A1Control spreadAvoid damageSpinal electrodesInternal osteosythesisMedicineNeural foramen
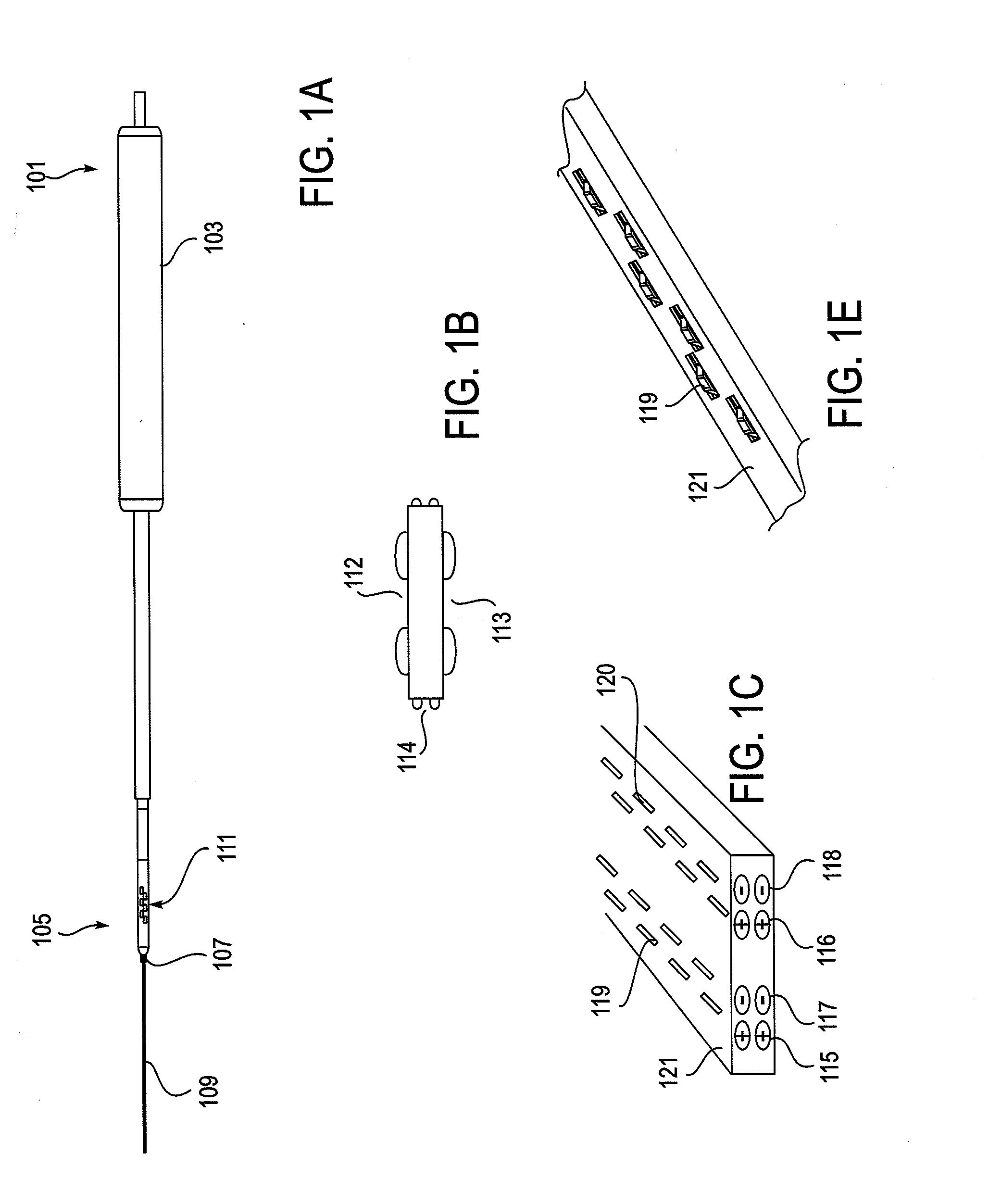
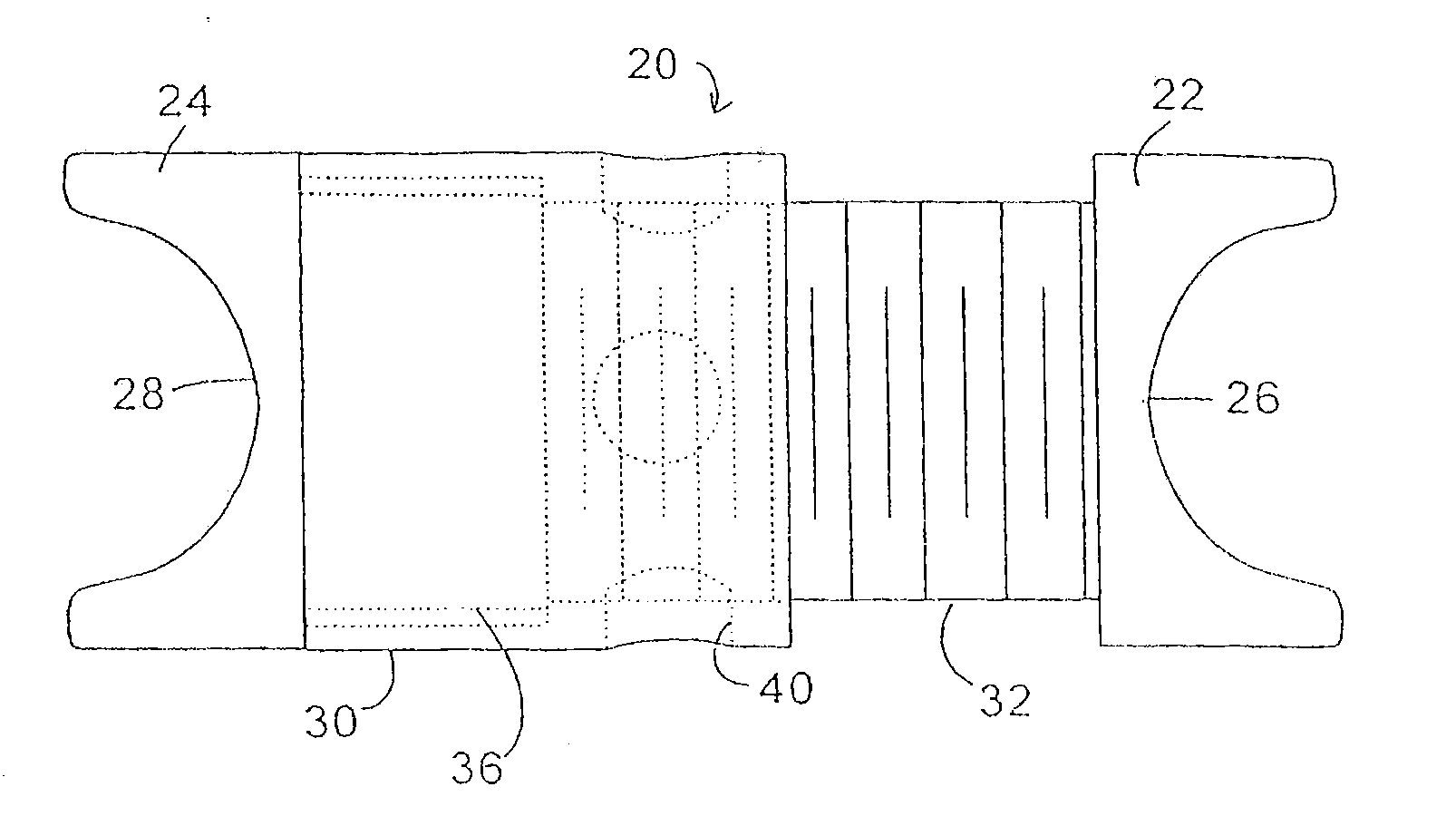
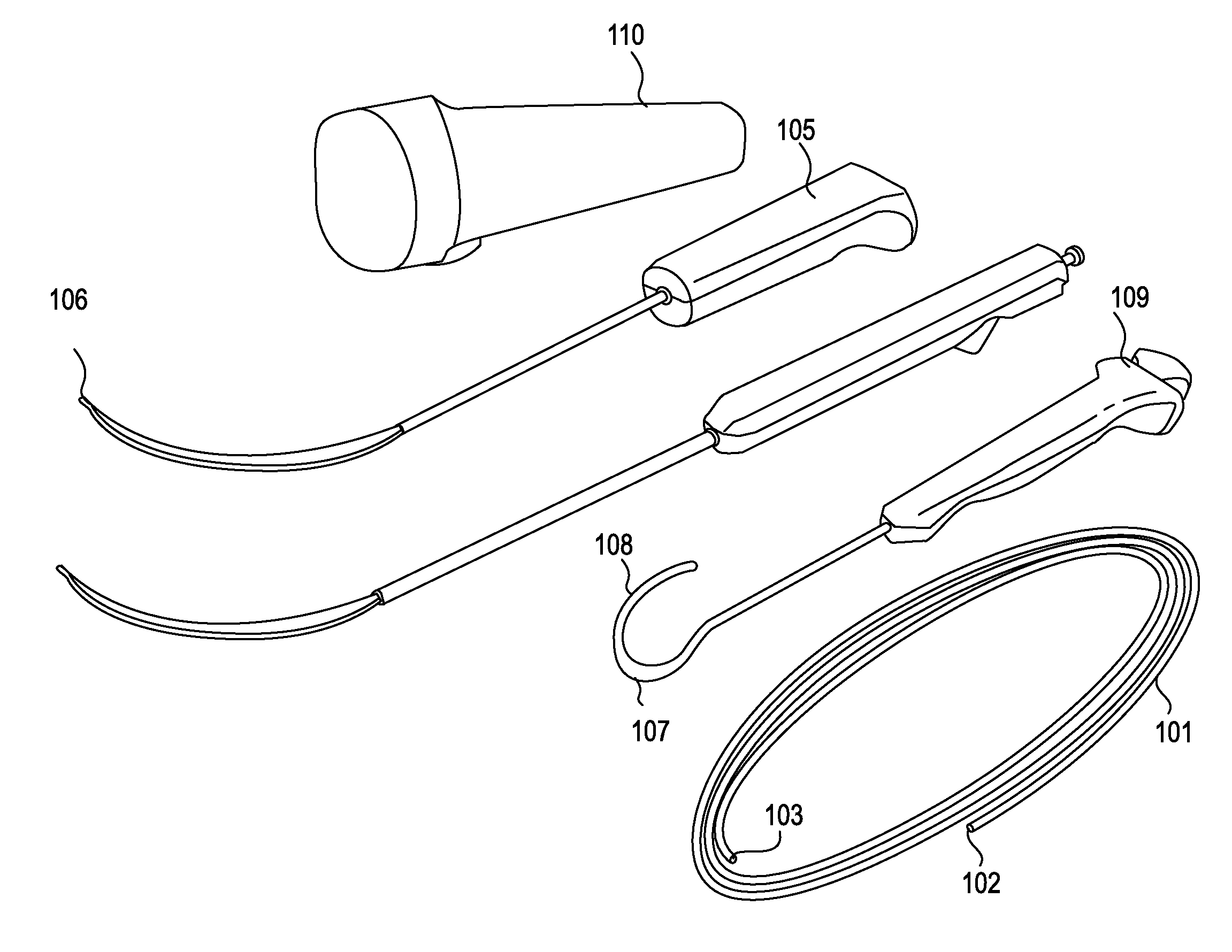
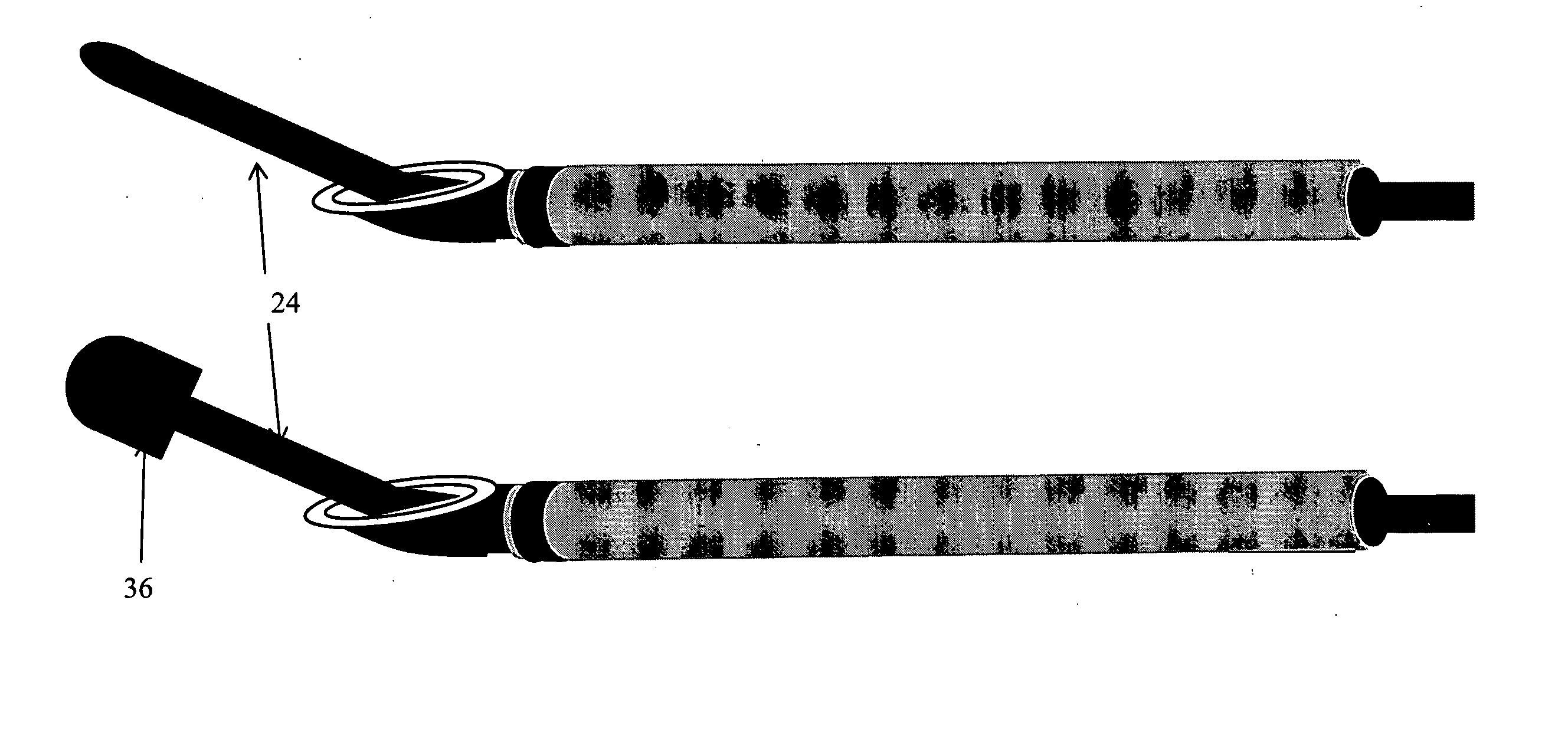
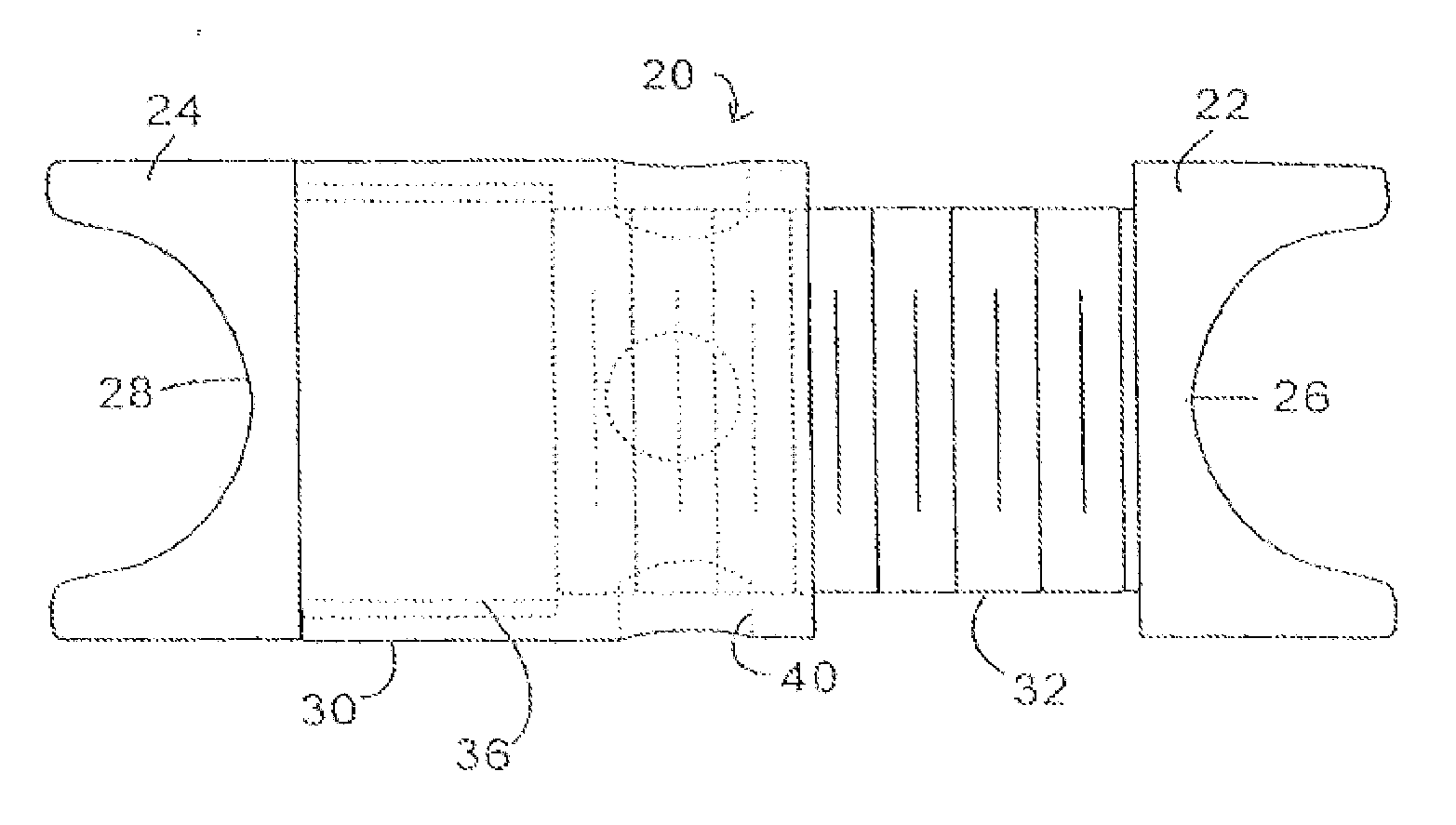
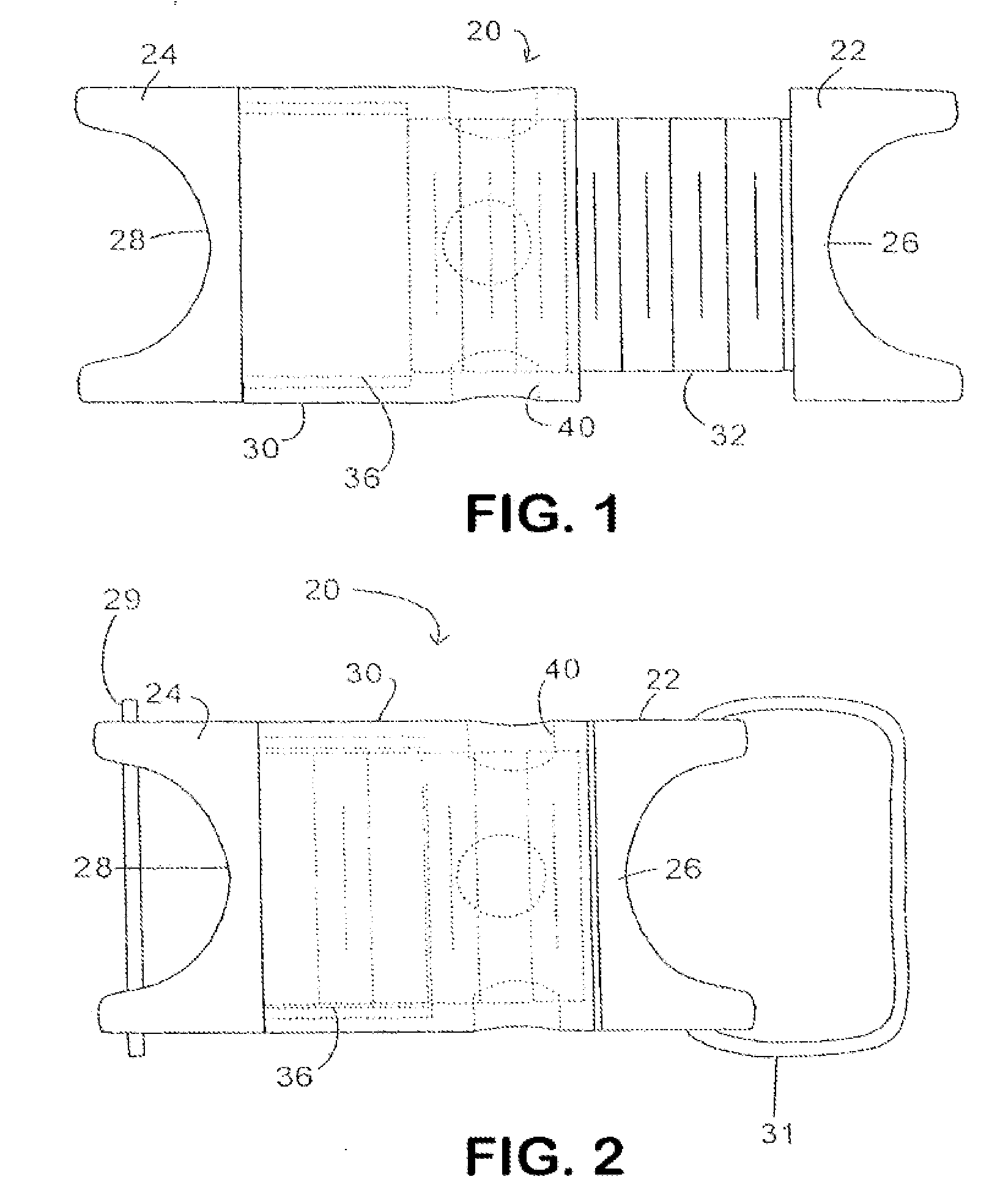
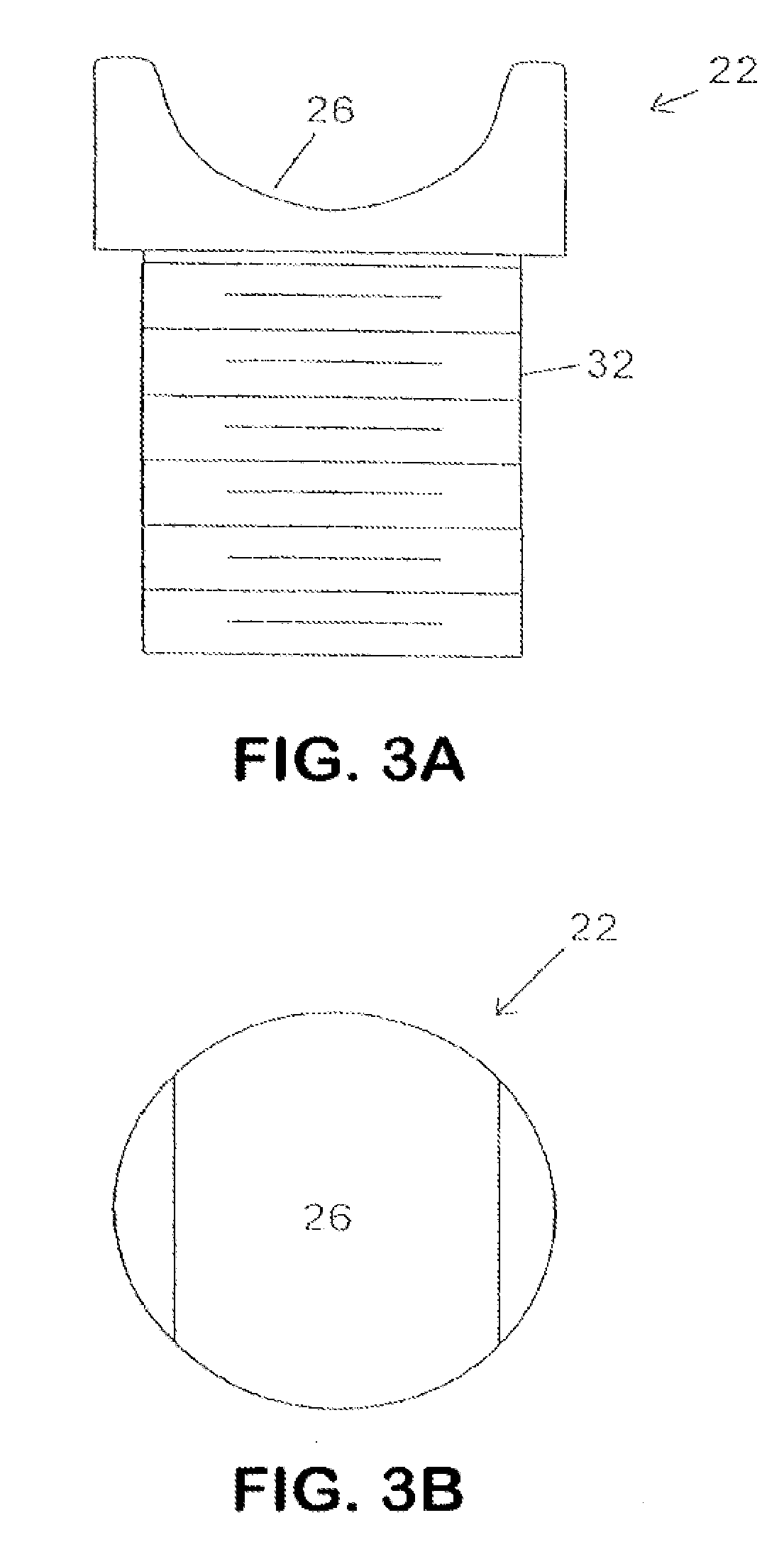
Described herein are devices, systems and methods for determining if a nerve is nearby a device or portion of a device. The neural stimulation tools described herein are configured to be flexible and low-profile, so that they can be used within body regions that may be tortuous or difficult to reach, such as within a compressed or partially occluded neural foramen. In most cases, these tools described herein are ribbon-shaped and adapted to be manipulated bimanually, for example, by applying force to the ends of the devices from separate locations outside of the patient's body. Thus, in some of the exemplary neural localization devices described herein, the distal end region of the device are configured to couple to the proximal end of a guidewire. One or more surfaces of the devices may include an electrode or multi-polar network of electrodes configured to stimulate only nerves within a predetermined distance of a particular face of the device.
