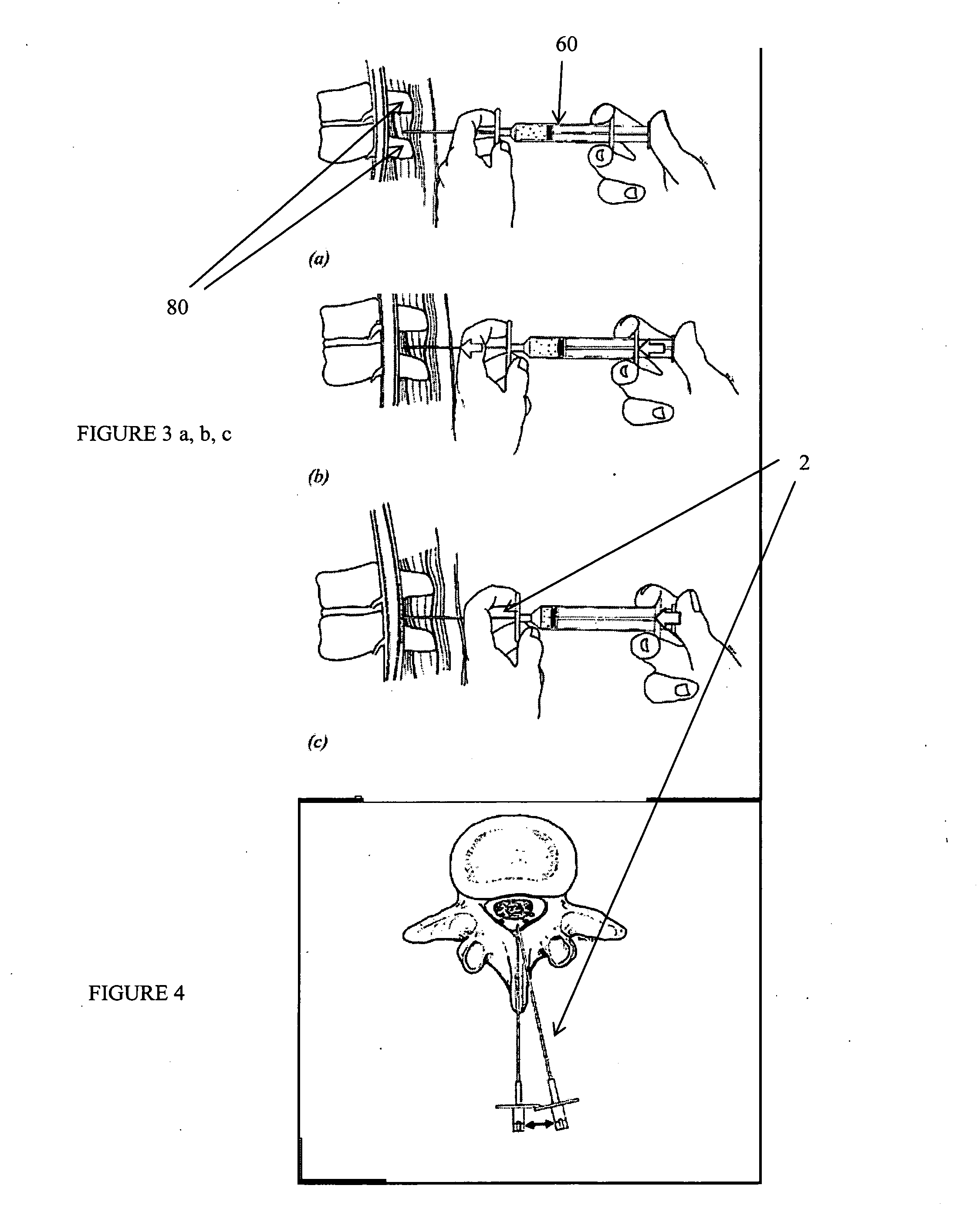
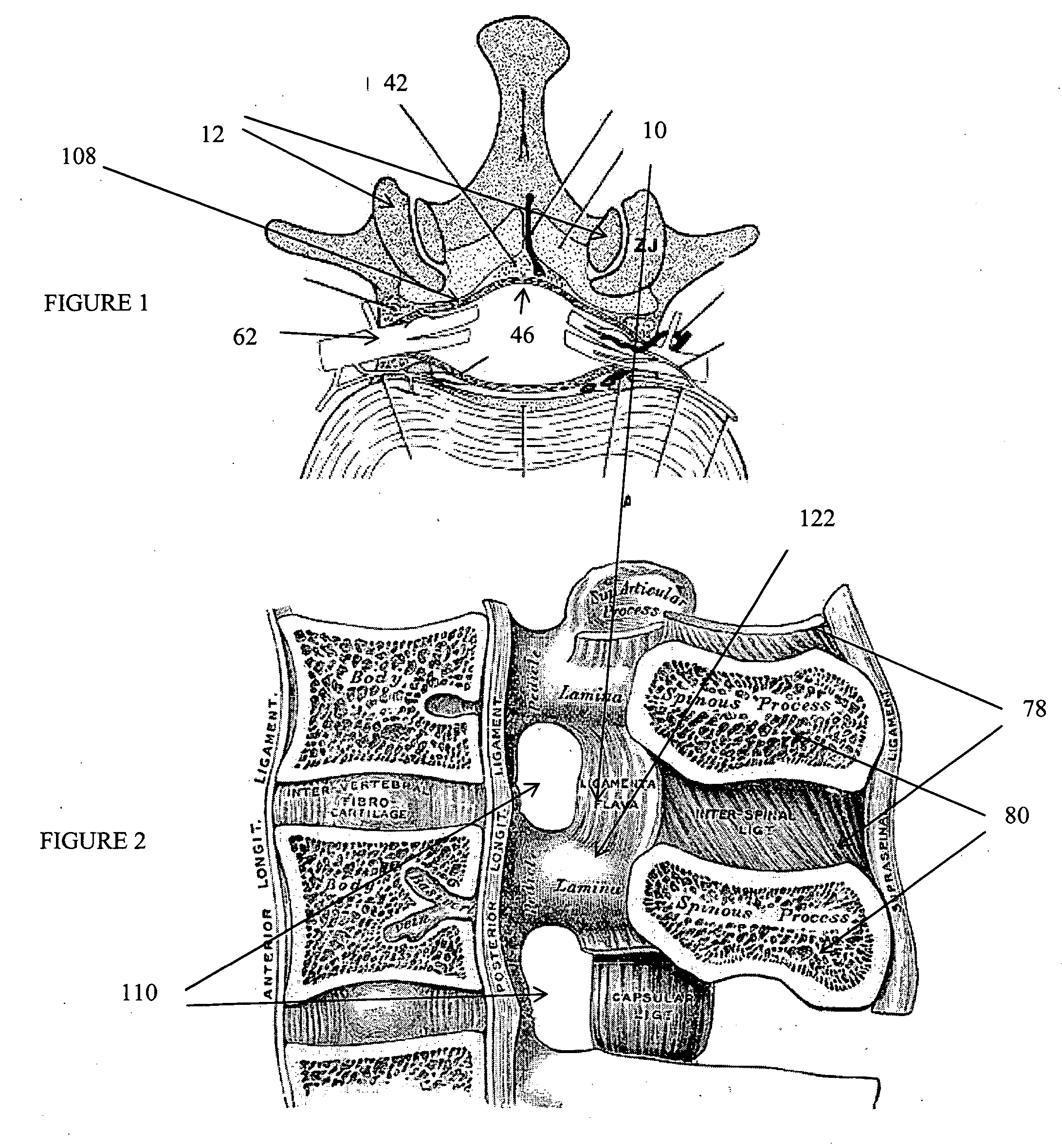
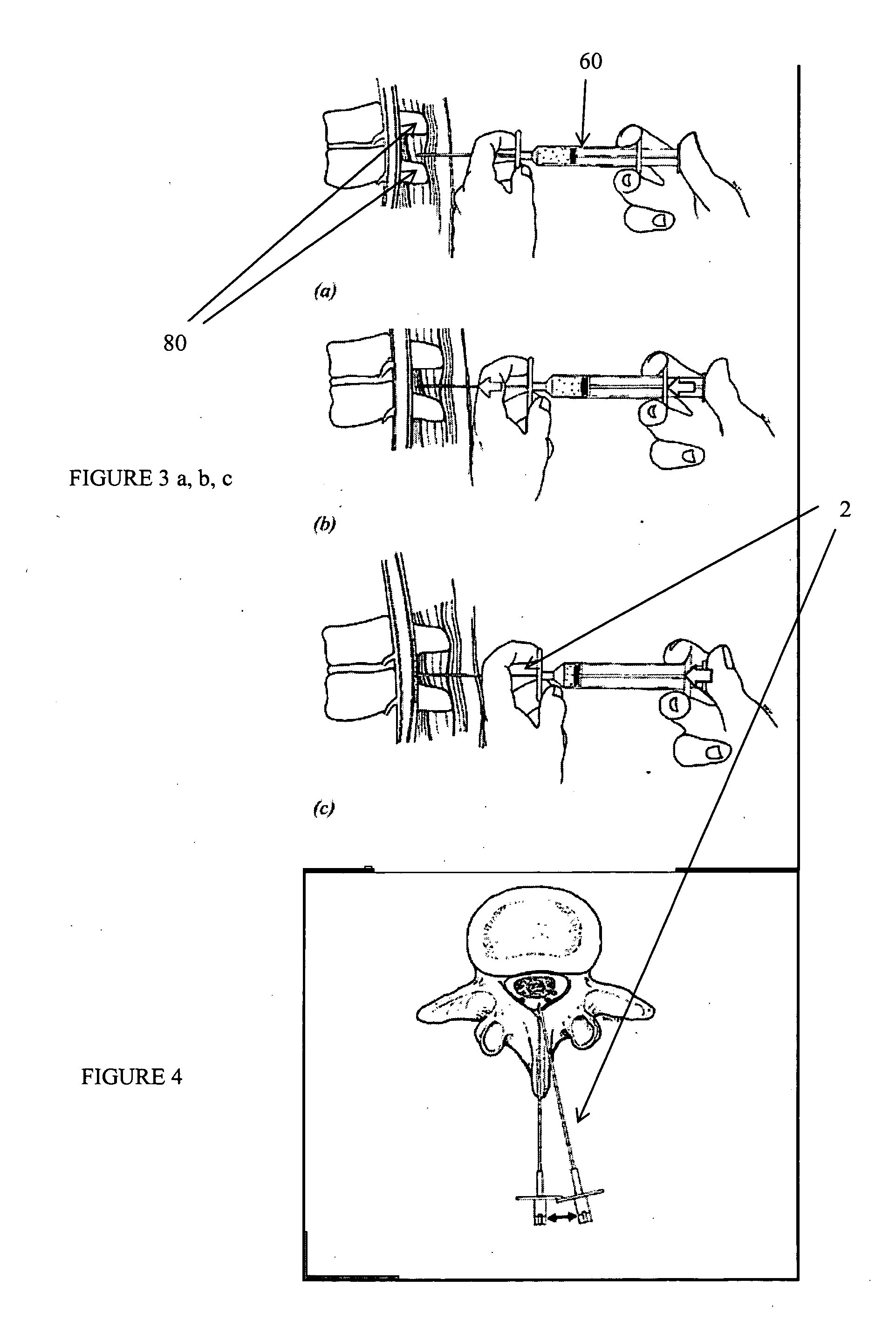
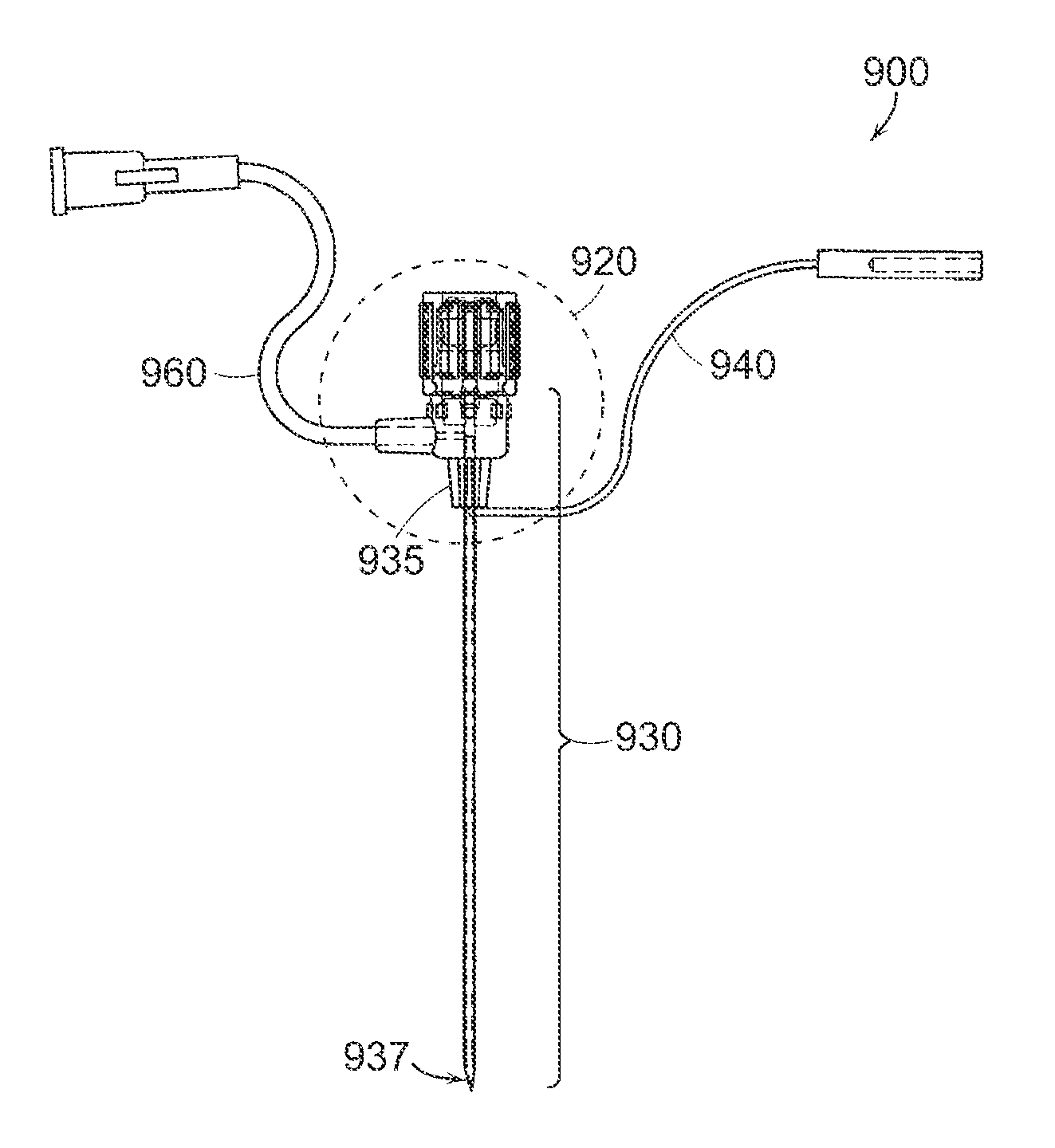
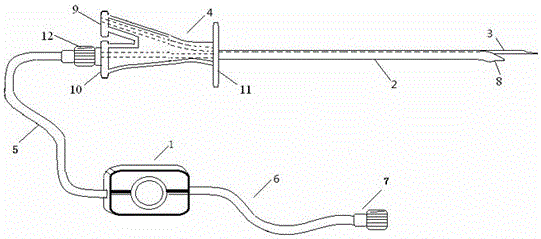
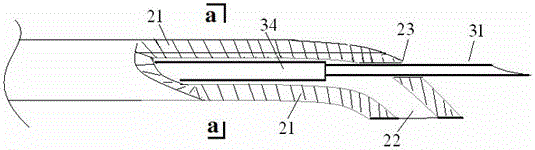
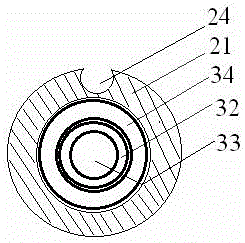
Patents
Literature
47 results about "Epidural needles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
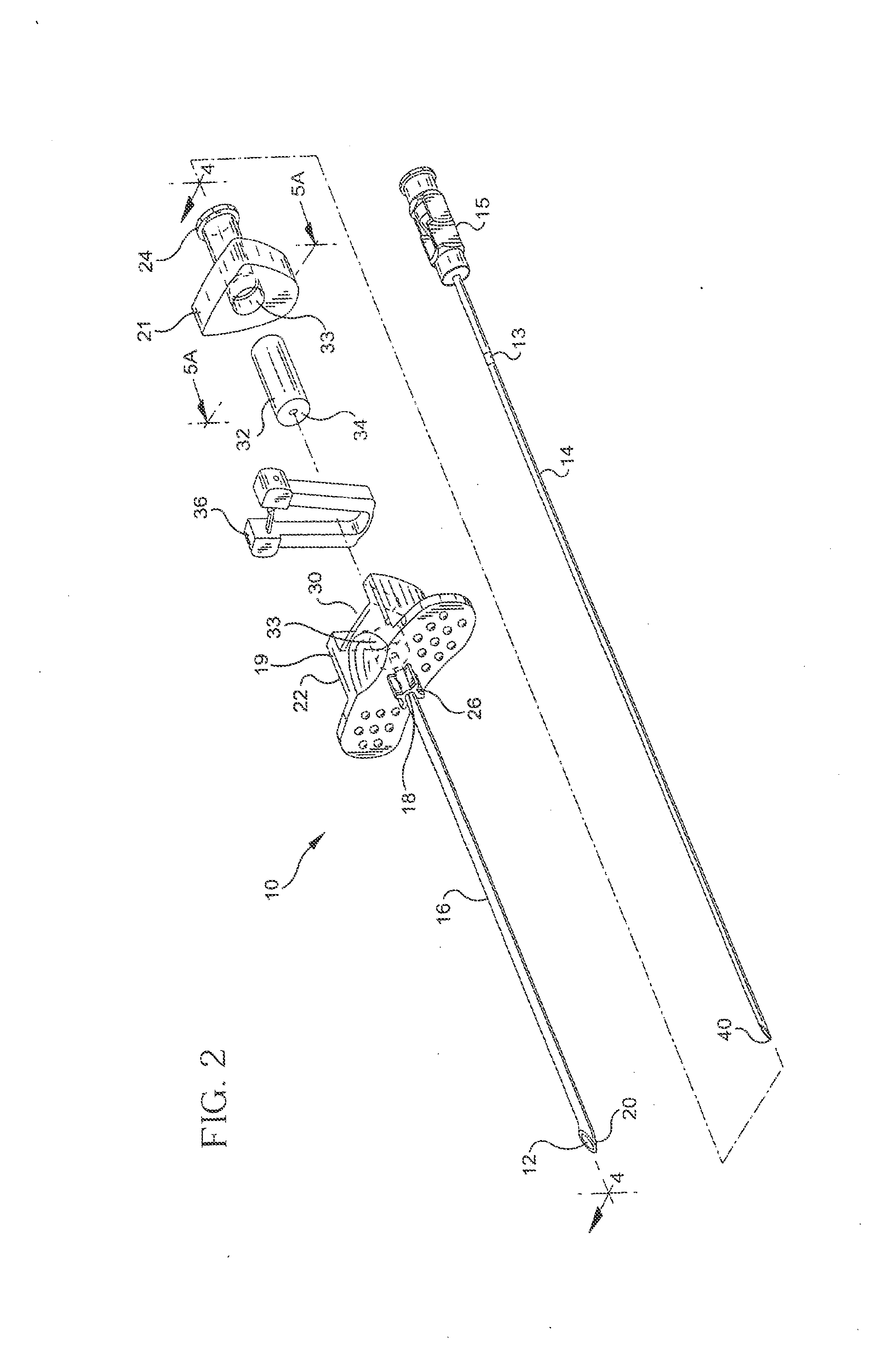
Devices and methods for selective surgical removal of tissue
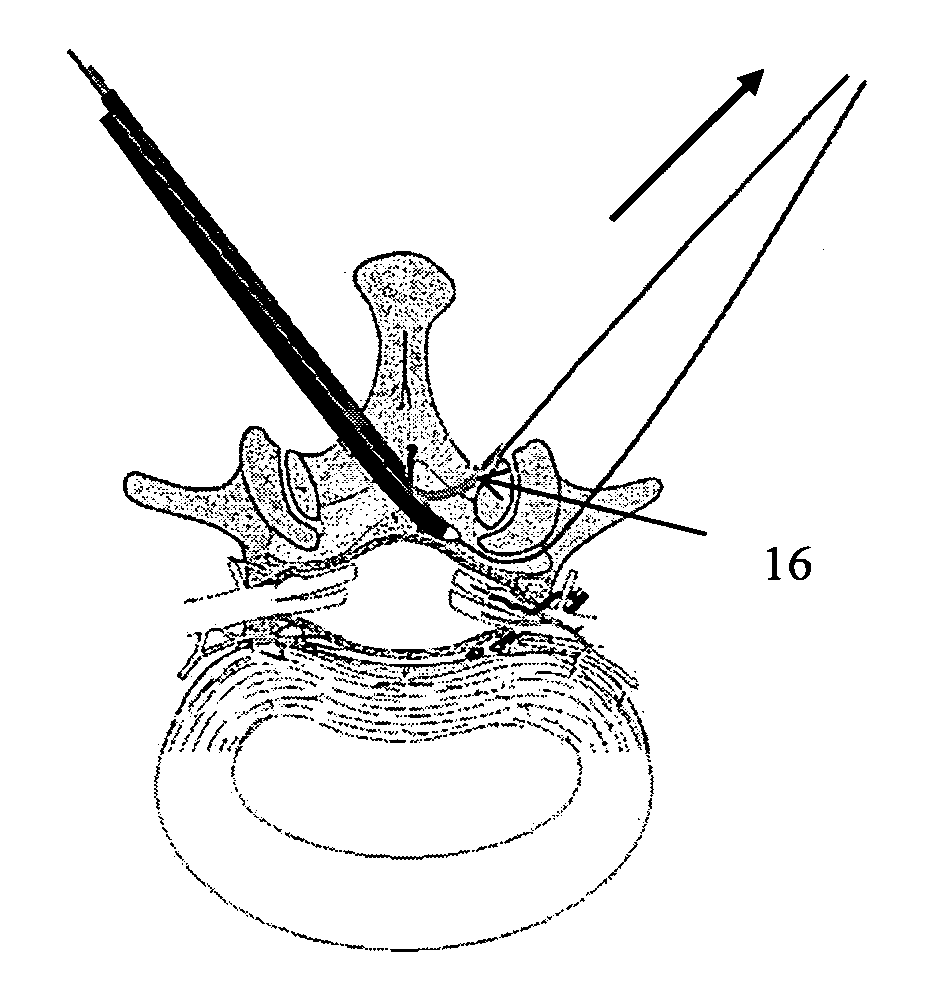
ActiveUS7738969B2Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalVascular structure
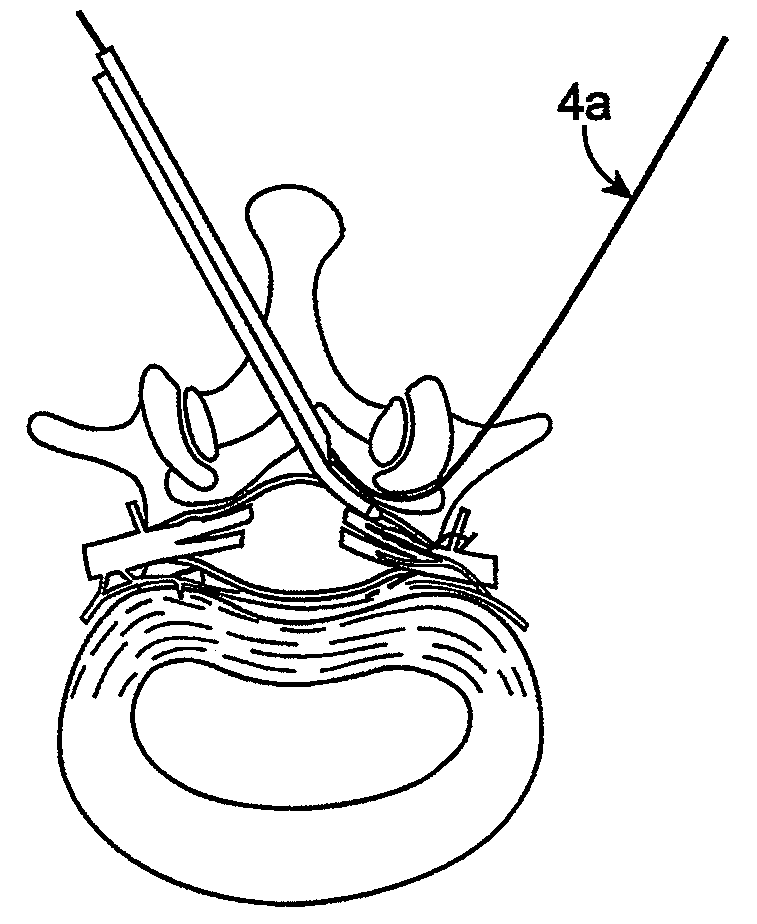
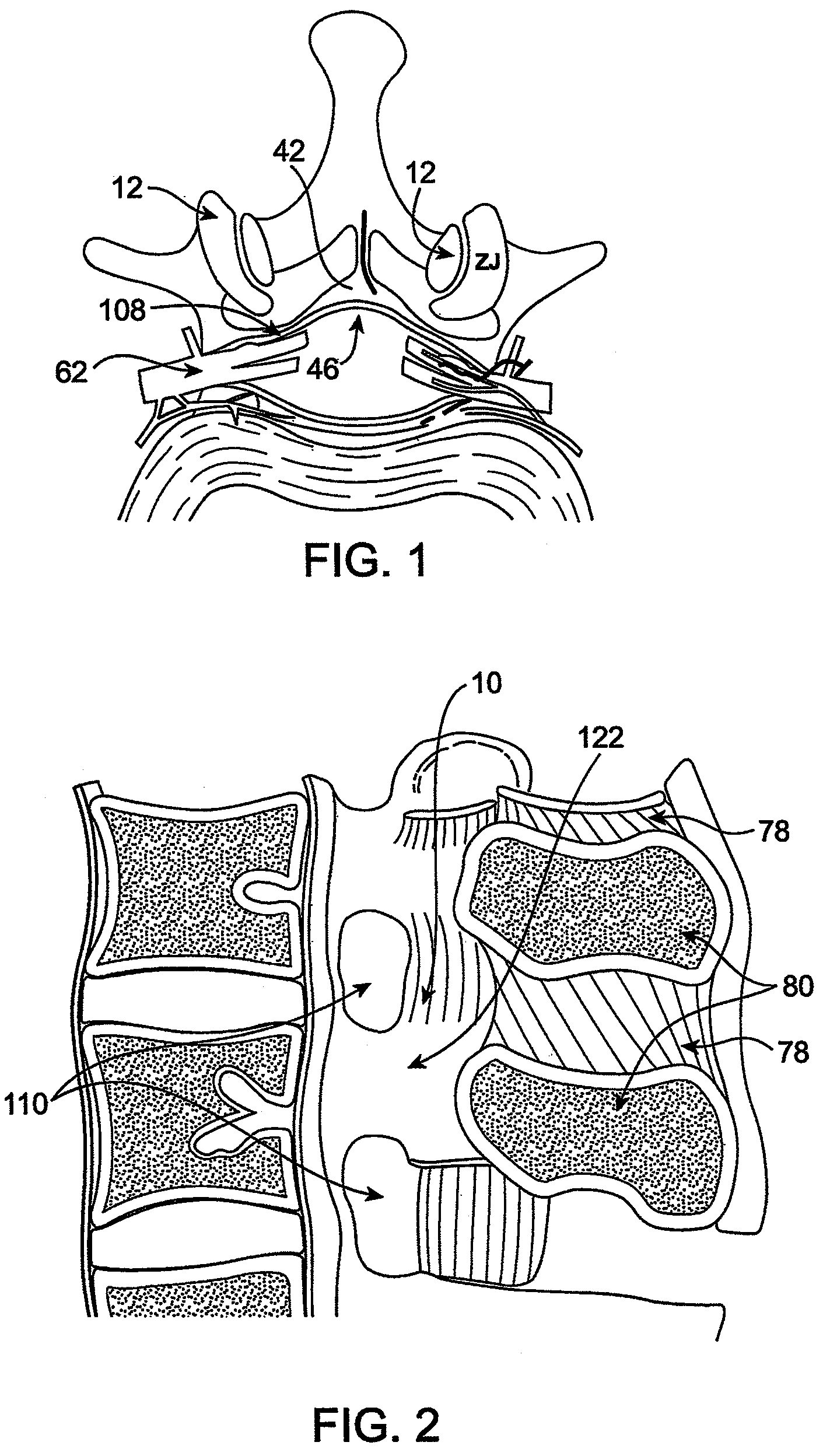
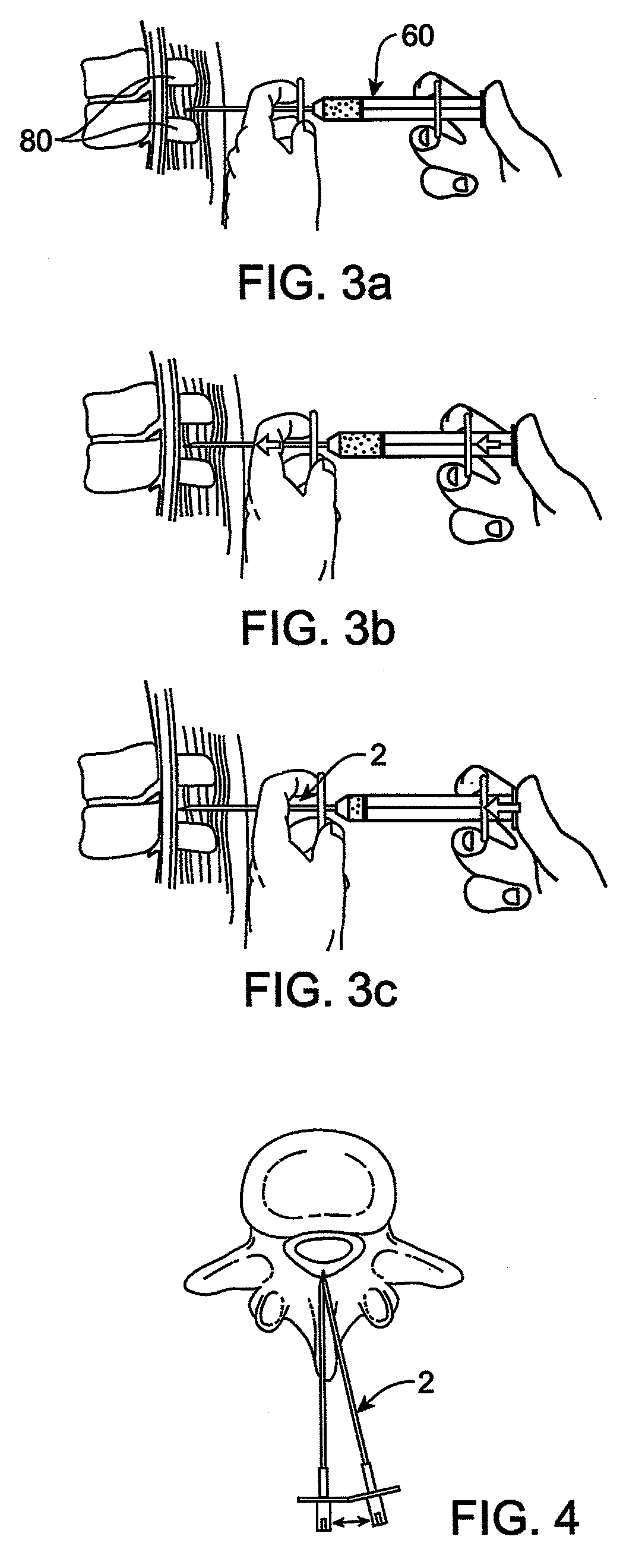
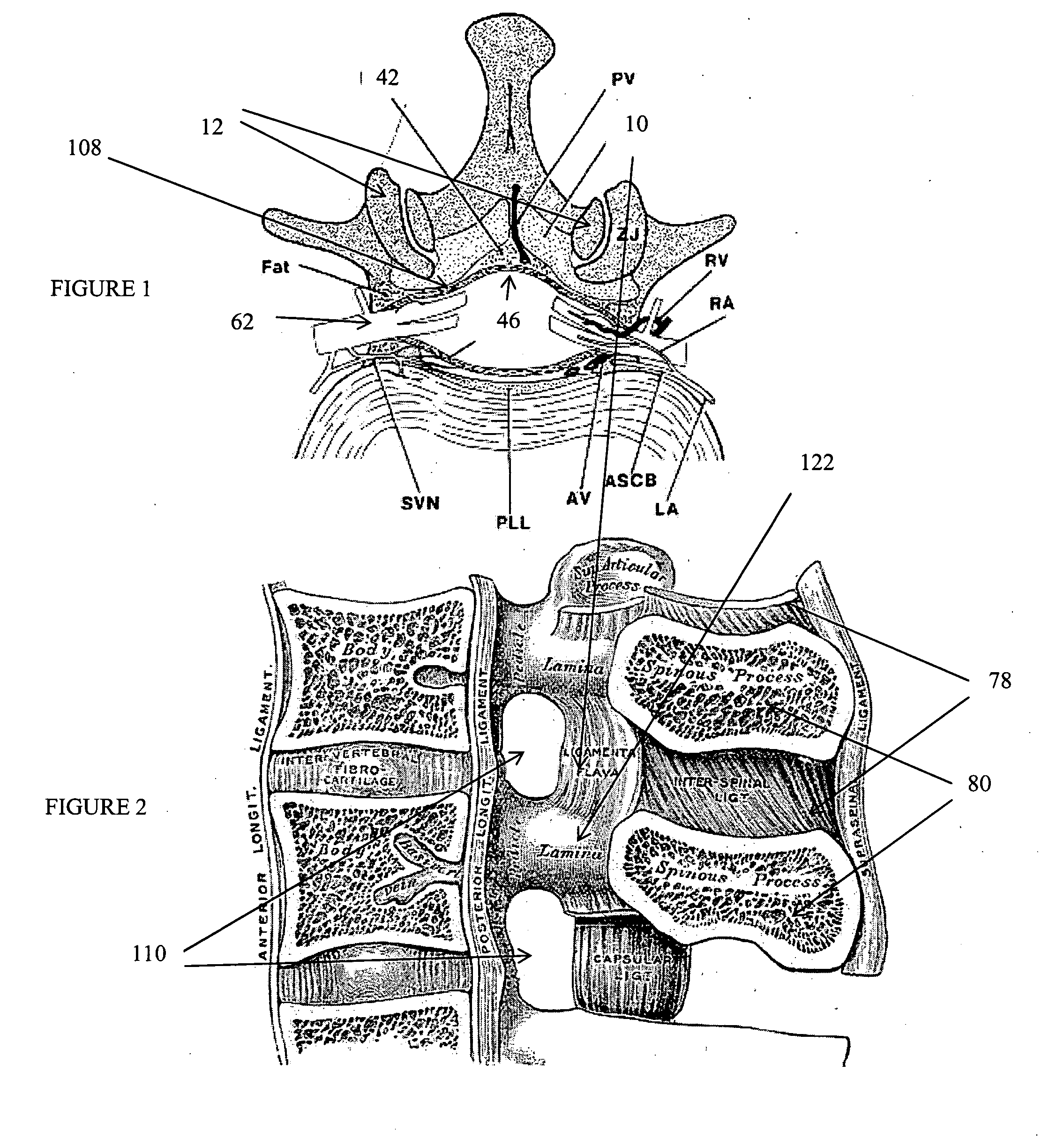
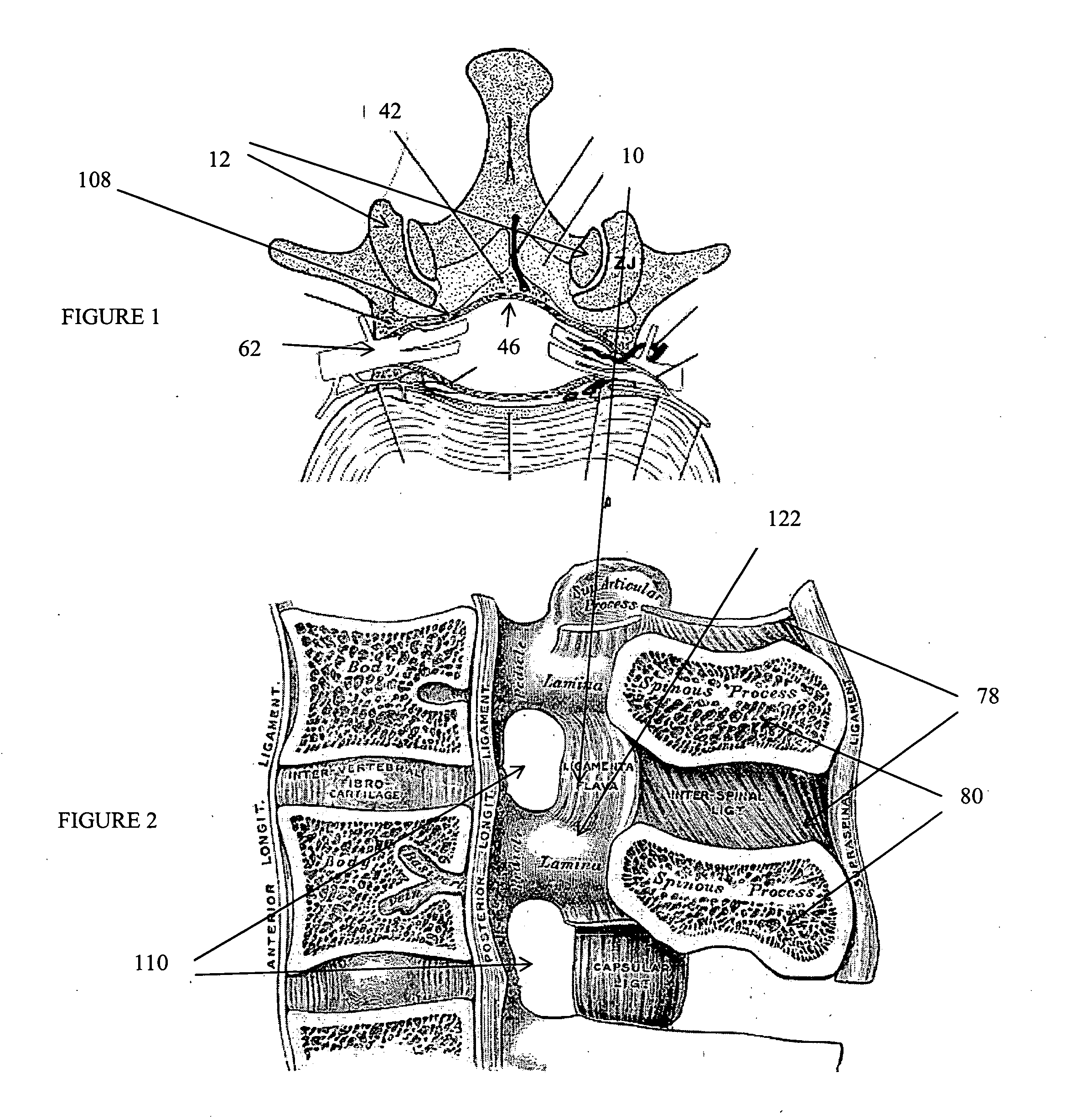
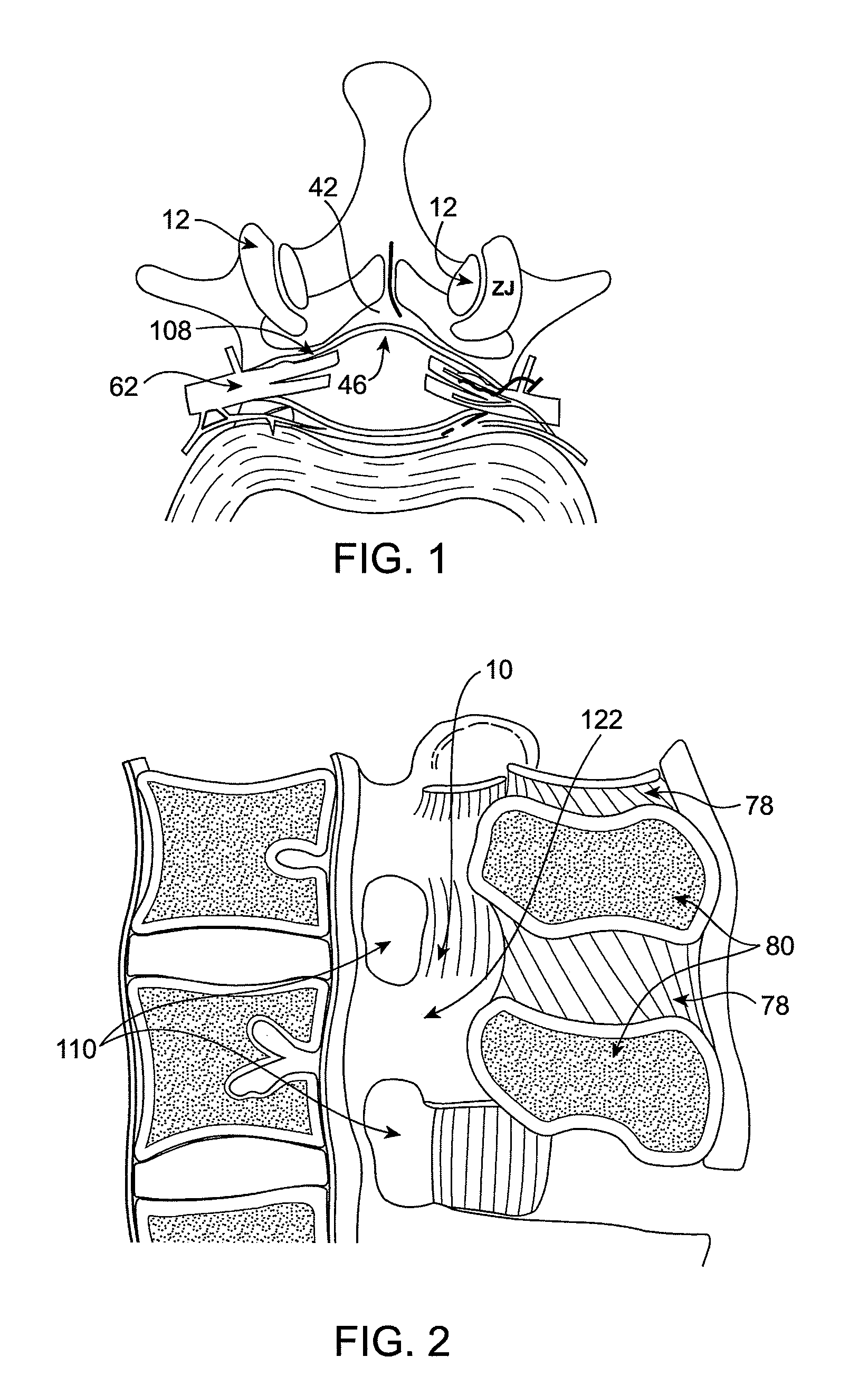
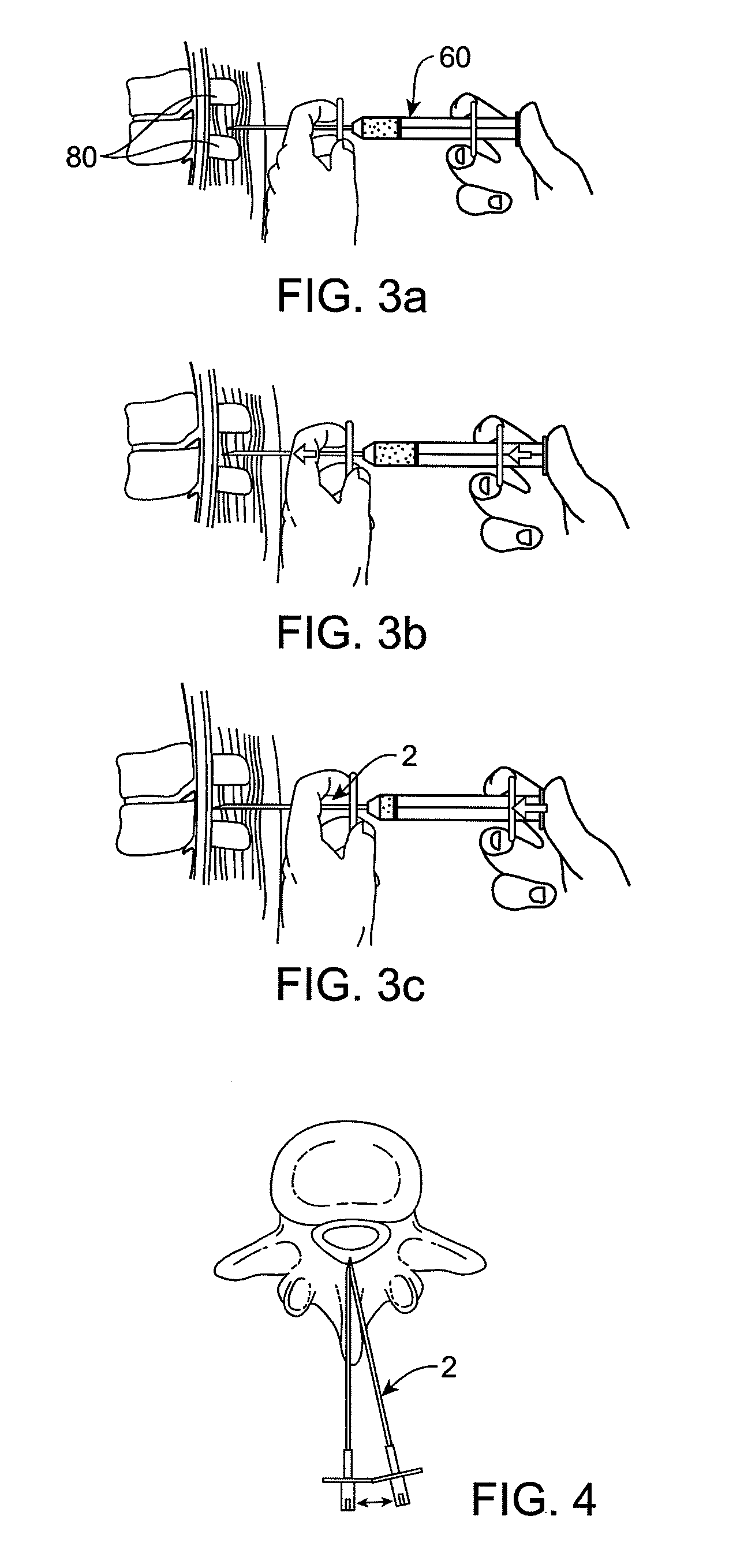
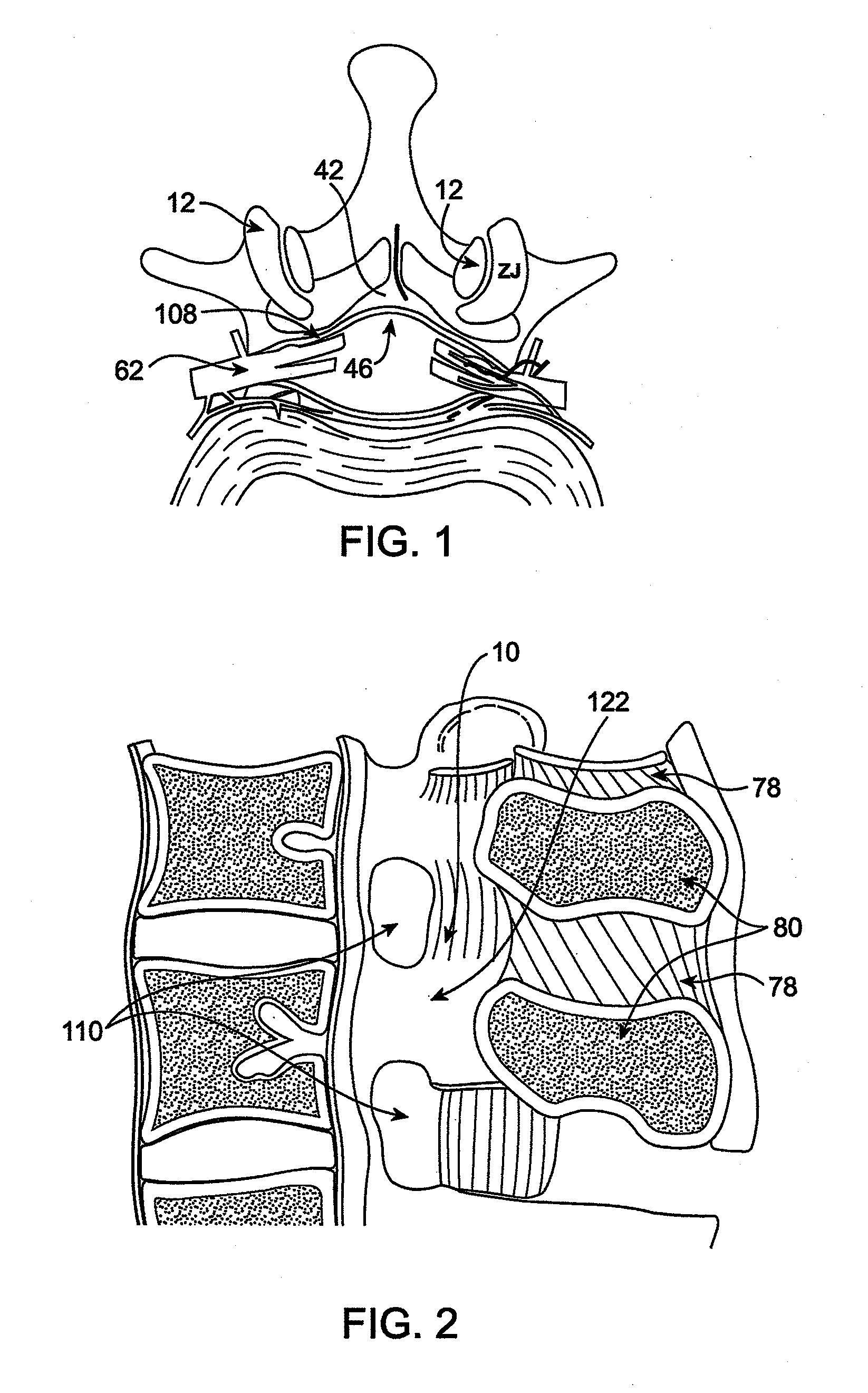
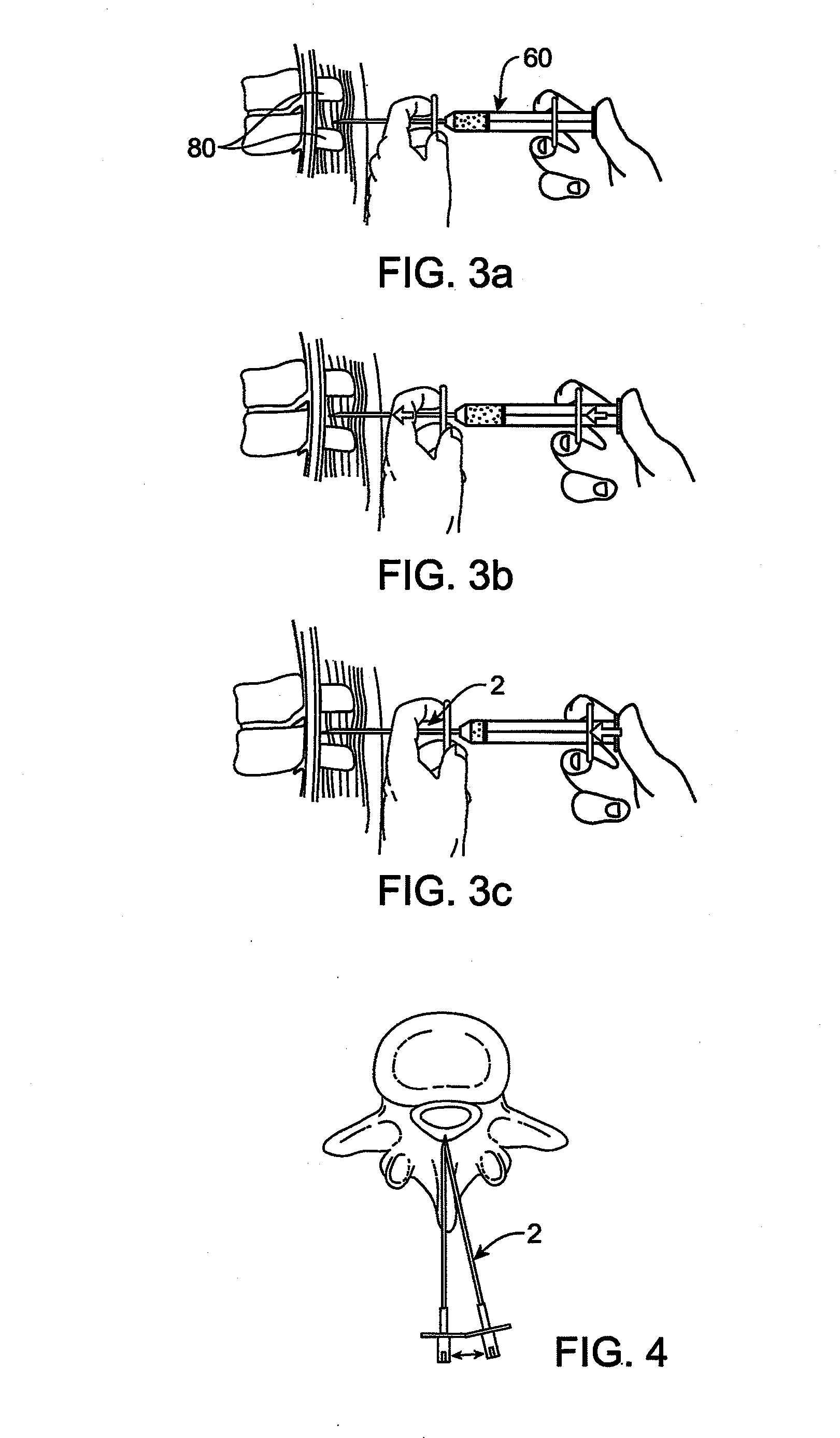
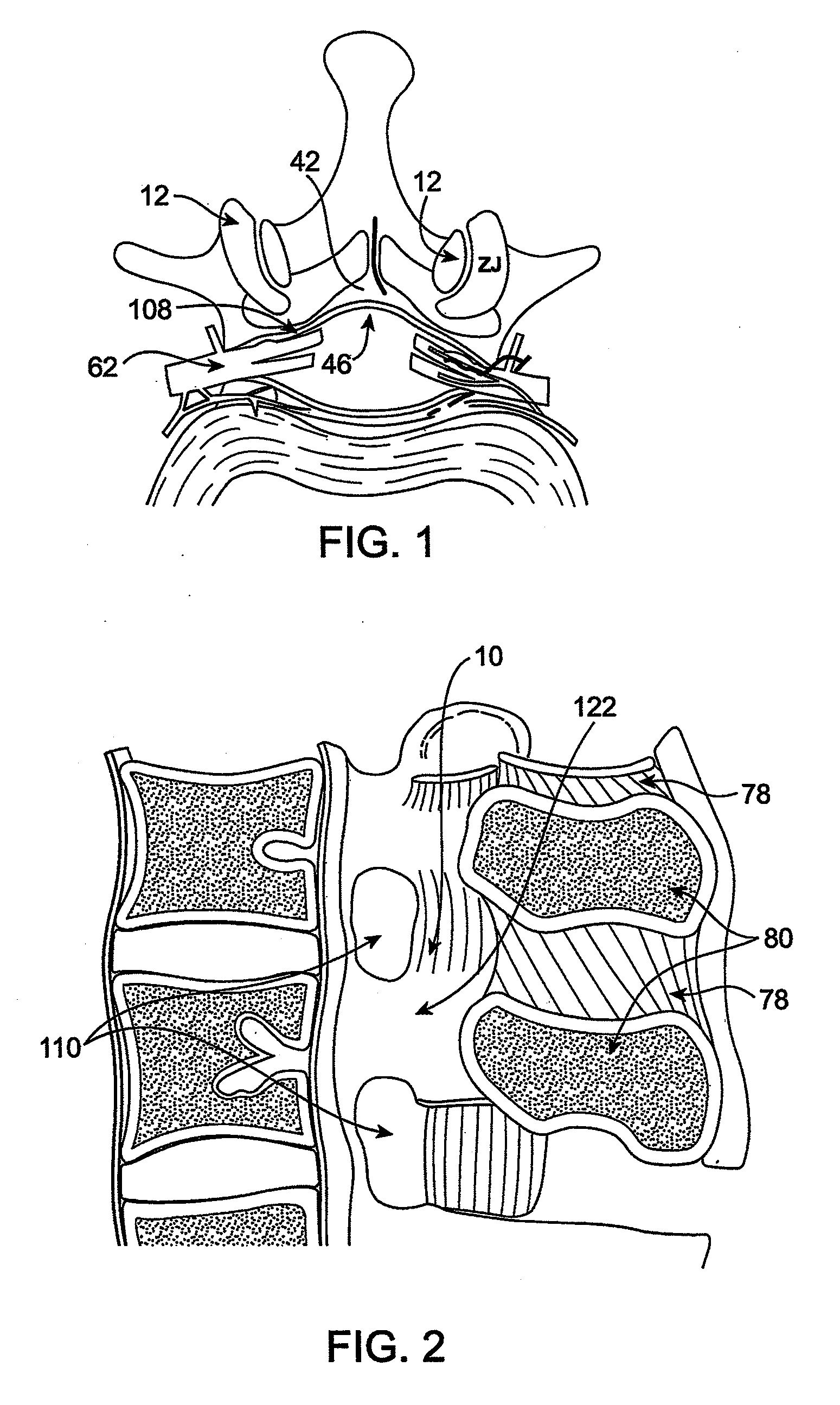
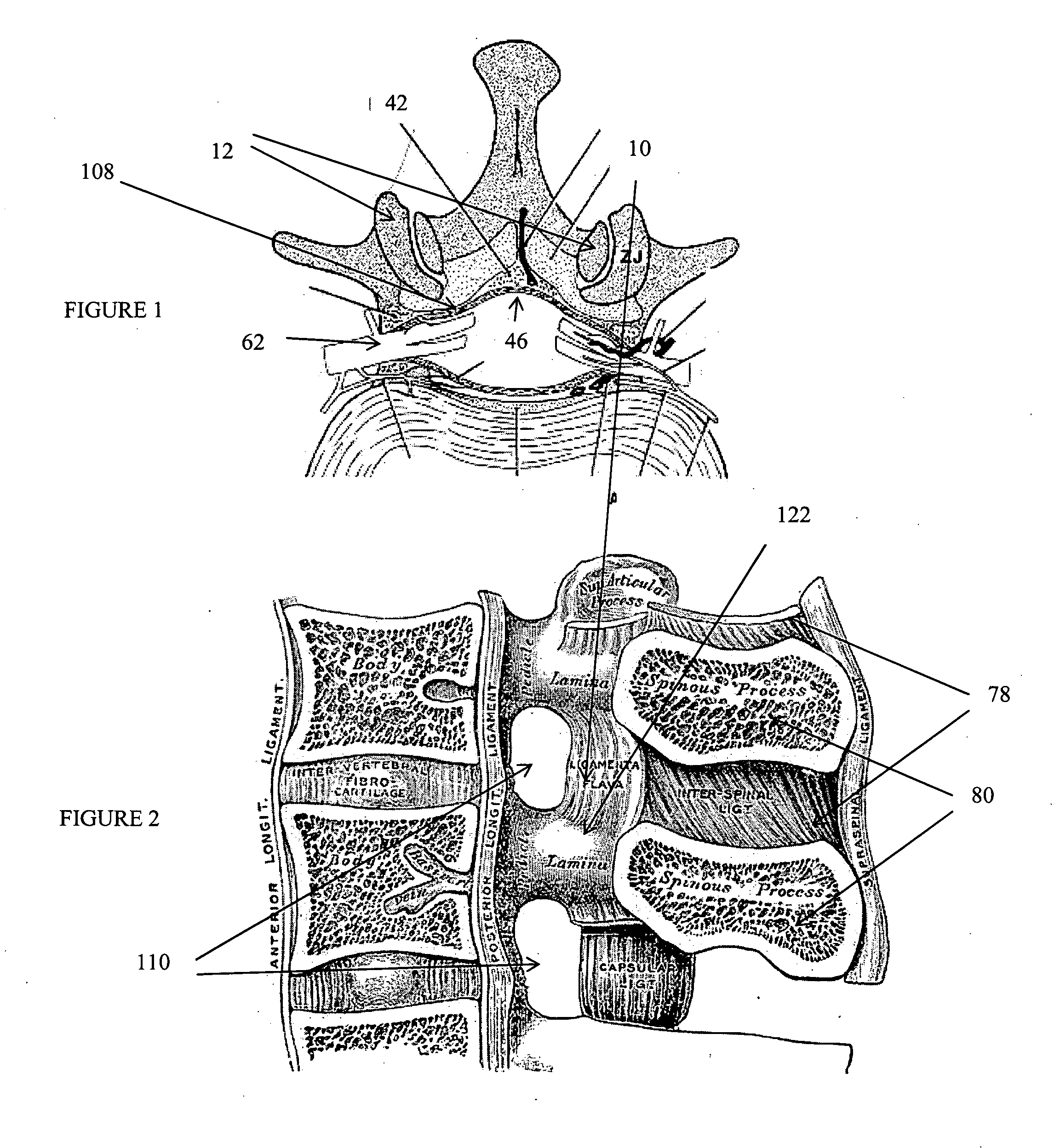
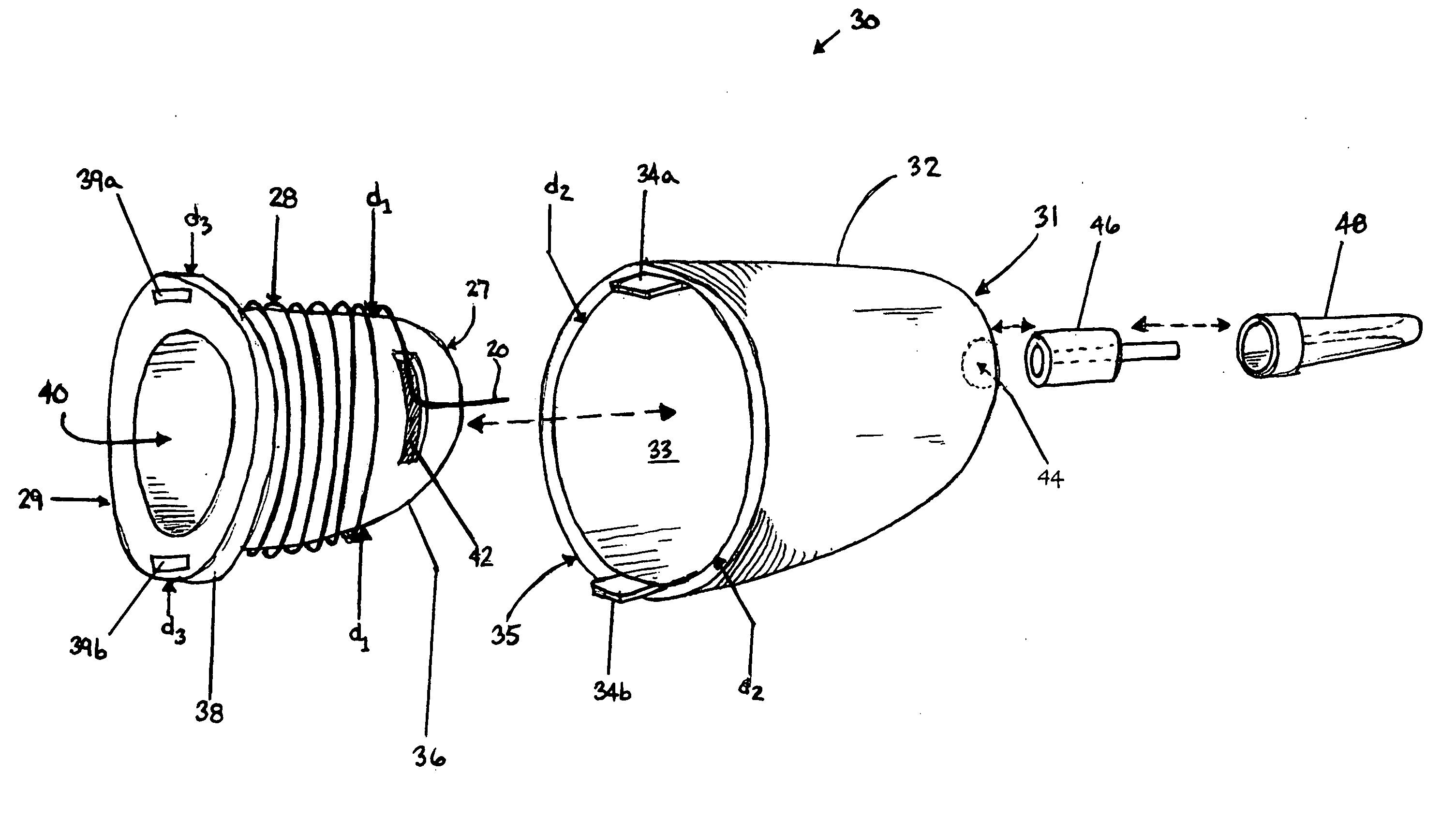
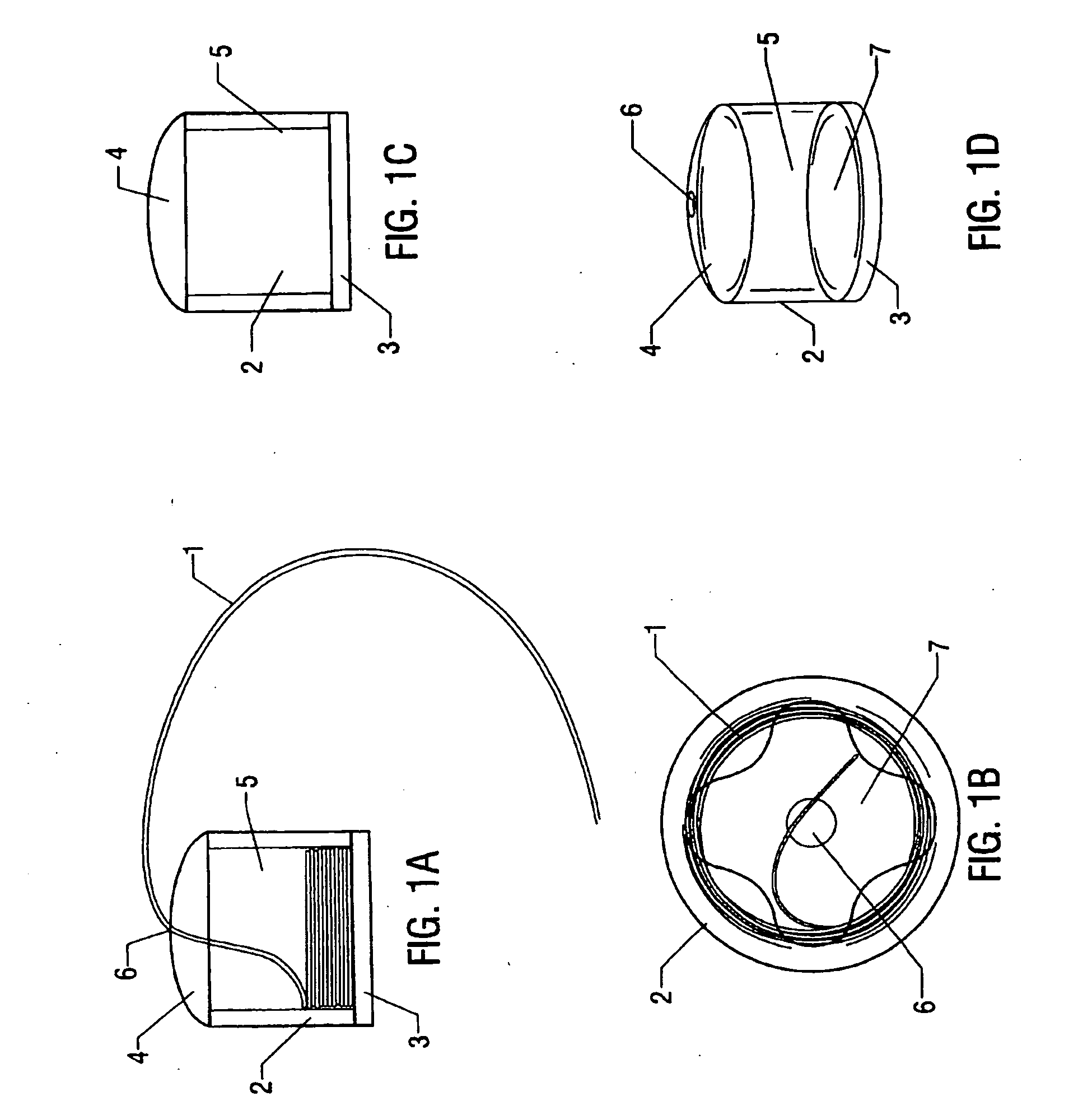
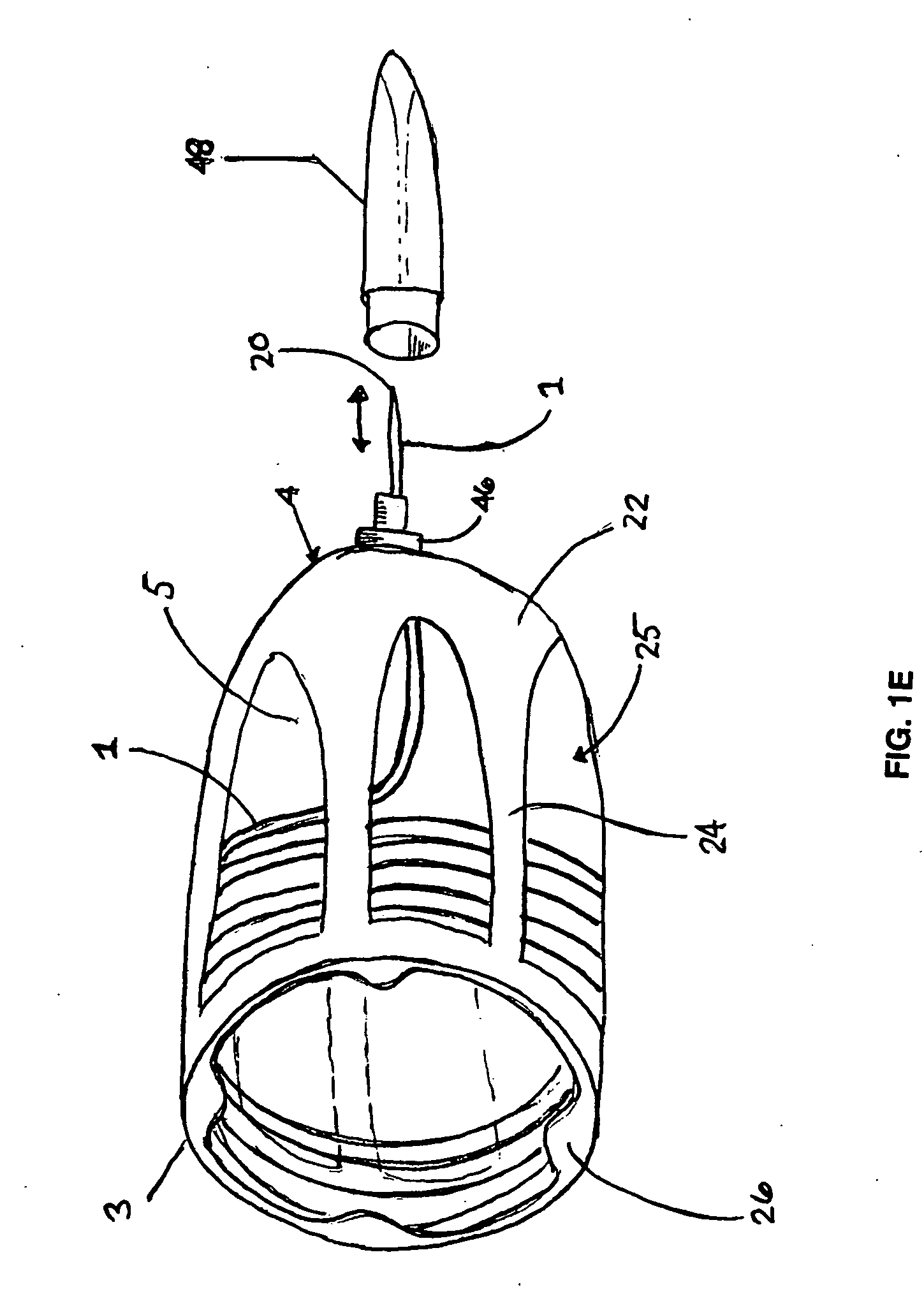
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue access
ActiveUS20060089633A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
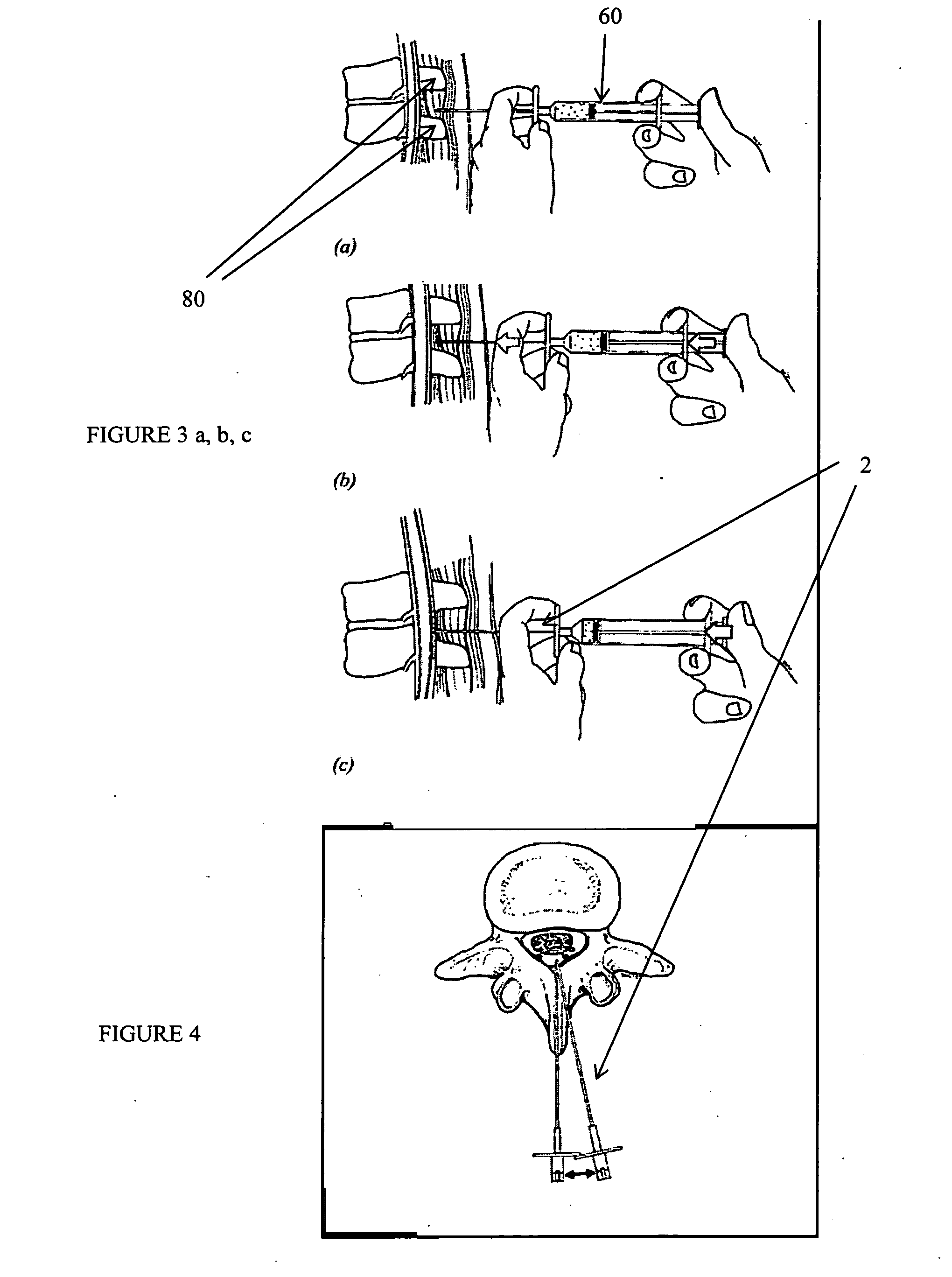
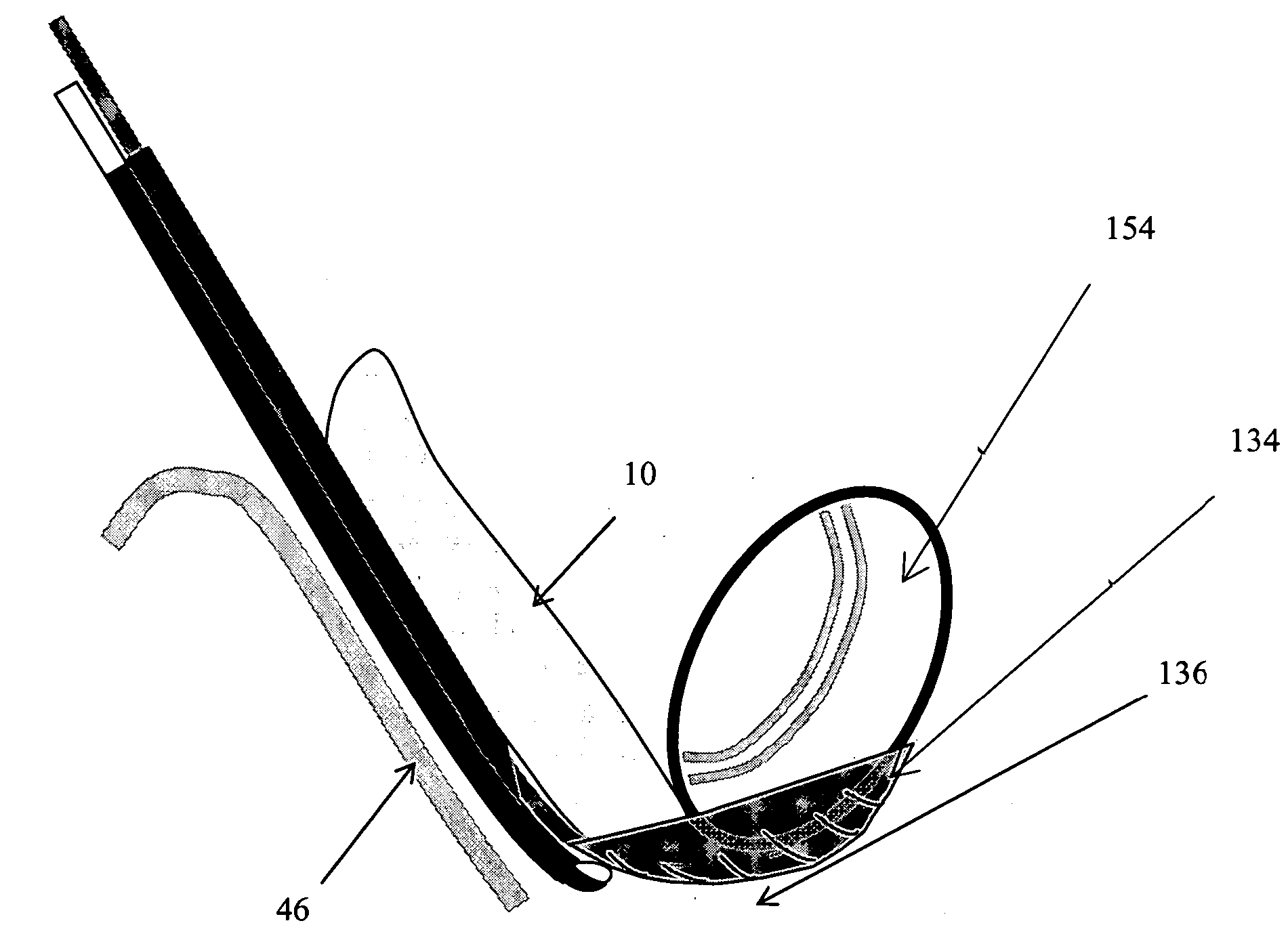
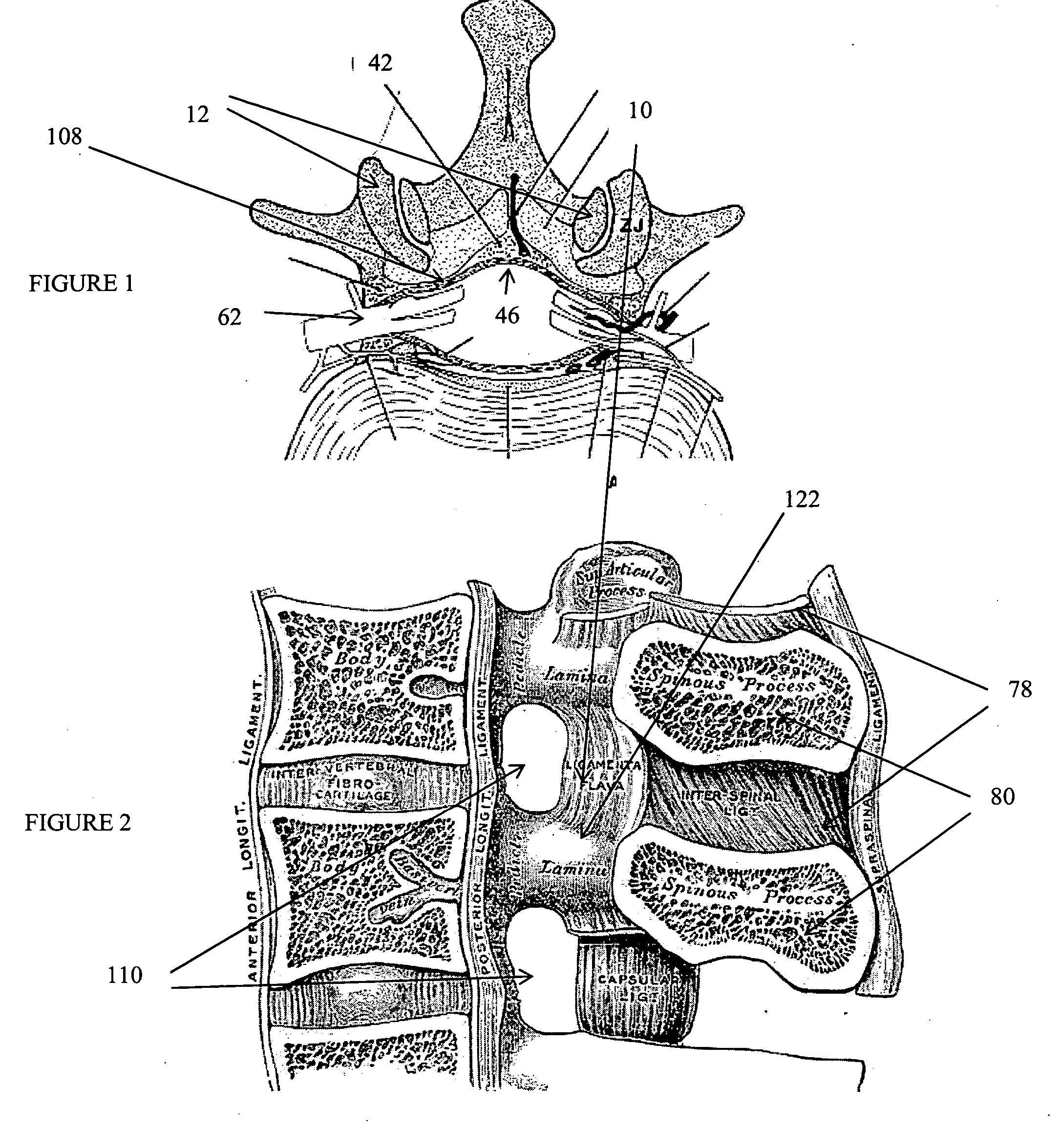
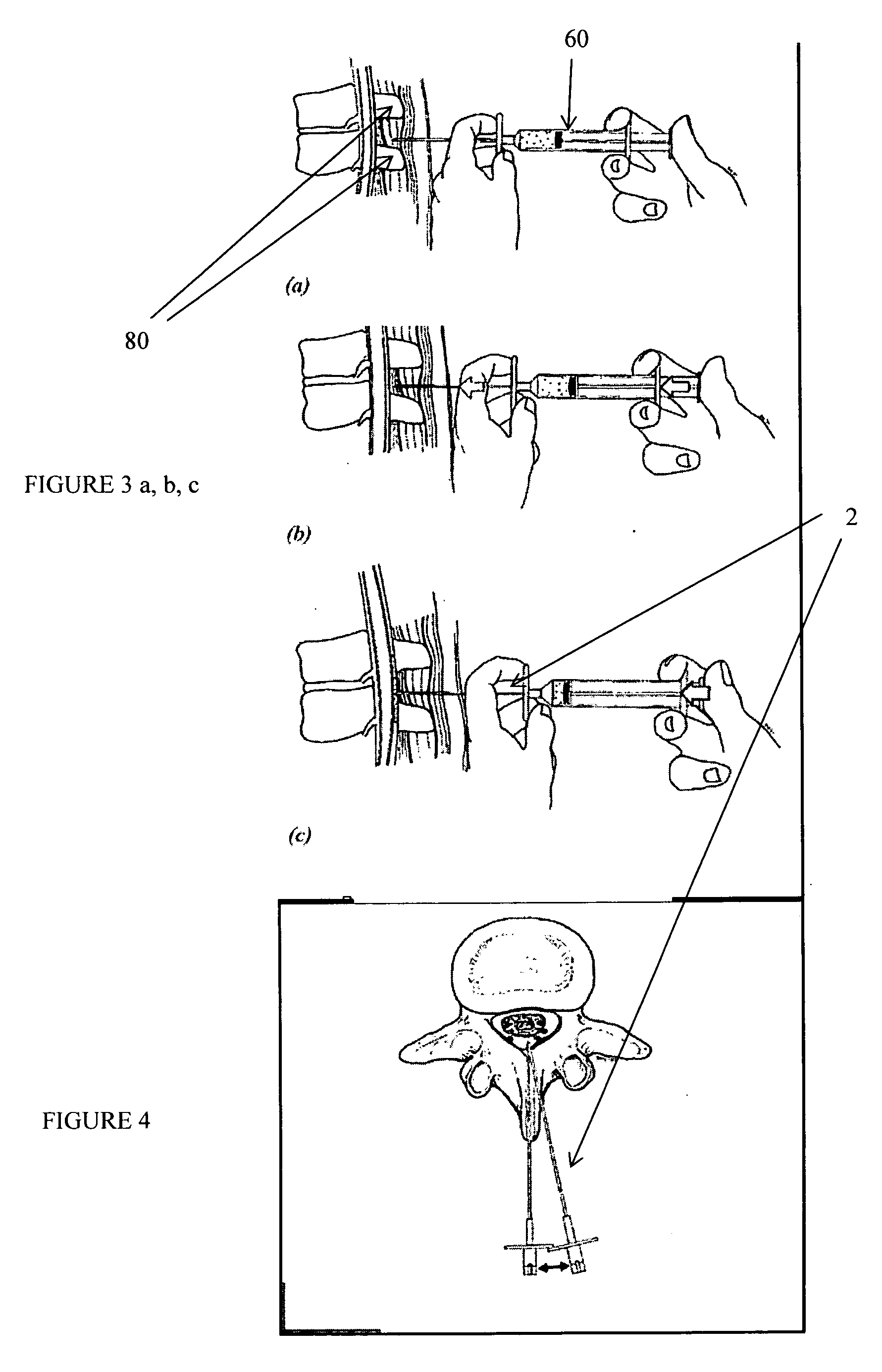
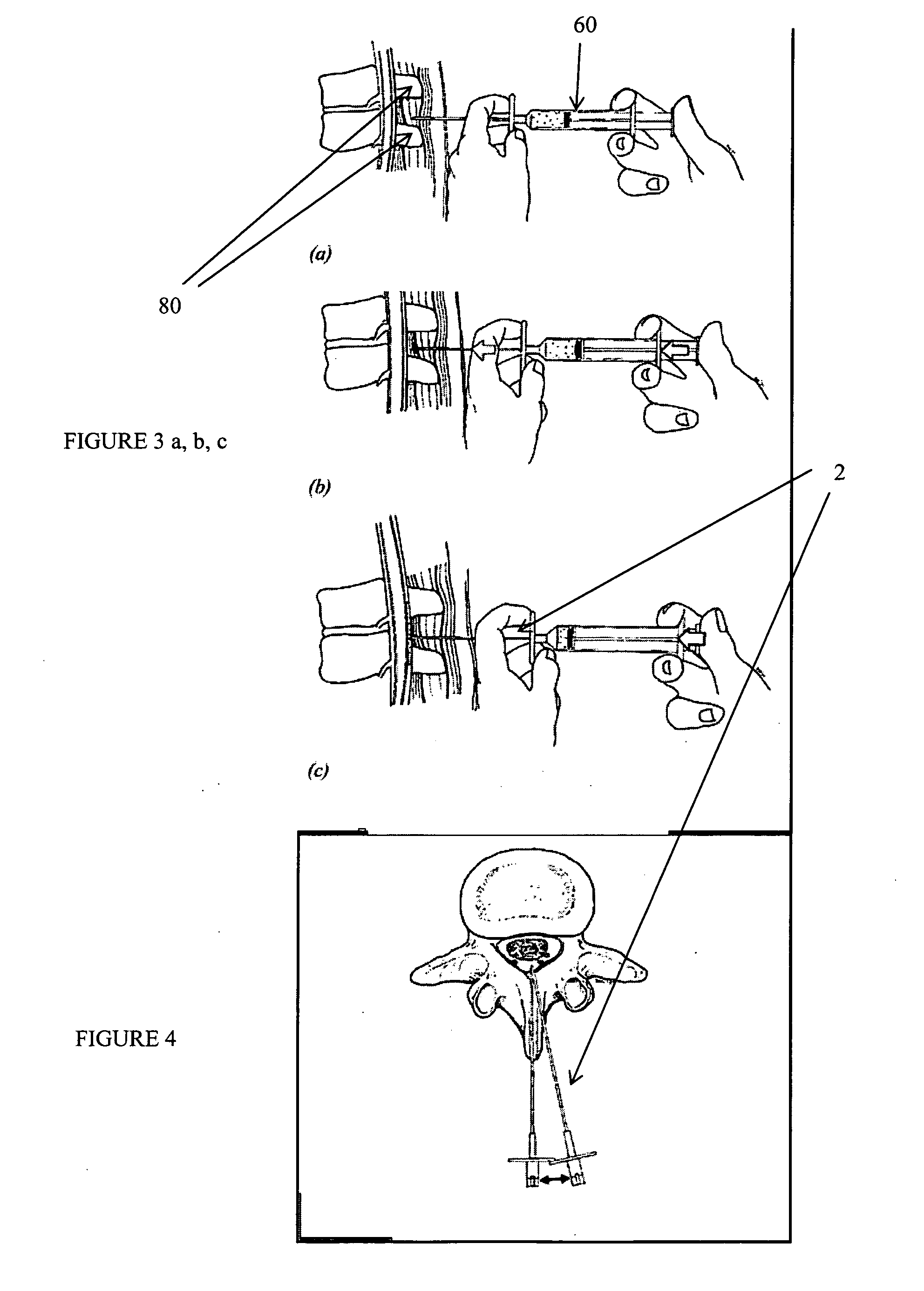
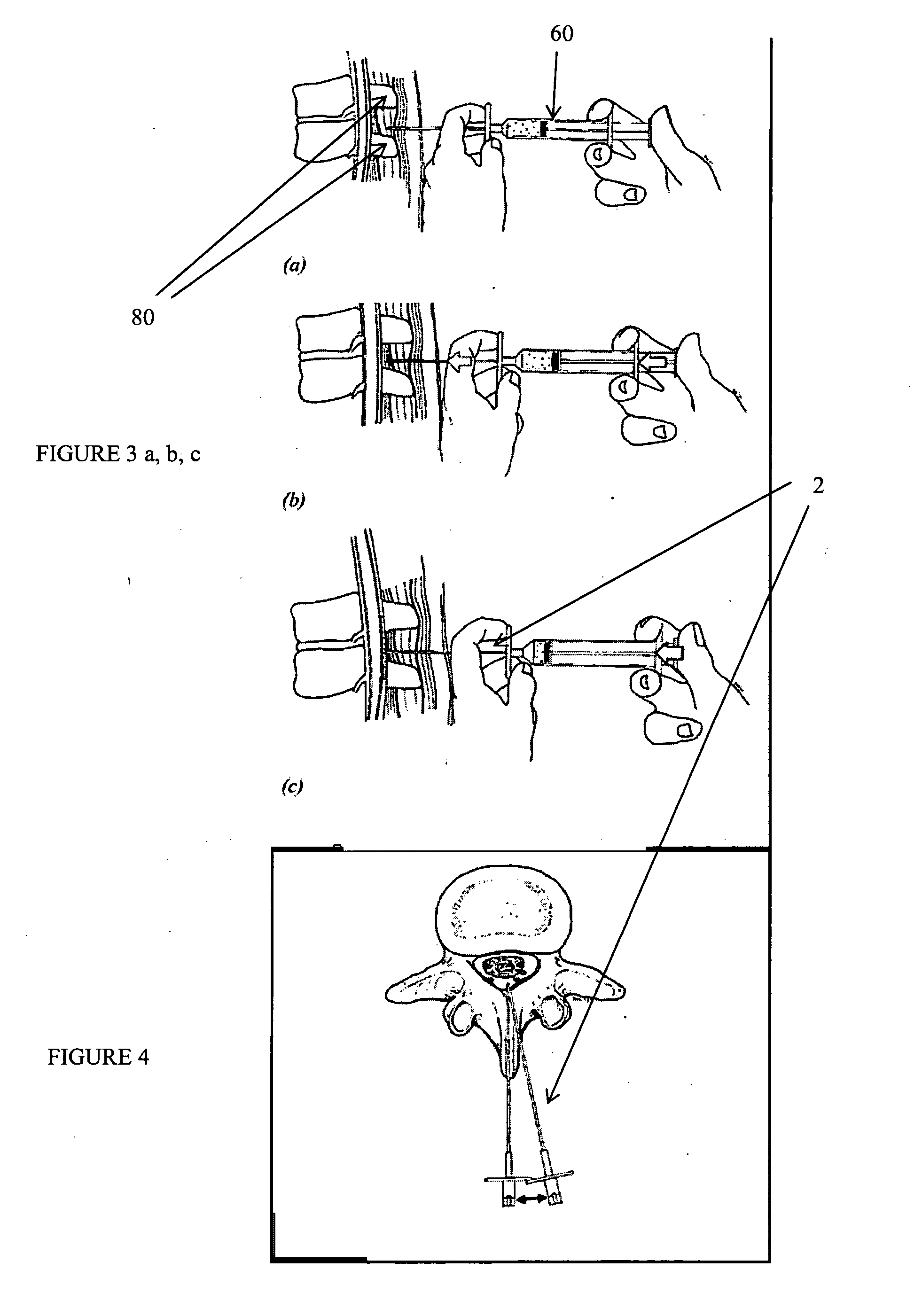
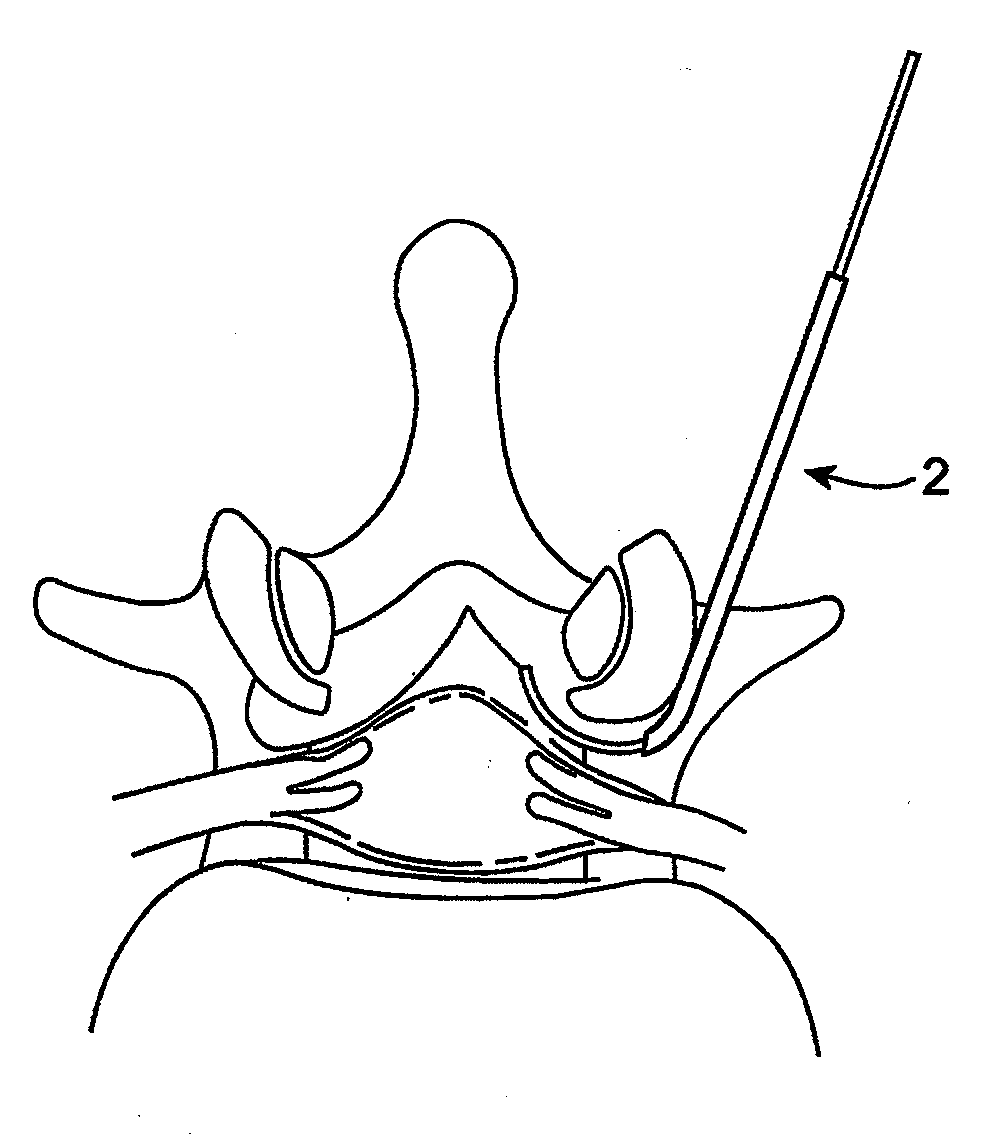
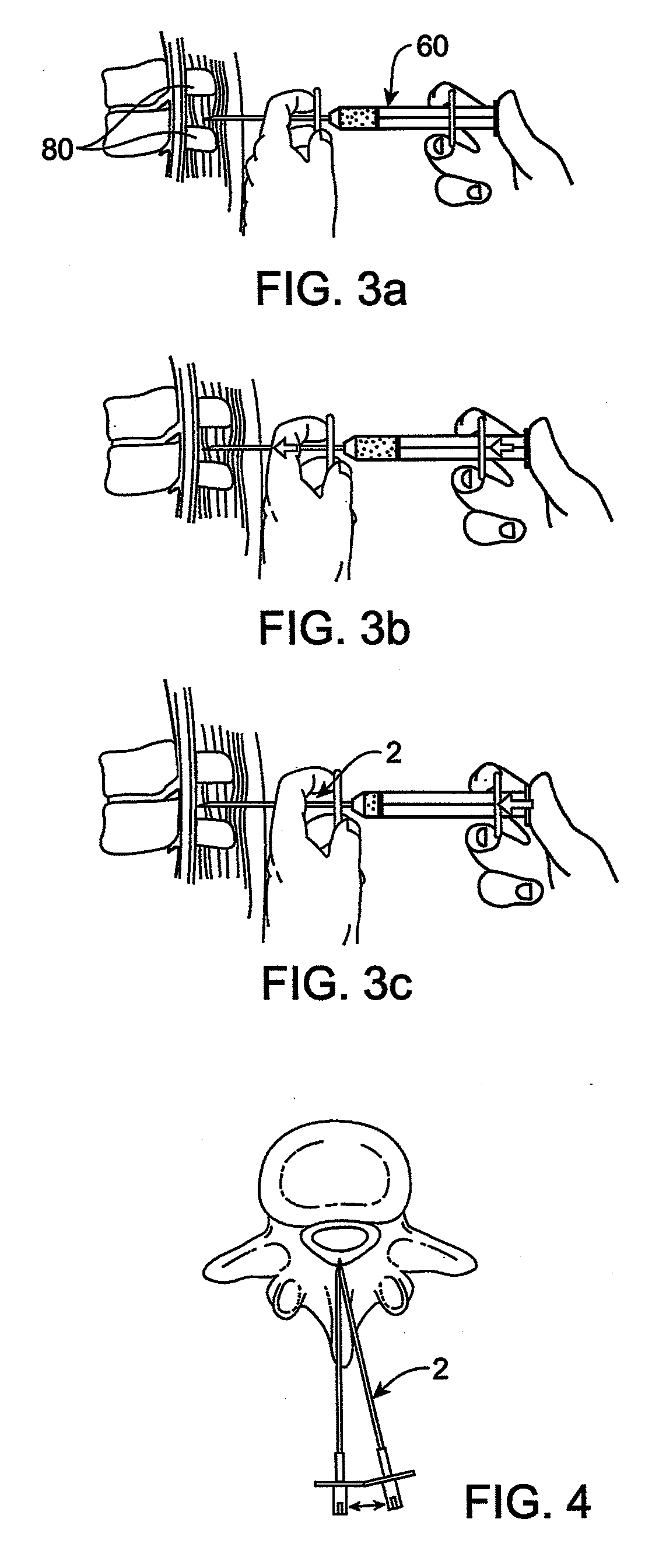