Owner:BAXANO SURGICAL
Devices and methods for tissue access
ActiveUS20110160731A1Easy to disassembleEliminate needElectrotherapyCannulasAccess methodLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In some embodiments, a surgical tissue removal device includes a flexible elongate body that is adapted to conform with the target anatomy and a guidewire connector at the distal end region of the flexible elongate body configured to removably connect to the end of a guidewire so that the guidewire and flexible elongate body can be pulled distally. The body may have at least one blade edge, and the flexible elongate body may be a thin, flat, ribbon shaped flexible body that comprises a profile having a width that is substantially greater than a height.
Owner:SPINAL ELEMENTS INC
Spine distraction implant
InactiveUS20080086212A1Increase volumeReduce restrictionsInternal osteosythesisSpinal implantsDistractionDevice implant
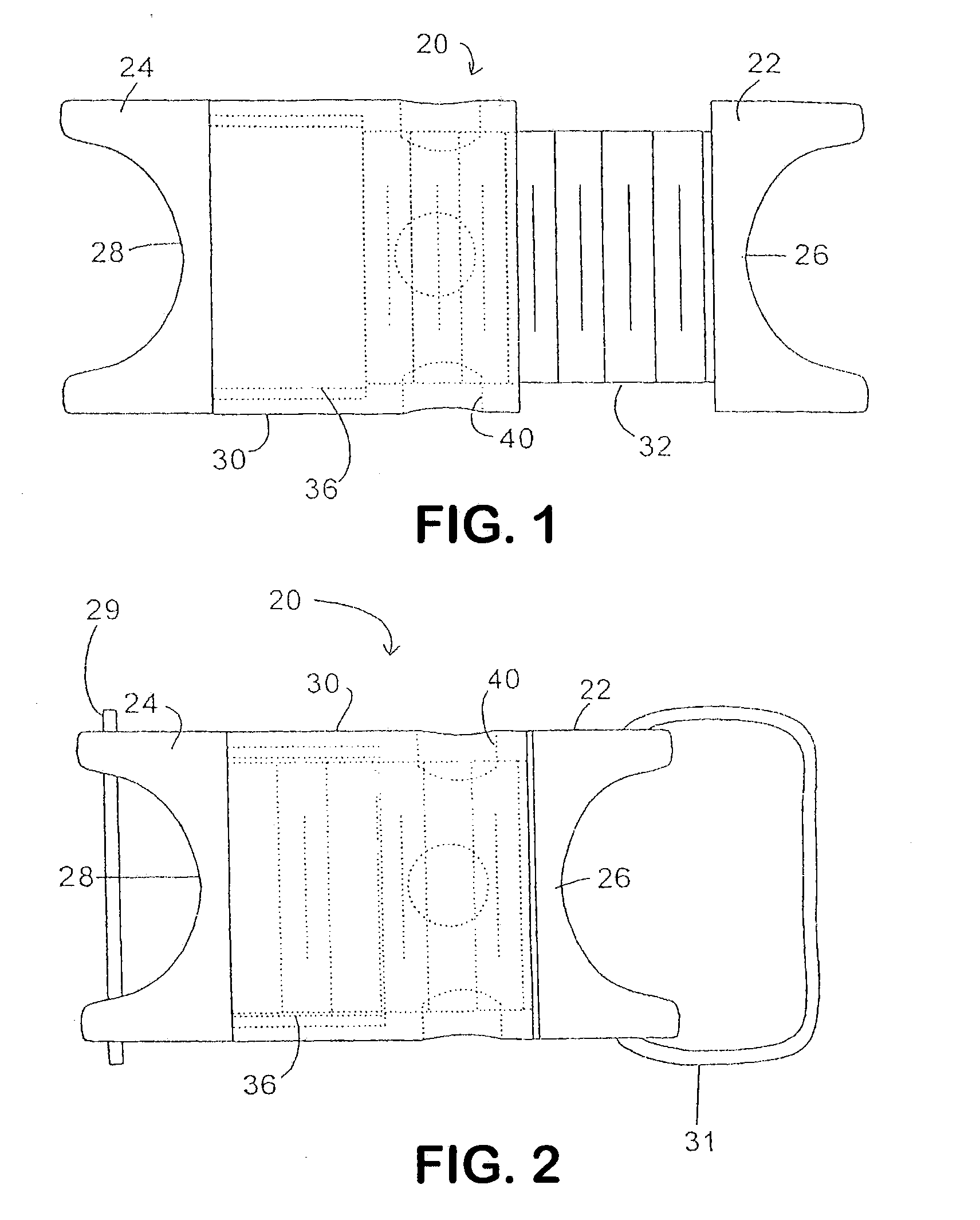
A spine distraction implant alleviates pain associated with spinal stenosis and facet arthropathy by expanding the volume in the spine canal and / or neural foramen. The implant provides a spinal extension stop while allowing freedom of spinal flexion. An interspinous process implant with a selectably expandable spacer can be placed between adjacent spinous processes. a device implanted between the spinous processes of adjacent vertebrae of the spine can be used for relieving pain associated with the vertebrae and surrounding tissues and structures by maintaining and / or adding distraction between adjacent vertebrae. A tissue expander can be adapted to move from a first insertion position, for ease of implantation between spinous processes, to a second retention position that prevents displacement of the implant. An embodiment of a system can include an implant having a spacer with a thickness and a wing, wherein a first configuration of the wing has a first height substantially similar to the thickness and wherein the wing is adapted to be selectably arranged in a second configuration such that the wing has a second height greater than the first height. A periphery of the implant has a shape generally conformal with a shape of an inner surface of a cannula and a cross-sectional diameter smaller than an inner diameter of the cannula. The cannula is inserted such that a proximal end of the cannula is arranged between the adjacent spinous processes. The implant is then urged into position between the adjacent spinous processes by way of the cannula, and subsequently arranged in a second configuration to fix the implant in position.
Owner:KYPHON
Devices and methods for selective surgical removal of tissue
ActiveUS20060094976A1Improve securityAvoid injuryCannulasAnti-incontinence devicesTissue remodelingLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for treating tissue
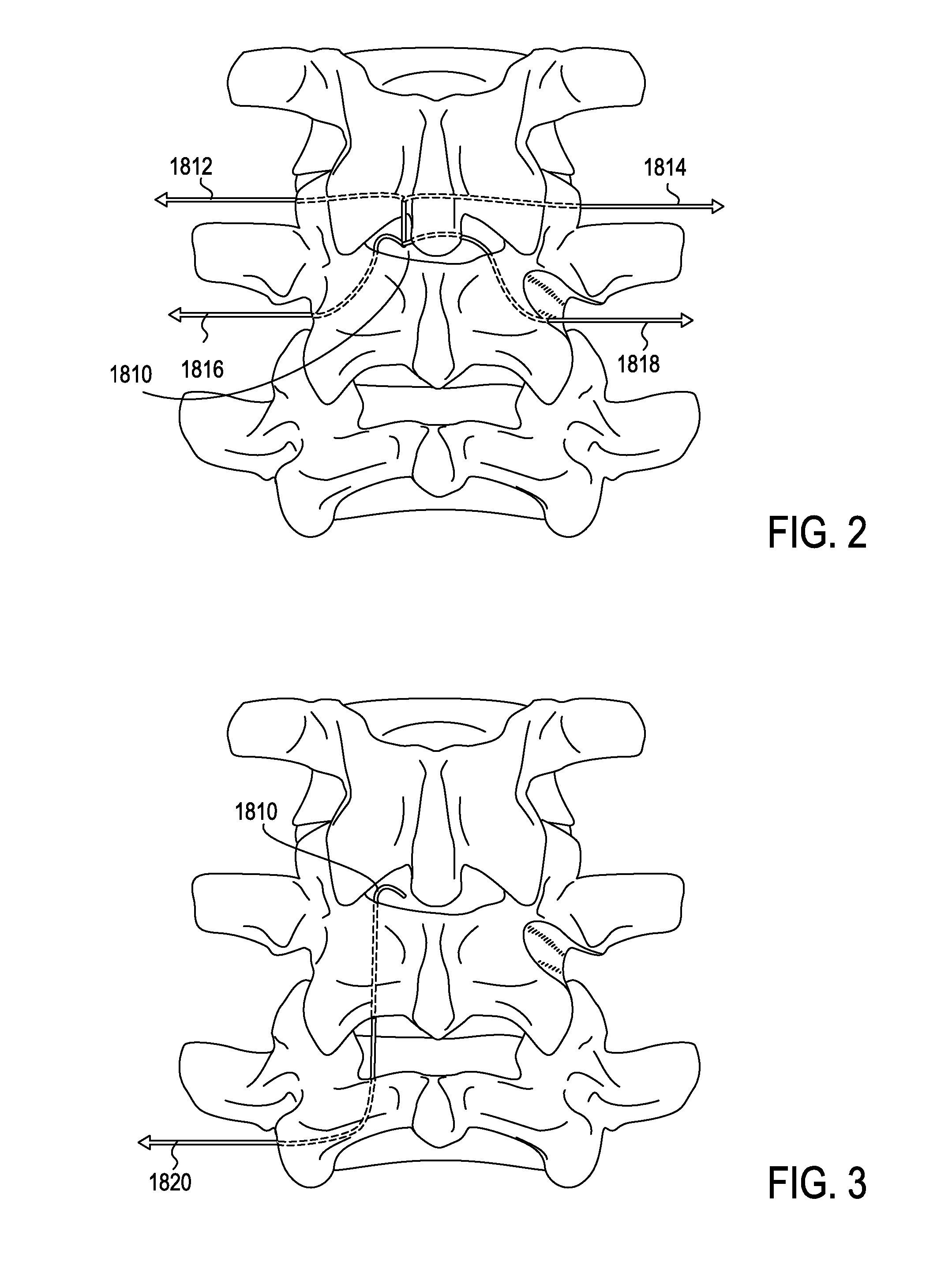
Described herein are devices, systems and methods for treating target tissue in a patient's spine. In general, the methods include the steps of advancing a wire into the patient from a first location, through a neural foramen, and out of the patient from a second location; connecting a tissue modification device to the wire; positioning the tissue modification device through the neural foramen using the wire; modifying target tissue in the spine by moving the tissue modification device against the target tissue; and delivering an agent to modified target tissue, wherein the agent is configured to inhibit blood flow from the modified target tissue. In some embodiments, the step of modifying target tissue comprises removing target tissue located ventral to the superior articular process while avoiding non-target tissue located lateral to the superior articular process.