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue abrasion device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the abrasive surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for tissue access
InactiveUS20060095028A1Improve securityAvoid injuryEar treatmentCannulasAccess methodSurgical removal
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:BAXANO
Devices and methods for tissue access
InactiveUS20060122458A1Enabling symptomatic reliefApproach can be quite invasiveCannulasDiagnosticsSurgical departmentNerve stimulation
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:BAXANO
Devices and methods for tissue modification
ActiveUS20060095059A1Easy to disassembleEliminate needCannulasAnti-incontinence devicesSurgical departmentNerve stimulation
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue modification
ActiveUS20060089609A1Enabling symptomatic reliefApproach can be quite invasiveCannulasAnti-incontinence devicesSurgical removalEpidural needles
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue modification
InactiveUS20100094231A1Improve securityAvoid injuryCannulasDiagnosticsSurgical removalEpidural needles
Owner:BAXANO
Devices and methods for tissue modification
ActiveUS20090204119A1Enabling symptomatic reliefApproach can be quite invasiveSpinal electrodesCannulasSurgical removalVascular structure
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for selective surgical removal of tissue
ActiveUS20060094976A1Improve securityAvoid injuryCannulasAnti-incontinence devicesTissue remodelingLateral recess
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for selective surgical removal of tissue
ActiveUS20090125036A1Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalVascular structure
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
Devices and methods for tissue access
InactiveUS20060100651A1Improve securityAvoid injuryEar treatmentCannulasTissue remodelingSpinal column
Methods and apparatus are provided for selective surgical removal of tissue, e.g., for enlargement of diseased spinal structures, such as impinged lateral recesses and pathologically narrowed neural foramen. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. Once the sharp tip of the needle is in the epidural space, it is converted to a blunt tipped instrument for further safe advancement. A specially designed epidural catheter that is used to cover the previously sharp needle tip may also contain a fiberoptic cable. Further embodiments of the current invention include a double barreled epidural needle or other means for placement of a working channel for the placement of tools within the epidural space, beside the epidural instrument. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. In one variation, a tissue removal device is provided including a thin belt or ribbon with an abrasive cutting surface. The device may be placed through the neural foramina of the spine and around the anterior border of a facet joint. Once properly positioned, a medical practitioner may enlarge the lateral recess and neural foramina via frictional abrasion, i.e., by sliding the tissue removal surface of the ribbon across impinging tissues. A nerve stimulator optionally may be provided to reduce a risk of inadvertent neural abrasion. Additionally, safe epidural placement of the working barrier and epidural tissue modification tools may be further improved with the use of electrical nerve stimulation capabilities within the invention that, when combined with neural stimulation monitors, provide neural localization capabilities to the surgeon. The device optionally may be placed within a protective sheath that exposes the abrasive surface of the ribbon only in the area where tissue removal is desired. Furthermore, an endoscope may be incorporated into the device in order to monitor safe tissue removal. Finally, tissue remodeling within the epidural space may be ensured through the placement of compression dressings against remodeled tissue surfaces, or through the placement of tissue retention straps, belts or cables that are wrapped around and pull under tension aspects of the impinging soft tissue and bone in the posterior spinal canal.