Owner:SPINAL ELEMENTS INC
Devices and methods for tissue access
InactiveUS20060100651A1Improve securityAvoid injuryEar treatmentCannulasTissue remodelingSpinal column
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Spine distraction implant
InactiveUS20100262243A1Increase volumeReduce restrictionsInternal osteosythesisJoint implantsDistractionDilator
Owner:MEDTRONIC EURO SARL
Flexible neural localization devices and methods
ActiveUS20120123294A1Control spreadAvoid damageSpinal electrodesInternal osteosythesisMedicineNeural foramen
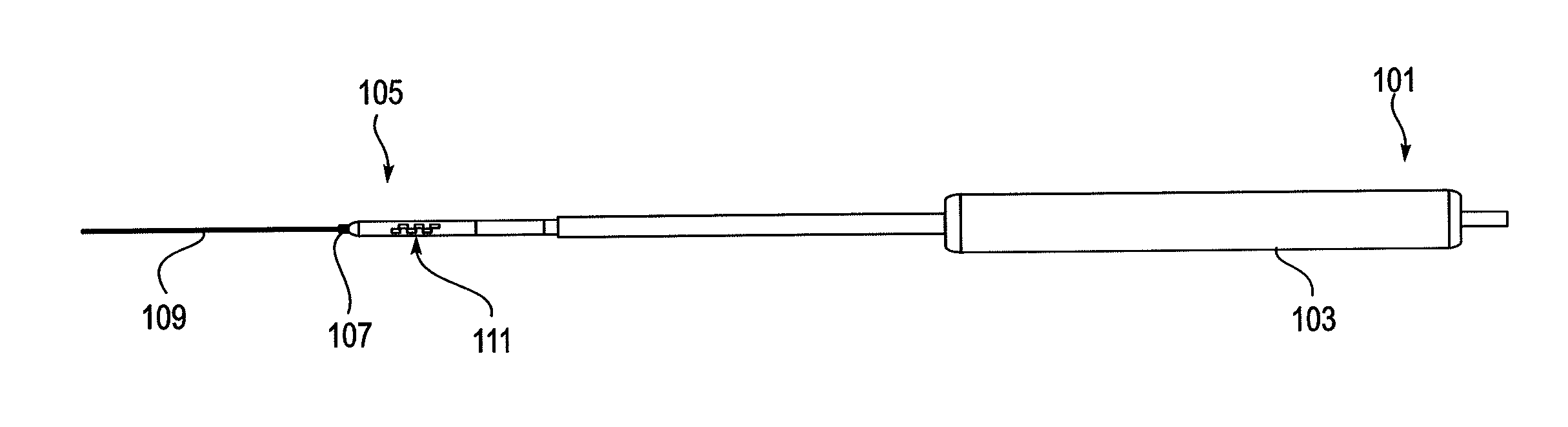
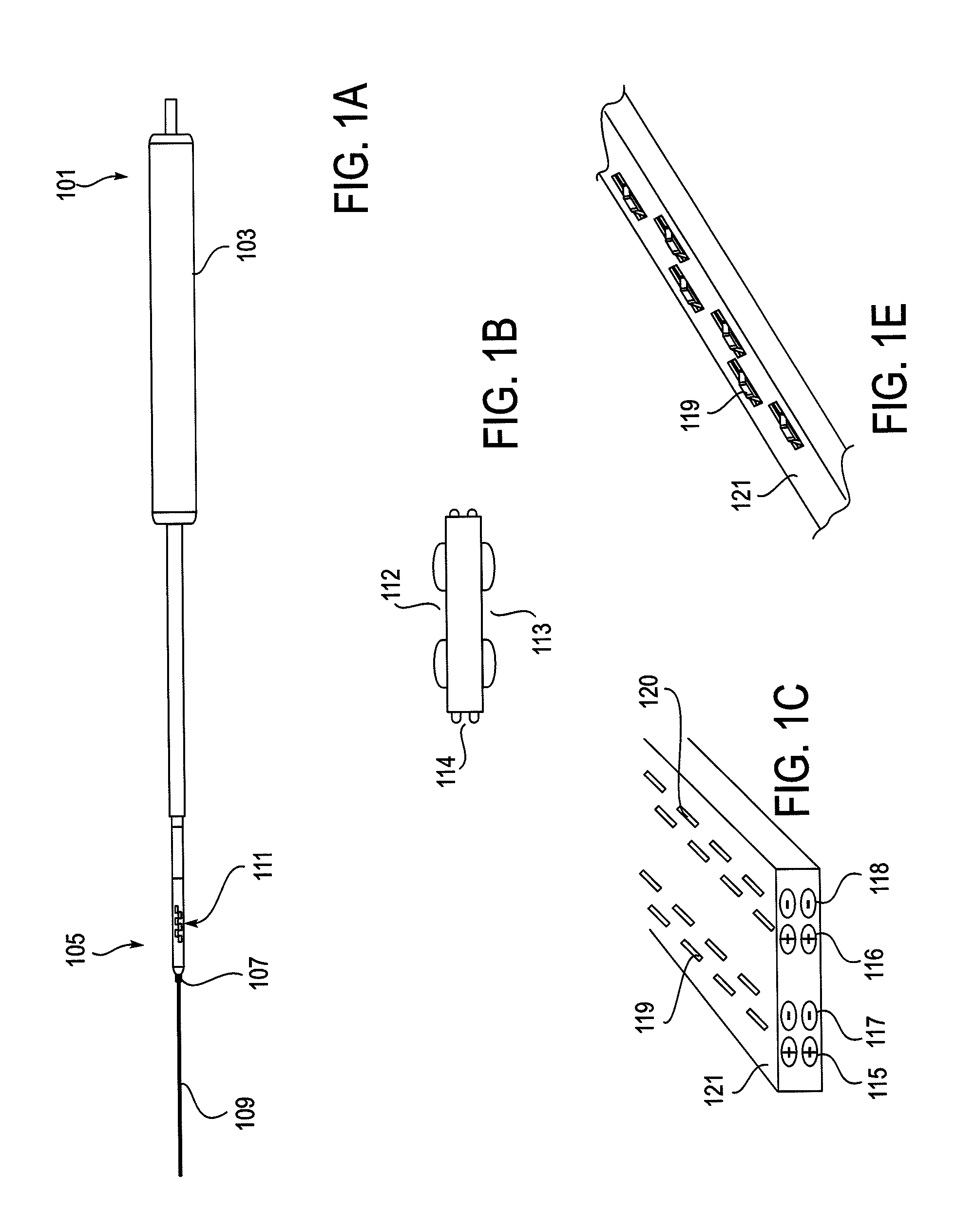
Methods for determining if a nerve is nearby a device. The neural stimulation tools described herein are configured to be flexible and low-profile, so that they can be used within body regions that may be tortuous or difficult to reach, such as within a compressed or partially occluded neural foramen. In most cases, these tools described herein are ribbon-shaped and adapted to be manipulated bimanually, applying force to the ends of the devices from separate locations outside of the patient's body. Thus, the distal end region of the device may be configured to couple to the proximal end of a guidewire. One or more surfaces of the devices may include an electrode or multi-polar network of electrodes configured to stimulate only nerves within a predetermined distance of a particular face of the device. Methods of using these devices are described.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue access
Owner:SPINAL ELEMENTS INC
Devices and methods for treating tissue
Owner:SPINAL ELEMENTS INC
Lumbar vertebra posterior approach fusion device
InactiveCN103610522AIncrease contact areaGuaranteed mechanical propertiesSpinal implantsBody right sideTransforaminal approach
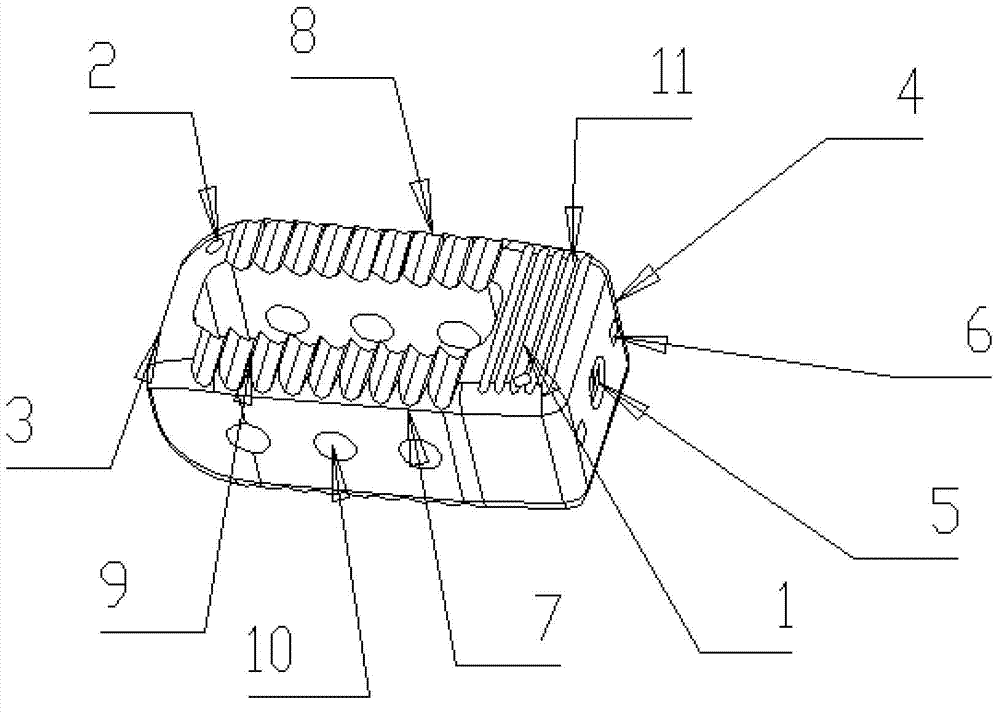
The invention relates to a lumbar vertebra posterior approach fusion device. The fusion device comprises a cuboid device body and a developing needle located on the device body. The device body is composed of a device body front end, a device body tail end, a device body upper surface, a device body lower surface, a device body left side wall and a device body right side wall. The device body is hollow inside and forms a device body inner cavity, thread through holes are formed in the device body tail end, and bone grafting windows are formed in the device body left side wall and the device body right side wall respectively and communicated with the device body inner cavity; the developing needle is exposed out of the device body upper surface and communicated with the device body lower surface. The lumbar vertebra posterior approach fusion device has the advantages of being suitable in self size, peripheral wall thickness and terminal plate contact area, fits the human body physiological structure, can be filled with enough sclerotin, has a good supporting function, further can ensure bone ingrowth, is convenient to implant and can meet two operation modes of lumbar vertebra posterior approach and lumbar vertebra posterior approach through intervertebral foramina.
Owner:广州聚生生物科技有限公司
Vertebral arch pedicle extension device
InactiveCN101695453AIncrease nerve compressionIncrease distanceInternal osteosythesisSurgical operationNeural foramen
The invention discloses a vertebral arch pedicle extension device, which comprises a front nail, a core bar, a rear nail and a fixer. The core bar has a hollow structure, wherein the middle part of the core bar is provided with a through hole, the front end of the core bar is connected with the front nail by screw thread, the rear end of the core bar is sleeved with the rear nail, and the core bar is glidingly connected with the rear nail; the fixer is fixedly connected at the rear end of the rear nail and enables the core bar and the rear nail to be fixed oppositely. The vertebral arch pedicle extension device can separate a centrum from a vertebral arch pedicle and extend the distance therebetween, finally achieve the aims of expanding a vertebral canal and an intervertebral foramina and reducing pressure, can be applied to surgical operation on stenosis of the lumbar spine and has good application prospect.
Owner:杨惠林 +3
Minimally invasive surgical instrument for treating spinal canal stenosis
InactiveCN104887295ABig spaceAvoid accidental damageSuture equipmentsInternal osteosythesisMini invasive surgerySurgical operation
The invention discloses a minimally invasive surgical instrument for treating spinal canal stenosis. The instrument comprises a casing pipe and a piezosurgery. The front end of the casing pipe is provided with a long main body, and the rear end is the insertion port of the piezosurgery. The side surface of the rear end of the long main body is provided with access ports, used to access a wash pipe, a water absorption pipe, an endoscope conduit, and a lamp line pipe. The casing pipe is placed into a position of spinal aperture or an intervertebral foramen through a minimally invasive method. The piezosurgery is guided by a channel in the casing pipe to perform bone gridding or bone cutting. Since the space limitation of the casing pipe, damages on nerves by mistake when the piezosurgery is operated can be prevented. The wash pipe, the water absorption pipe, the endoscope conduit, and the lamp line pipe are guided by the casing pipe, preventing wound caused by passing into a body. When the wash pipe performs washing, the piezosurgery is cooled down. The minimally invasive surgical instrument changes an open method for treating spinal canal stenosis by a conventional instrument, and a minimally invasive surgical method is used to treat spinal canal stenosis, so as to satisfy requirements of surgical operation on accuracy and security.
Owner:BONE MEDICAL TECH OF SUZHOU CO LTD
Devices and methods for treating tissue
Described herein are devices, systems and methods for treating target tissue in a patient's spine. In general, the methods include the steps of advancing a wire into the patient from a first location, through a neural foramen, and out of the patient from a second location; connecting a tissue modification device to the wire; positioning the tissue modification device through the neural foramen using the wire; modifying target tissue in the spine by moving the tissue modification device against the target tissue; and delivering an agent to modified target tissue, wherein the agent is configured to inhibit blood flow from the modified target tissue. In some embodiments, the step of modifying target tissue comprises removing target tissue located ventral to the superior articular process while avoiding non-target tissue located lateral to the superior articular process.
Owner:SPINAL ELEMENTS INC
Methods and devices for spinal correction
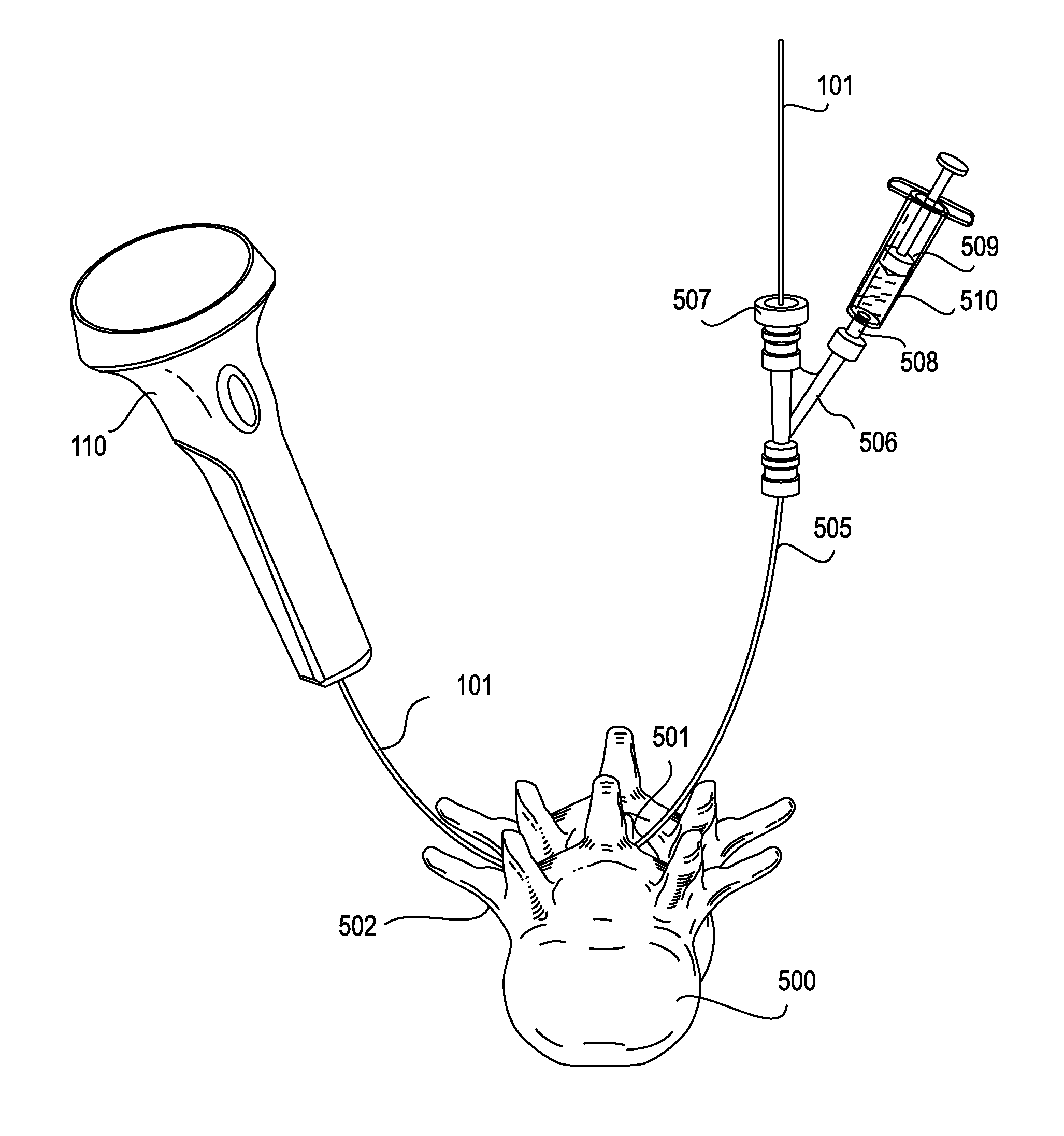
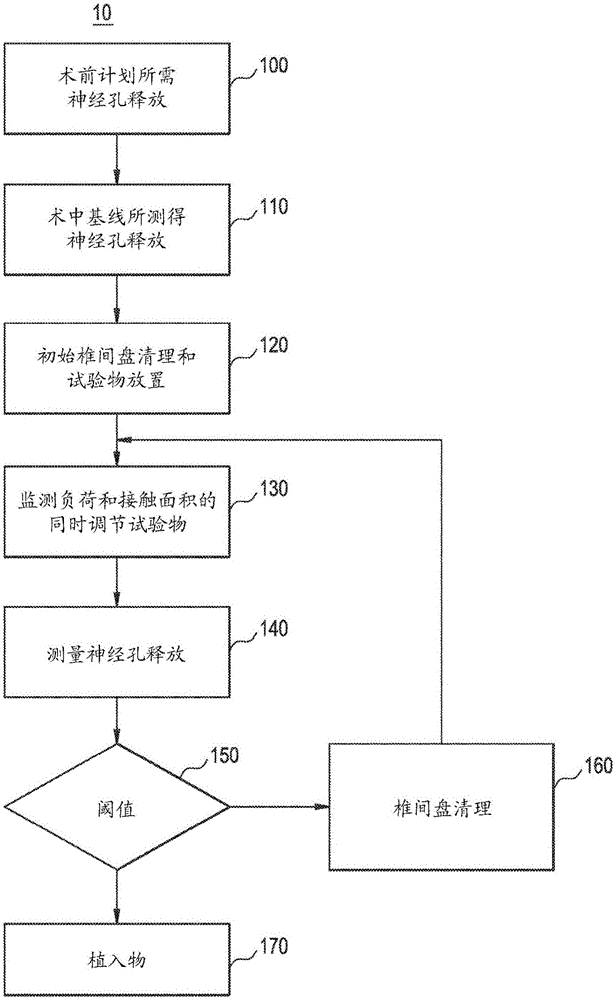
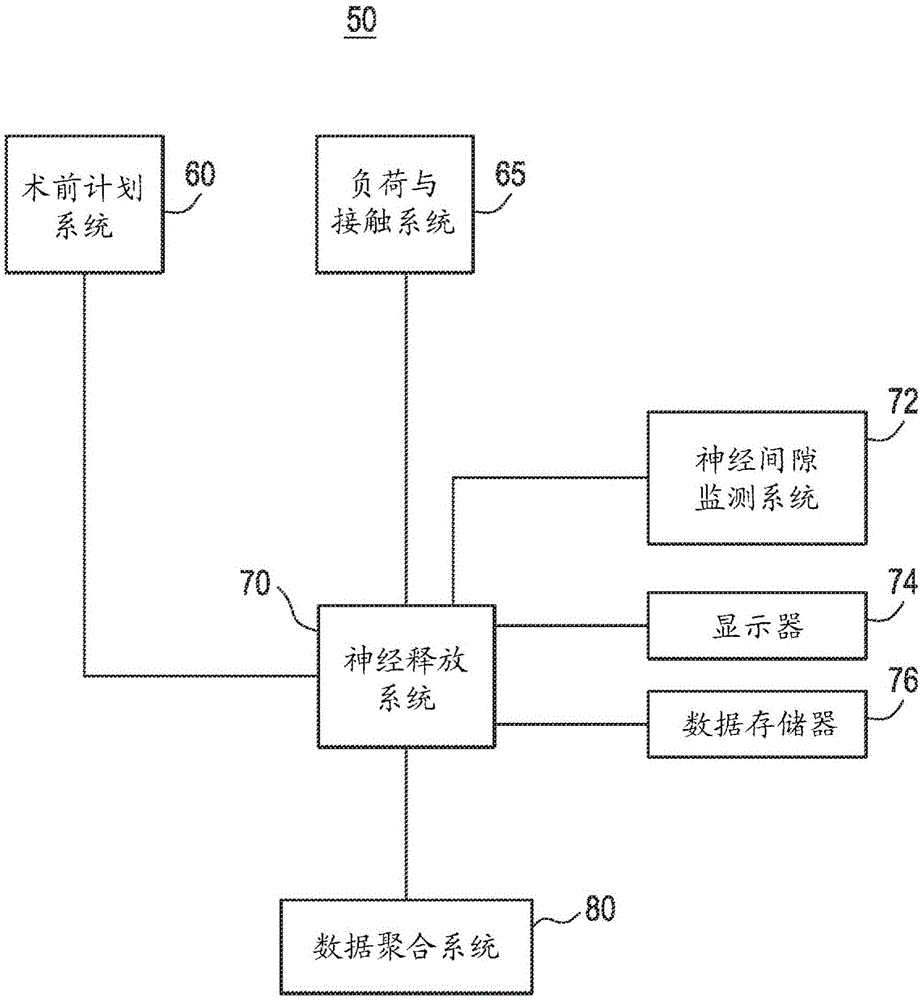
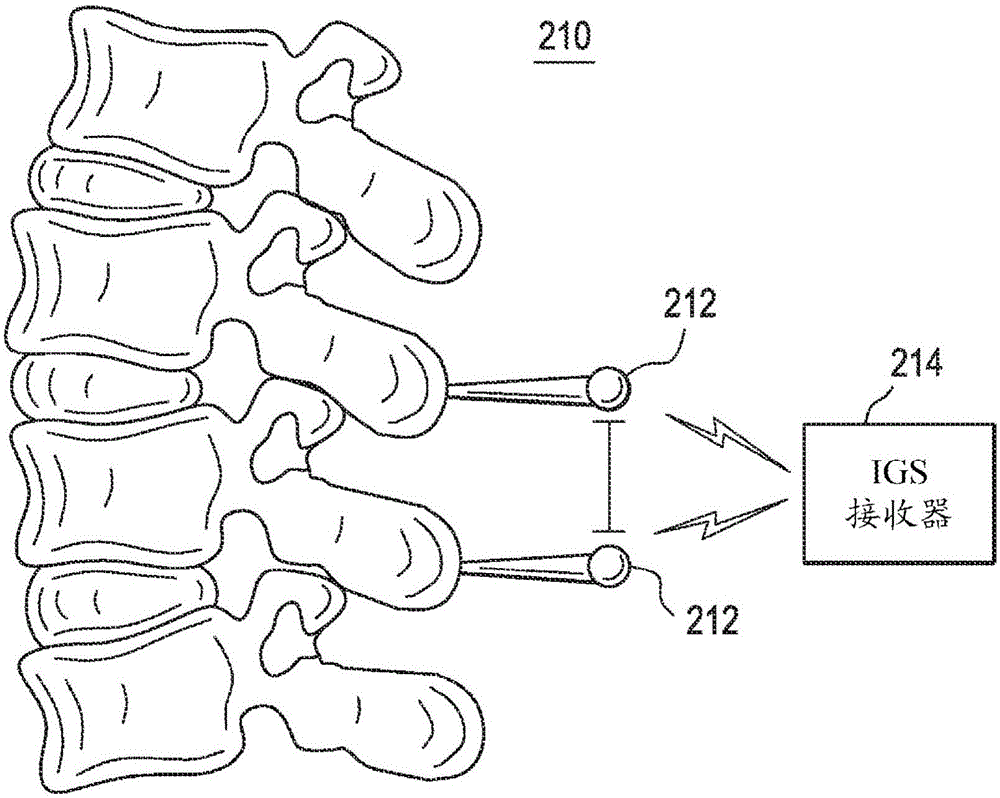
An expandable trial can include an inferior portion, a superior portion, and a middle expanding portion as well as load cells for monitoring the load on the trial. The trial may also include recesses on its lateral sides to provide spacing to accommodate a disc removal tool so tissue can be cleared monitoring load. In addition, neural foramen spacing can be monitoring to provide information about how much neural release has been achieved as the disc is cleaned and the spine is positioned and repositioned.
Owner:DEPUY SYNTHES PROD INC
Supporting device of percutaneous expanding crest
The invention discloses a supporting device of a percutaneous expanding crest, comprising a supporting piece provided with a via hole, an expansion piece and a back blocking piece, wherein the expansion piece comprises a shaft and a first end of the shaft to be pivoted to one or a plurality of vanes, and the back blocking piece is matched with a second end of the shaft of the expansion piece. The shaft of the expansion piece is sleeved in the via hole of the supporting piece in a penetrating way, the second end of the shaft of the expansion piece is provided with a long slide hole in an axial direction, and is pivoted to one or a plurality of vanes in a sliding way through the long slide hole. The supporting piece can be movably contacted with the vanes which are pivoted to the both ends of the expansion piece to form a tip type ellipse, the expansion piece and the supporting piece forming form the tip type ellipse are inserted into a vertebrae spinous process interval position together, and the vanes at the both ends strentch by being pushed or pushing against a pivot to be respectively arranged at both sides of the vertebrae spinous process interval position in a locking way. The device can lower the infection risk, can efficiently form support to vertebrae, can stabilize a vertebrae neural foramen space after an operation, and has the advantages of simpleness and efficiency as a device for treating pains caused by compressing vertebrae nerves.
Owner:A SPINE ASIA
Supporting device of percutaneous expanding crest
The invention discloses a supporting device of a percutaneous expanding crest, comprising a supporting piece provided with a via hole, an expansion piece and a back blocking piece, wherein the expansion piece comprises a shaft and a first end of the shaft to be pivoted to one or a plurality of vanes, and the back blocking piece is matched with a second end of the shaft of the expansion piece. Theshaft of the expansion piece is sleeved in the via hole of the supporting piece in a penetrating way, the second end of the shaft of the expansion piece is provided with a long slide hole in an axialdirection, and is pivoted to one or a plurality of vanes in a sliding way through the long slide hole. The supporting piece can be movably contacted with the vanes which are pivoted to the both ends of the expansion piece to form a tip type ellipse, the expansion piece and the supporting piece forming form the tip type ellipse are inserted into a vertebrae spinous process interval position together, and the vanes at the both ends strentch by being pushed or pushing against a pivot to be respectively arranged at both sides of the vertebrae spinous process interval position in a locking way. Thedevice can lower the infection risk, can efficiently form support to vertebrae, can stabilize a vertebrae neural foramen space after an operation, and has the advantages of simpleness and efficiencyas a device for treating pains caused by compressing vertebrae nerves.
Owner:A SPINE ASIA
Lockable Spinal Implant
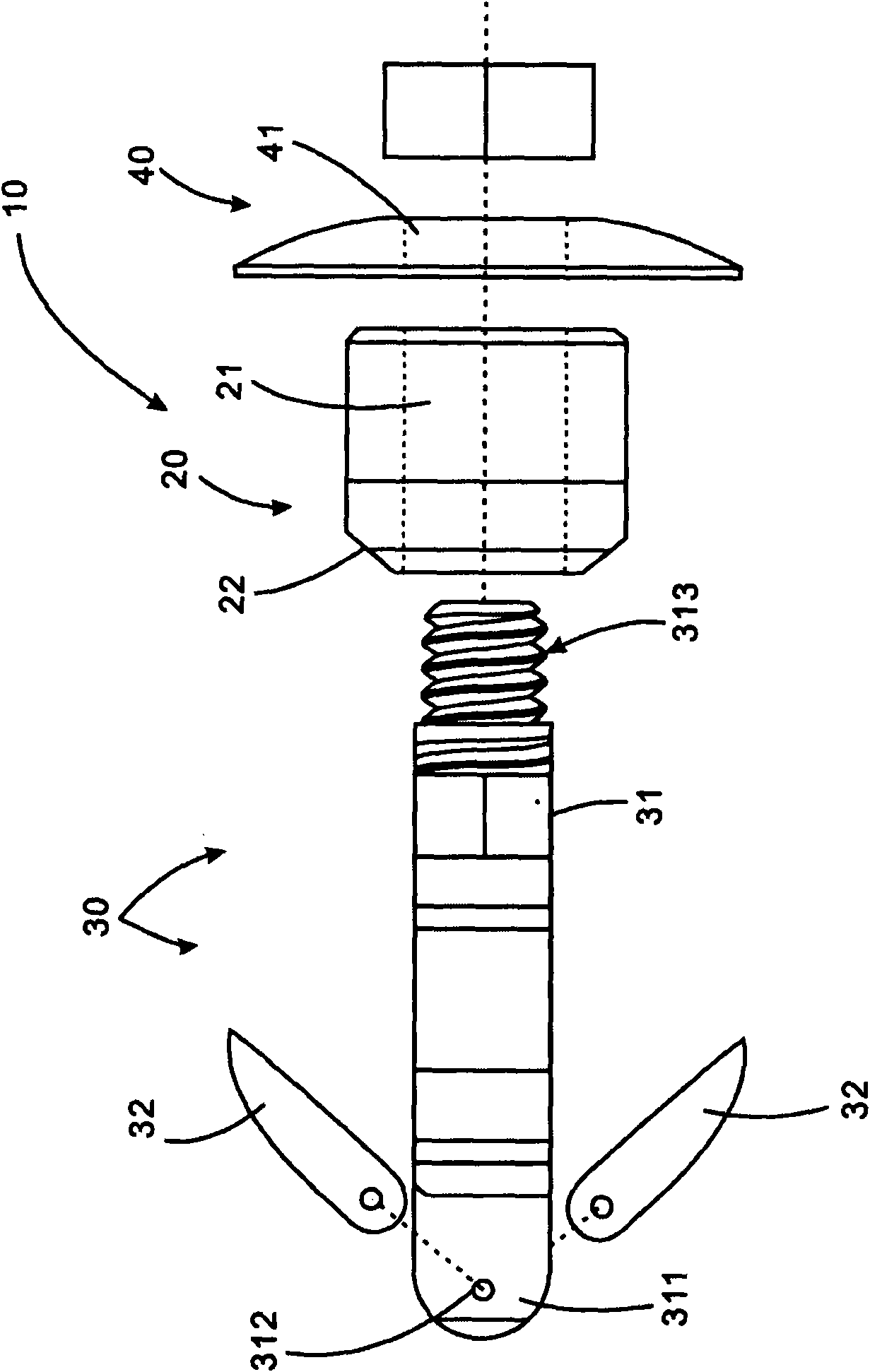
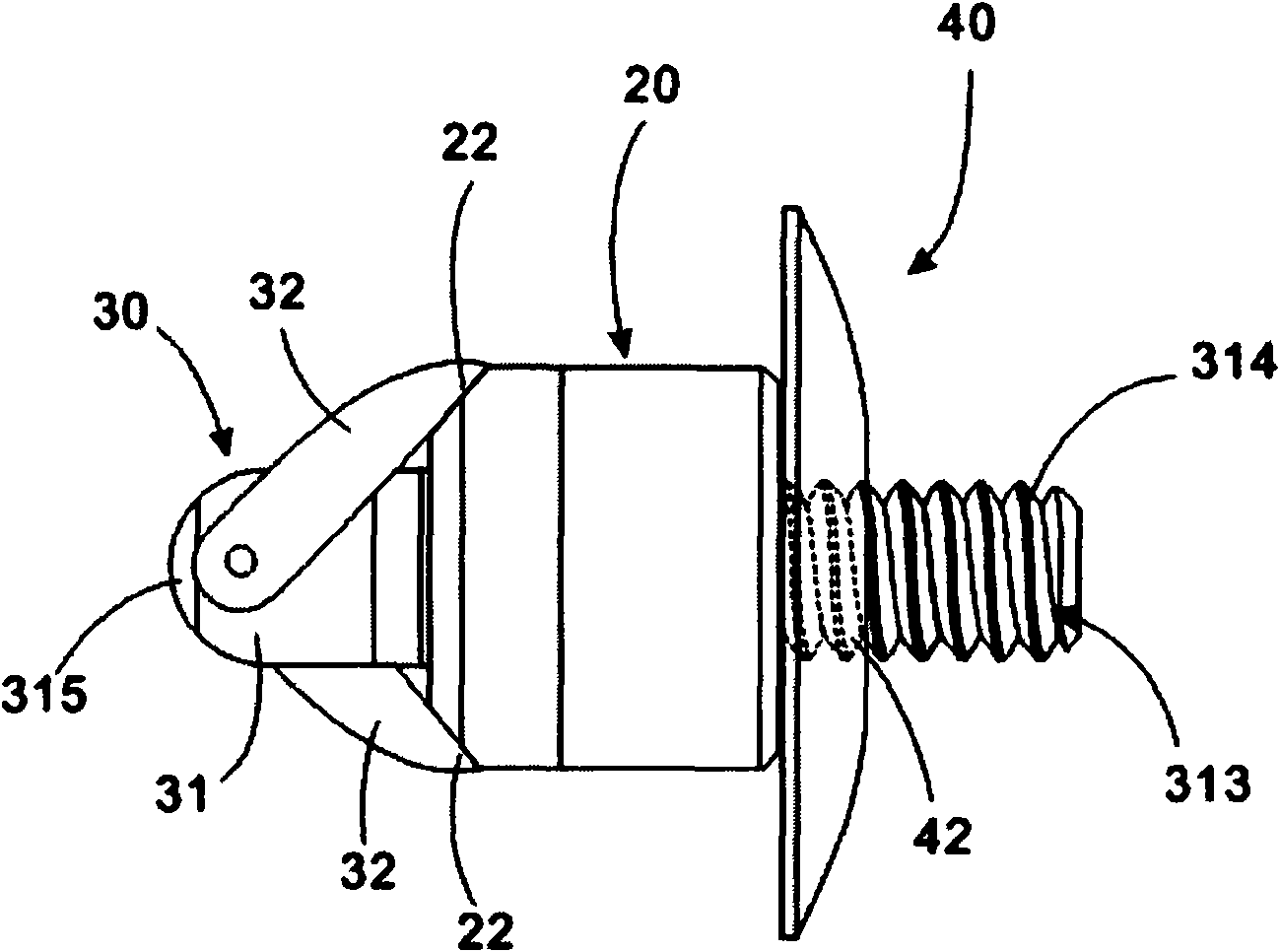
A spinal implant which is configured to be deployed between adjacent vertebral bodies. The implant has at least one extendable support element with a retracted configuration to facilitate deployment of the implant and an extended configuration so as to expand the implant and effectively distract the disc space, stabilize the motion segments and eliminate pathologic spine motion. The implant has a minimal dimension in its unexpanded state that is smaller than the dimensions of the neuroforamen through which it typically passes to be deployed within the intervertebral space. The implant is provided with a locking system having a plurality of linked locking elements that work in unison to lock the implant in an extended configuration. Bone engaging anchors also may be provided to ensure secure positioning.
Owner:HOWMEDICA OSTEONICS CORP
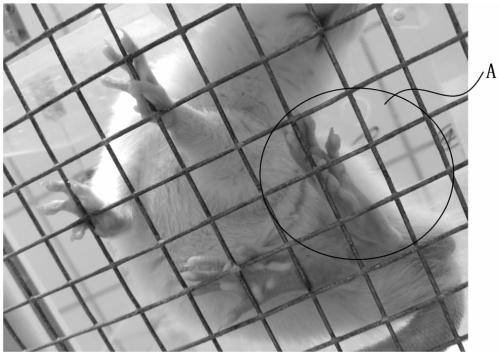
Preparation method and application of rat chronic dorsal root neurothlipsis model
InactiveCN111280124AEasy to measure objectivelyAvoid damageAnimal husbandryEtiologyLumbar intervertebral disc
The invention discloses a preparation method and application of a rat chronic dorsal root neurothlipsis model. The method includes the steps: step one, narcotizing a rat; step two, cutting a longitudinal incision on the horizontal left or horizontal right of parts from the 4th lumbar vertebra to the 1st sacrrum, cutting the skin, performing blunt dissection on paraspinal muscles, and exposing the4th lumbar intervertebral foramina and the 5th lumbar intervertebral foramina; and step three, inserting compression elements into the 4th lumbar intervertebral foramen and the 5th lumbar intervertebral foramen on the left or the right so as to obtain the rat chronic dorsal root neurothlipsis model. The compression elements are inserted into the lumbar intervertebral foramina so that continuous and chronic mechanical compression symptoms, caused by protrusions of lumbar disc herniation, of nerve roots are simulated, and the etiology of the symptoms is close to clinical situations; and the preparation method is simple, and has small damage to the animal, good stability and a high success rate. The model which is obtained by using the method can be applied to research of treatment methods and / or imaging detection of lumbar disc herniation.
Owner:嘉升(上海)生物科技有限公司
Instrument tools for performing percutaneous extraforaminotomy with foraminal ligament resection
InactiveUS20170311977A1Easy to useInsert smoothlyCannulasSurgical needlesEpidural neurolysisNeural foramen
Provided is an instrument tool for performing a percutaneous extraforaminotomy with foraminal ligament resection, which examines various ligaments around intervertebral foramen, artificially expands the intervertebral foramen blocked by adhesive fibrosis through repeated inflammatory reactions using epidural neurolysis or percutaneous extraforaminotomy, transmits chemical materials efficacious in pain treatment to the periphery of nervous branches causing pain through a catheter, and smoothly discharges an inflammatory material existing in spinal canal with the chemical materials through the intervertebral foramen.
Owner:PARK KYUNG WOO
Vertebral arch pedicle extension device
InactiveCN101695453BIncrease nerve compressionIncrease distanceInternal osteosythesisSurgical operationNeural foramen
The invention discloses a vertebral arch pedicle extension device, which comprises a front nail, a core bar, a rear nail and a fixer. The core bar has a hollow structure, wherein the middle part of the core bar is provided with a through hole, the front end of the core bar is connected with the front nail by screw thread, the rear end of the core bar is sleeved with the rear nail, and the core bar is glidingly connected with the rear nail; the fixer is fixedly connected at the rear end of the rear nail and enables the core bar and the rear nail to be fixed oppositely. The vertebral arch pedicle extension device can separate a centrum from a vertebral arch pedicle and extend the distance therebetween, finally achieve the aims of expanding a vertebral canal and an intervertebral foramina and reducing pressure, can be applied to surgical operation on stenosis of the lumbar spine and has good application prospect.
Owner:杨惠林 +3
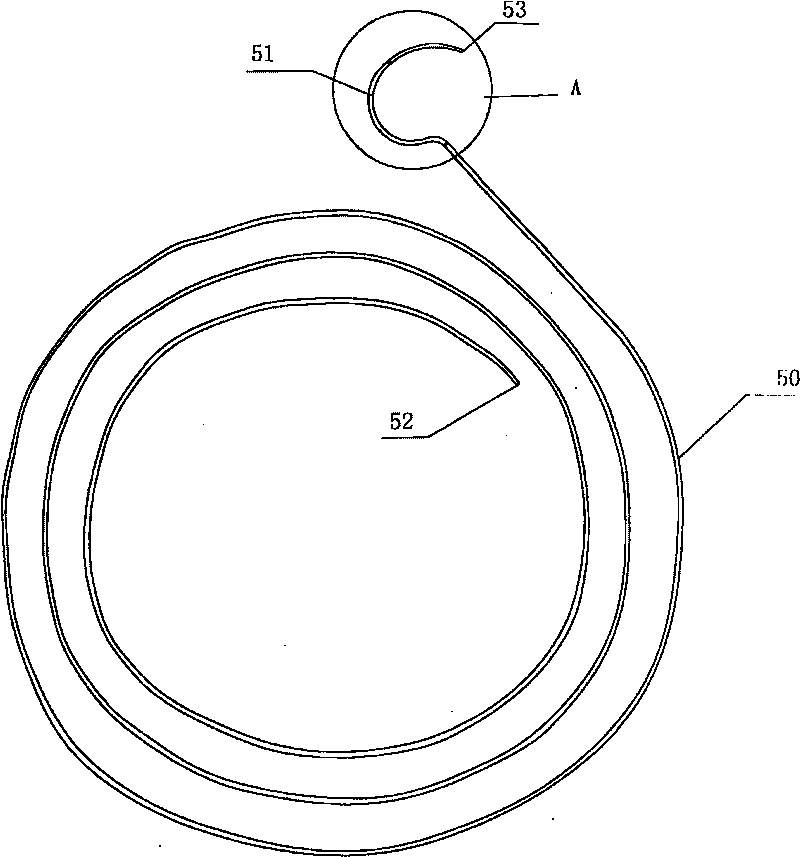
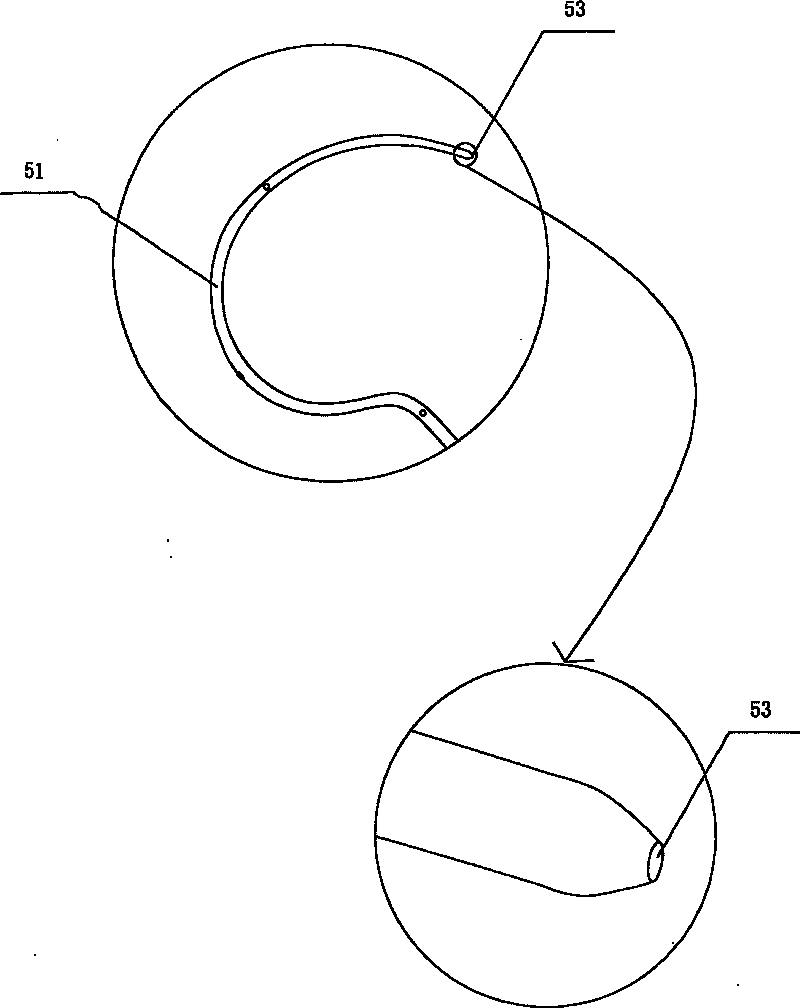
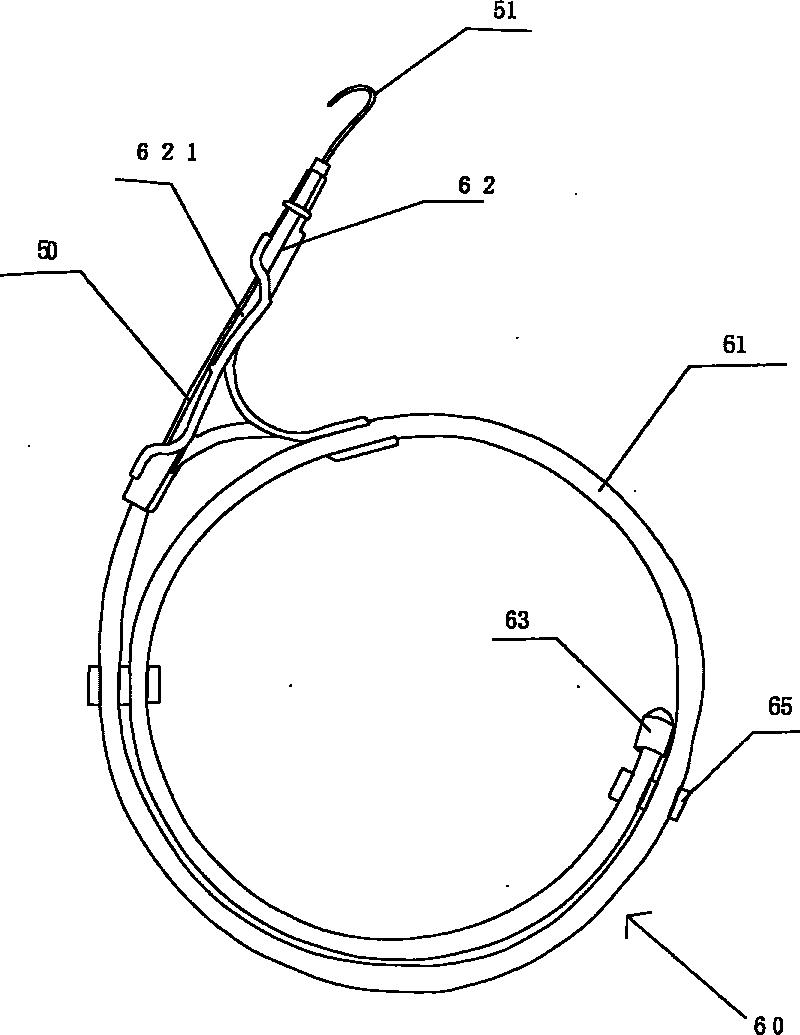
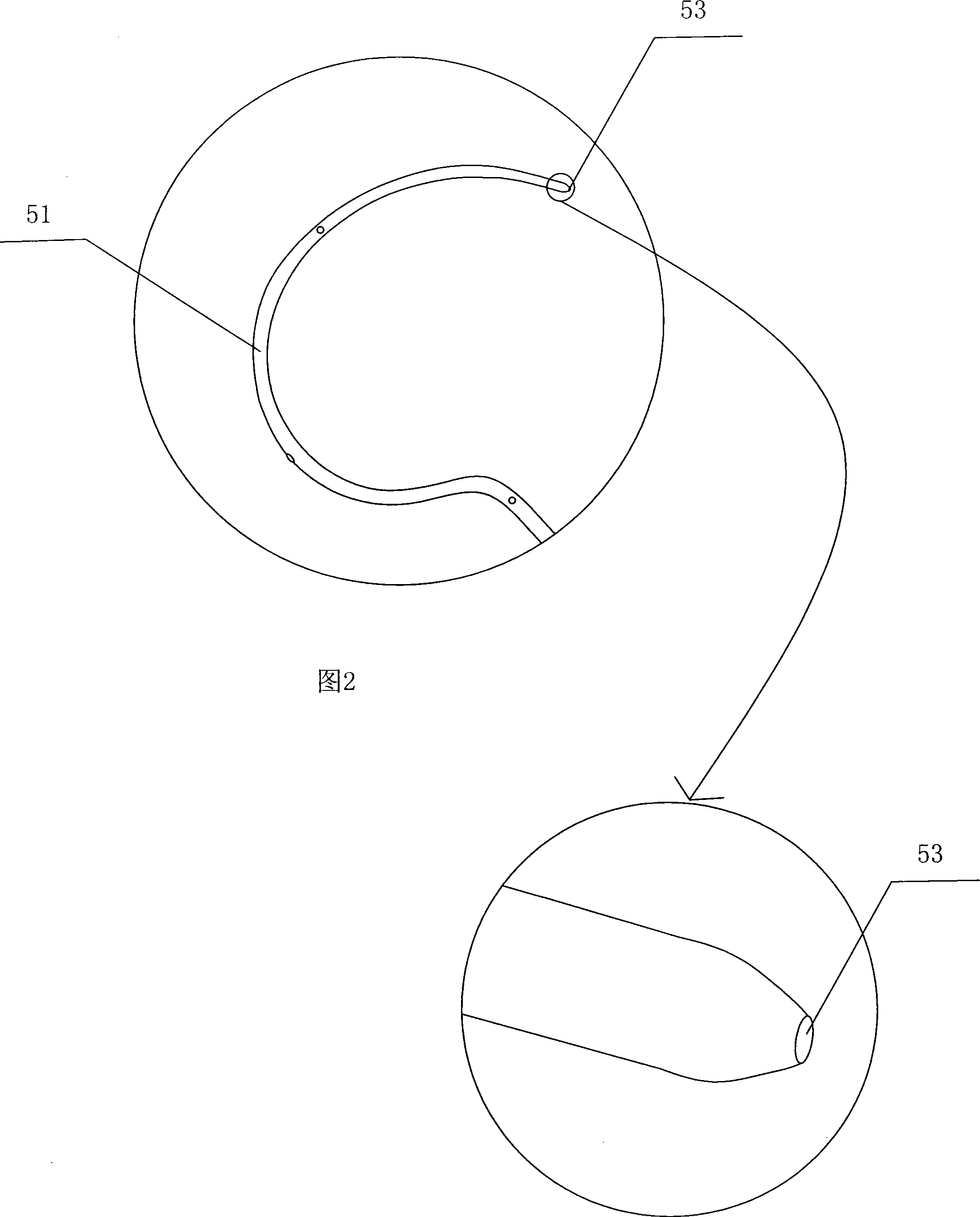
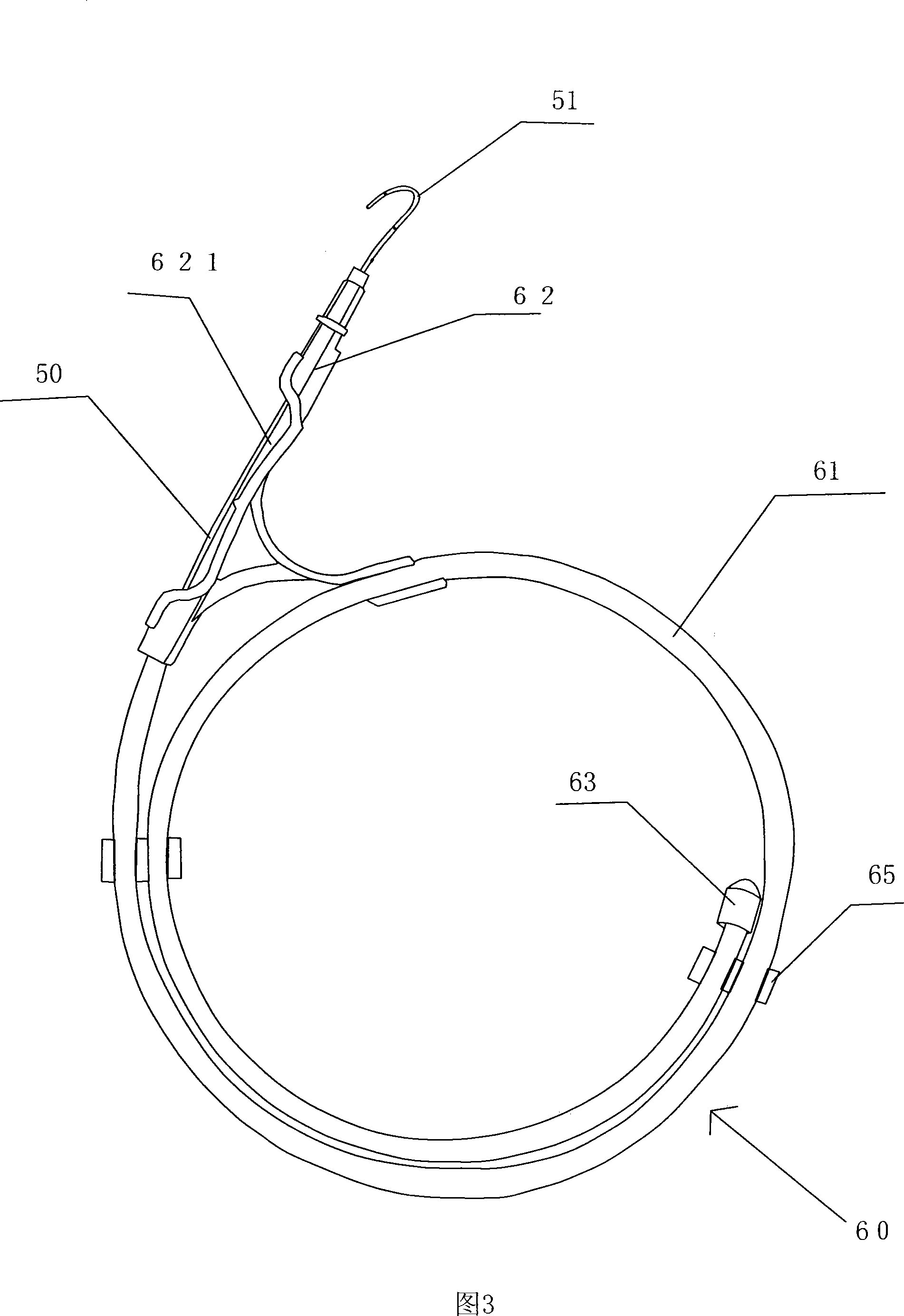
Exterior pipe of hard film
The invention relates to a medical treatment apparatus, in particular to an epidural guiding tube which comprises a malleable rubber tube. One end of the pipe is a hook bending tip and the other end is an open end. 1 to 6 orifice(s) for drug injection is / are arranged on the hook bending tip and an orifice for drug injection is arranged on the front end of the hook bending tip. A sleeve is arrangedon the outside of the malleable rubber tube. The tip of the epidural guiding tube is shaped in a bending hook, so that the epidural guiding tube is not easy to penetrate putamen or blood vessel for progressing in an epidural space in the shape of a hook one-eighth bend. The diameter of the hook is bigger than (or equal to) the diameter of an intervertebral foramina and the epidural guiding tube isalso strayed into the intervertebral foramina. Being positioned in a joint of the sleeve, the tip of the malleable rubber tube is in the state of extension, thus being convenient for putting the mall eable rubber tube into an inner chamber of an epidural needle and favorable to protect the guiding tube away from the pollution of thimerosal, talcum powder on sterile gloves, etc.
Owner:赵阳 +1
Adjustable spine distraction implant
ActiveUS20190090915A1Less discomfortRelief the painInternal osteosythesisSpinal implantsSpinal columnDistraction
An adjustable spine distraction implant alleviates pain associated with spinal stenosis and facet arthropathy by expanding the volume and / or cross sectional area in the spinal canal and / or neural foramen. The adjustable implant provides a spinal extension inhibitor. The implant includes elliptical or oval shaped adjustable member or spacer for positioning between and adjustably spacing apart the spinous processes.
Owner:LIFE SPINE INC
Leads for extraforaminal stimulation of dorsal roots and dorsal root ganglia and related methods
ActiveUS20180169405A1Easy to implantSpinal electrodesSubcutaneous electrodesNeural foramenElectrical stimulations
The present disclosure generally relates to extraforaminal electrical stimulation systems and leads for electrical stimulation of the dorsal root and dorsal root ganglion (DRG), minimally invasive implantation methods therefore, and related methods of providing extraforaminal electrical stimulation of the dorsal root and DRG for the treatment of a medical condition. In accordance with certain aspects, the extraforaminal electrical stimulation leads and methods are particularly suited for stimulation of dorsal roots and DRG of the cervical and thoracic spine.
Owner:PACESETTER INC
Application of cdn1163 in the preparation of drugs for alleviating or treating neuropathic pain
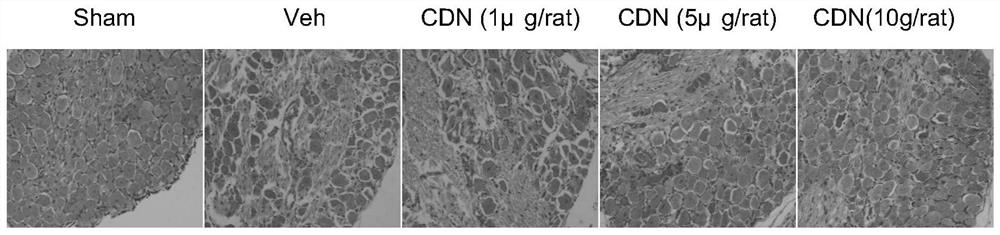
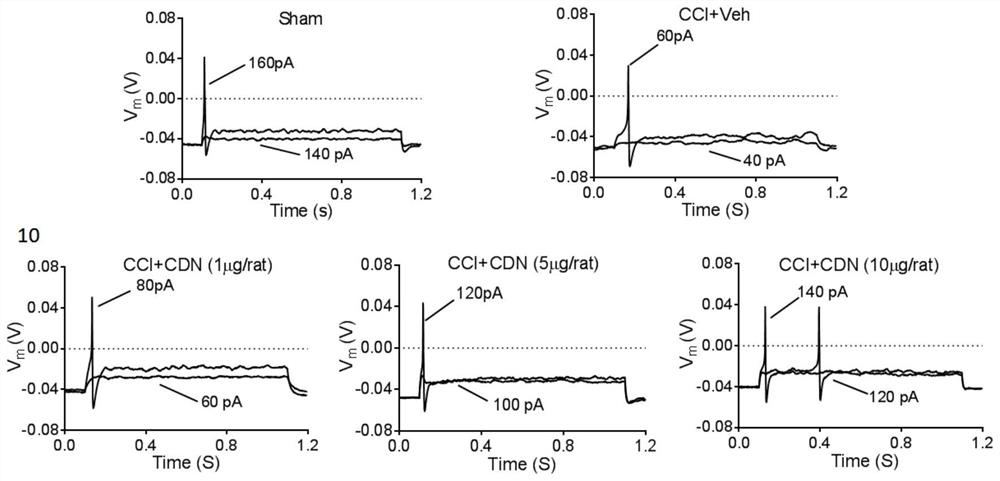
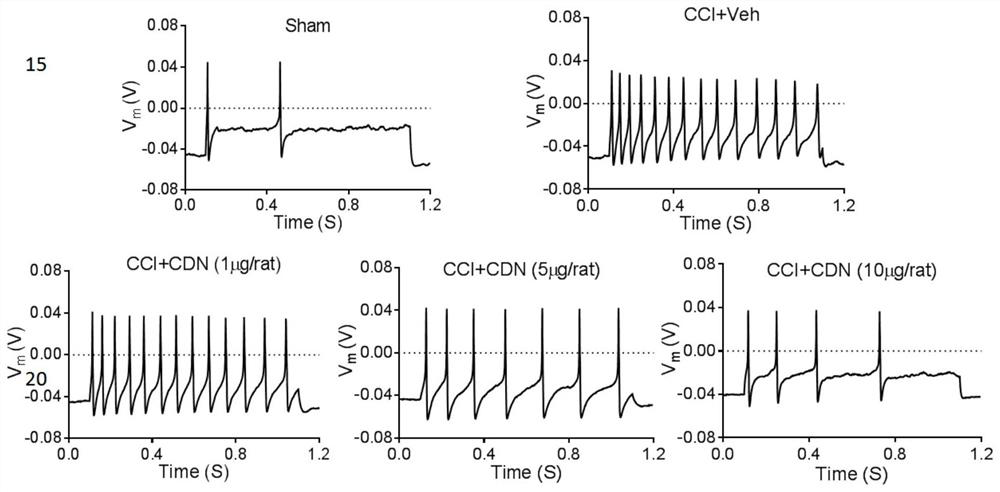
ActiveCN112716953BPromote activationInhibition of elevated excitabilityOrganic active ingredientsNervous disorderDrug withdrawalNeural foramen
The invention discloses the application of CDN1163 in preparing a medicine for relieving or treating neuropathic pain. The CDN1163 can act on neuropathic pain including one or more of central neuropathic pain and peripheral neuropathic pain, and improve chronic sciatic nerve compression in rats. Mechanical pain sensitivity and thermal pain sensitivity caused by sexual injury, improve neuronal shrinkage and astrocyte activation in lumbar intervertebral foramen 5 dorsal root ganglia, inhibit neuronal excitation in lumbar intervertebral foramina 4-6 dorsal root ganglia It can effectively reduce the symptoms of neuropathic pain, and repeated injections can significantly reverse mechanical hyperalgesia and thermal hyperalgesia, and there is no sign of tolerance, and the therapeutic effect can be maintained after drug withdrawal. and difficult-to-treat neuropathic pain medications.
Owner:CHINA PHARM UNIV
Leads for extraforaminal stimulation of dorsal roots and dorsal root ganglia and related methods
The present disclosure generally relates to extraforaminal electrical stimulation systems and leads for electrical stimulation of the dorsal root and dorsal root ganglion (DRG), minimally invasive implantation methods therefore, and related methods of providing extraforaminal electrical stimulation of the dorsal root and DRG for the treatment of a medical condition. In accordance with certain aspects, the extraforaminal electrical stimulation leads and methods are particularly suited for stimulation of dorsal roots and DRG of the cervical and thoracic spine.
Owner:PACESETTER INC
Exterior pipe of hard film
The invention relates to a medical treatment apparatus, in particular to an epidural guiding tube which comprises a malleable rubber tube. One end of the pipe is a hook bending tip and the other end is an open end. 1 to 6 orifice(s) for drug injection is / are arranged on the hook bending tip and an orifice for drug injection is arranged on the front end of the hook bending tip. A sleeve is arranged on the outside of the malleable rubber tube. The tip of the epidural guiding tube is shaped in a bending hook, so that the epidural guiding tube is not easy to penetrate putamen or blood vessel for progressing in an epidural space in the shape of a hook one-eighth bend. The diameter of the hook is bigger than (or equal to) the diameter of an intervertebral foramina and the epidural guiding tube is also strayed into the intervertebral foramina. Being positioned in a joint of the sleeve, the tip of the malleable rubber tube is in the state of extension, thus being convenient for putting the malleable rubber tube into an inner chamber of an epidural needle and favorable to protect the guiding tube away from the pollution of thimerosal, talcum powder on sterile gloves, etc.
Owner:赵阳 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com