Owner:SPINAL ELEMENTS INC +1
Devices and methods for tissue modification
ActiveUS20060089640A1Improve securityAvoid injuryCannulasAnti-incontinence devicesSurgical removalEpidural needles
Methods and apparatus are provided for selective surgical removal of tissue. In one variation, tissue may be ablated, resected, removed, or otherwise remodeled by standard small endoscopic tools delivered into the epidural space through an epidural needle. The sharp tip of the needle in the epidural space, can be converted to a blunt tipped instrument for further safe advancement. The current invention includes specific tools that enable safe tissue modification in the epidural space, including a barrier that separates the area where tissue modification will take place from adjacent vulnerable neural and vascular structures. A nerve stimulator may be provided to reduce a risk of inadvertent neural abrasion.
Owner:MIS IP HLDG LLC +1
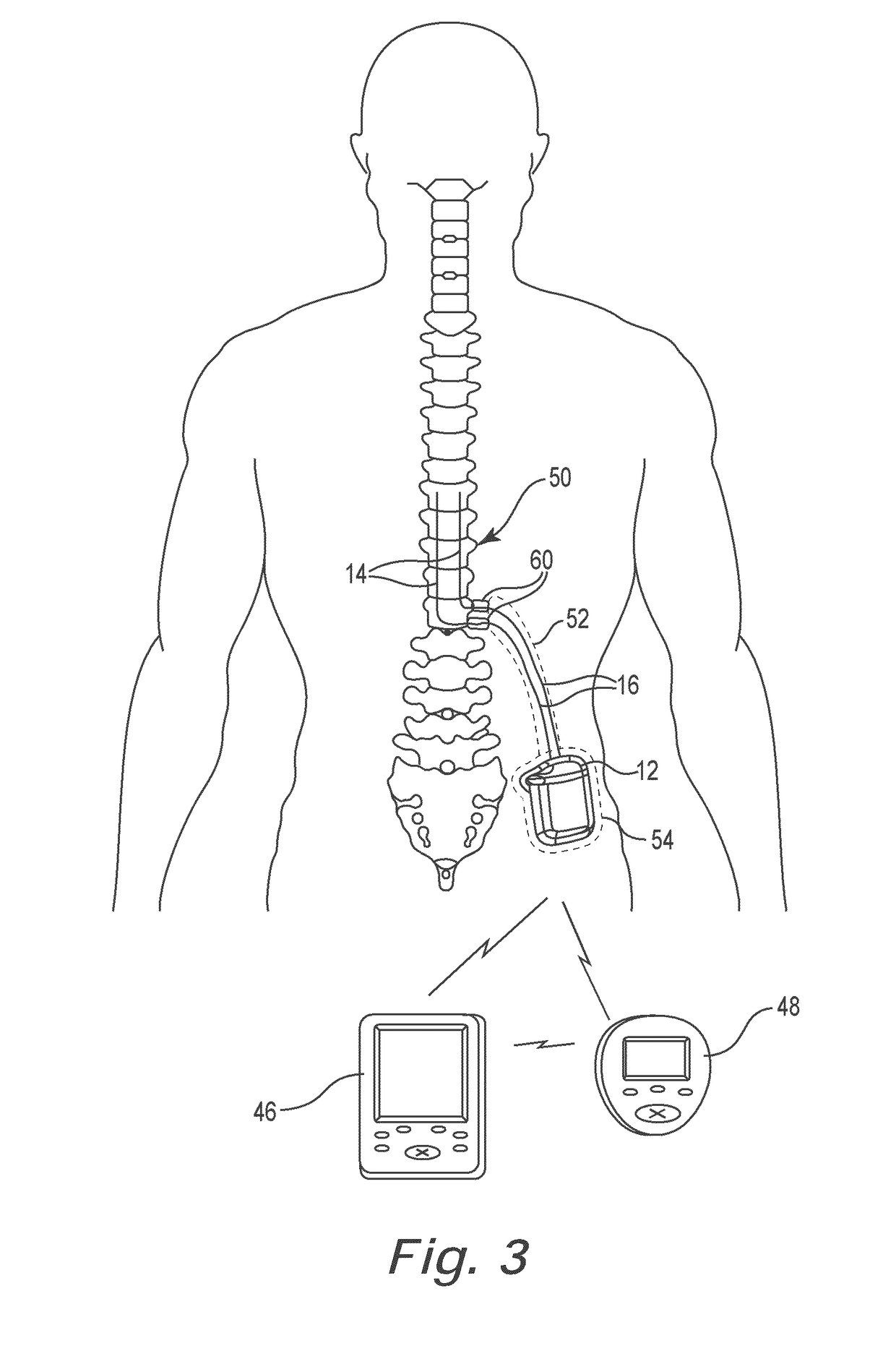
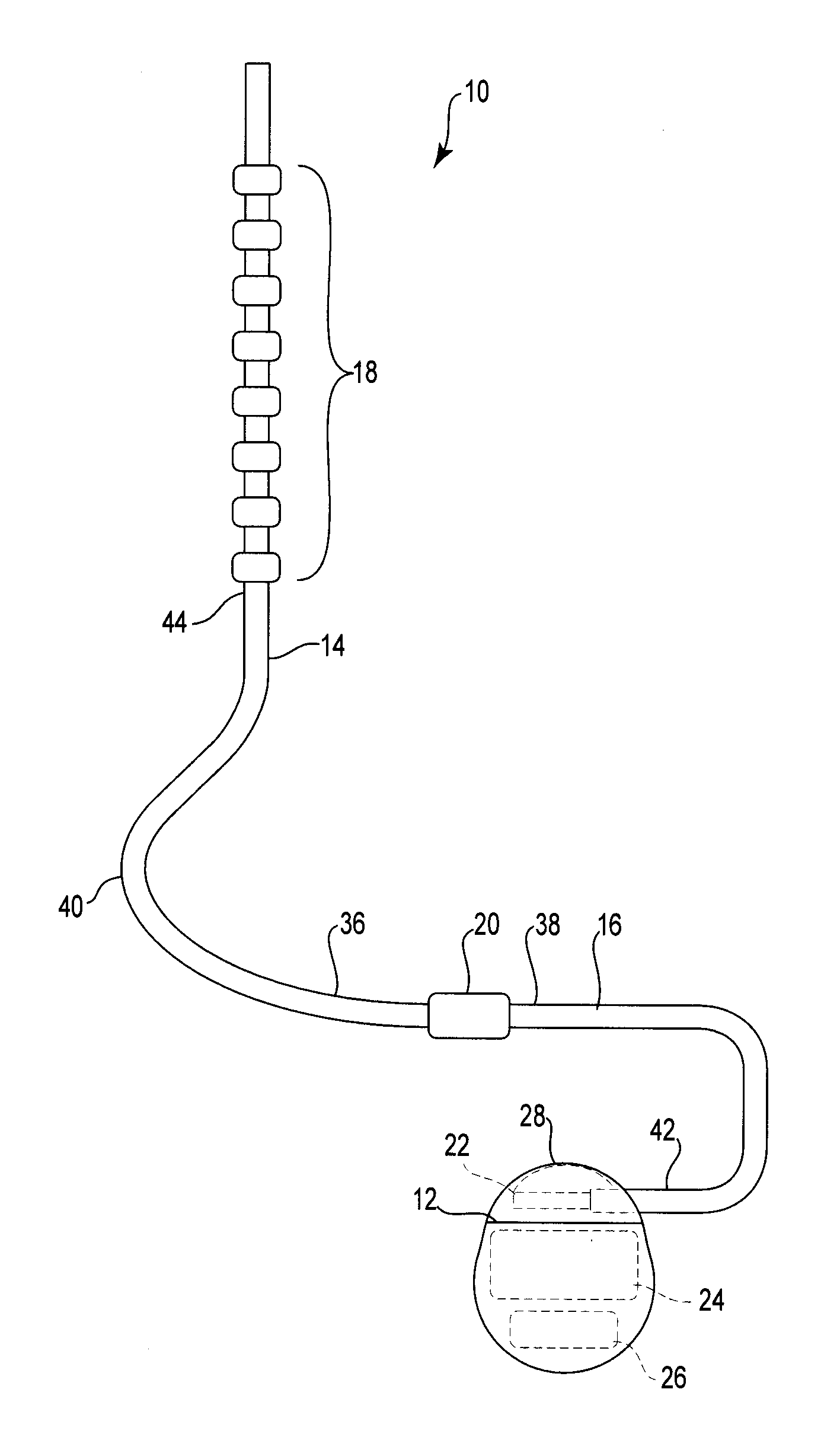
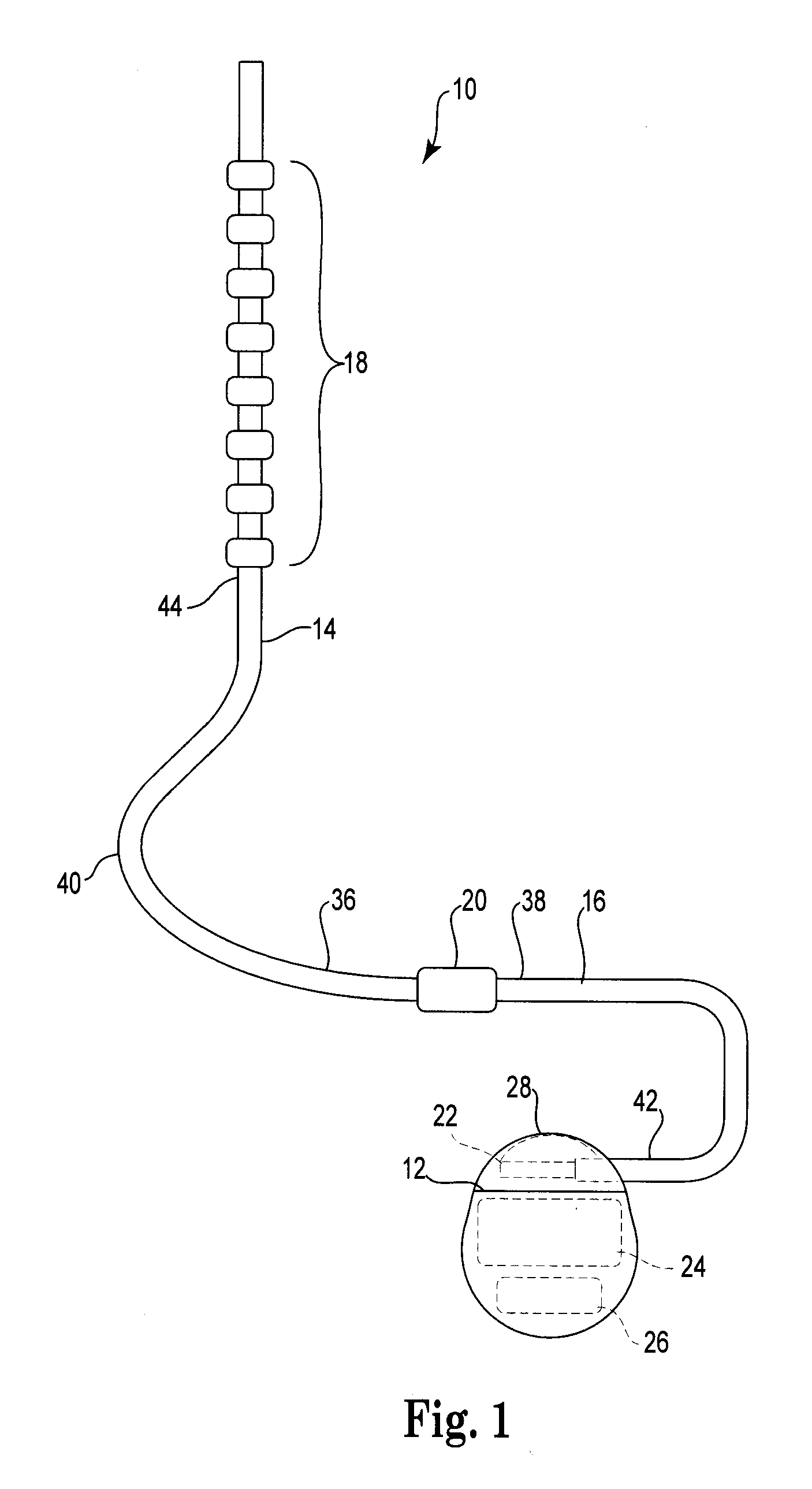
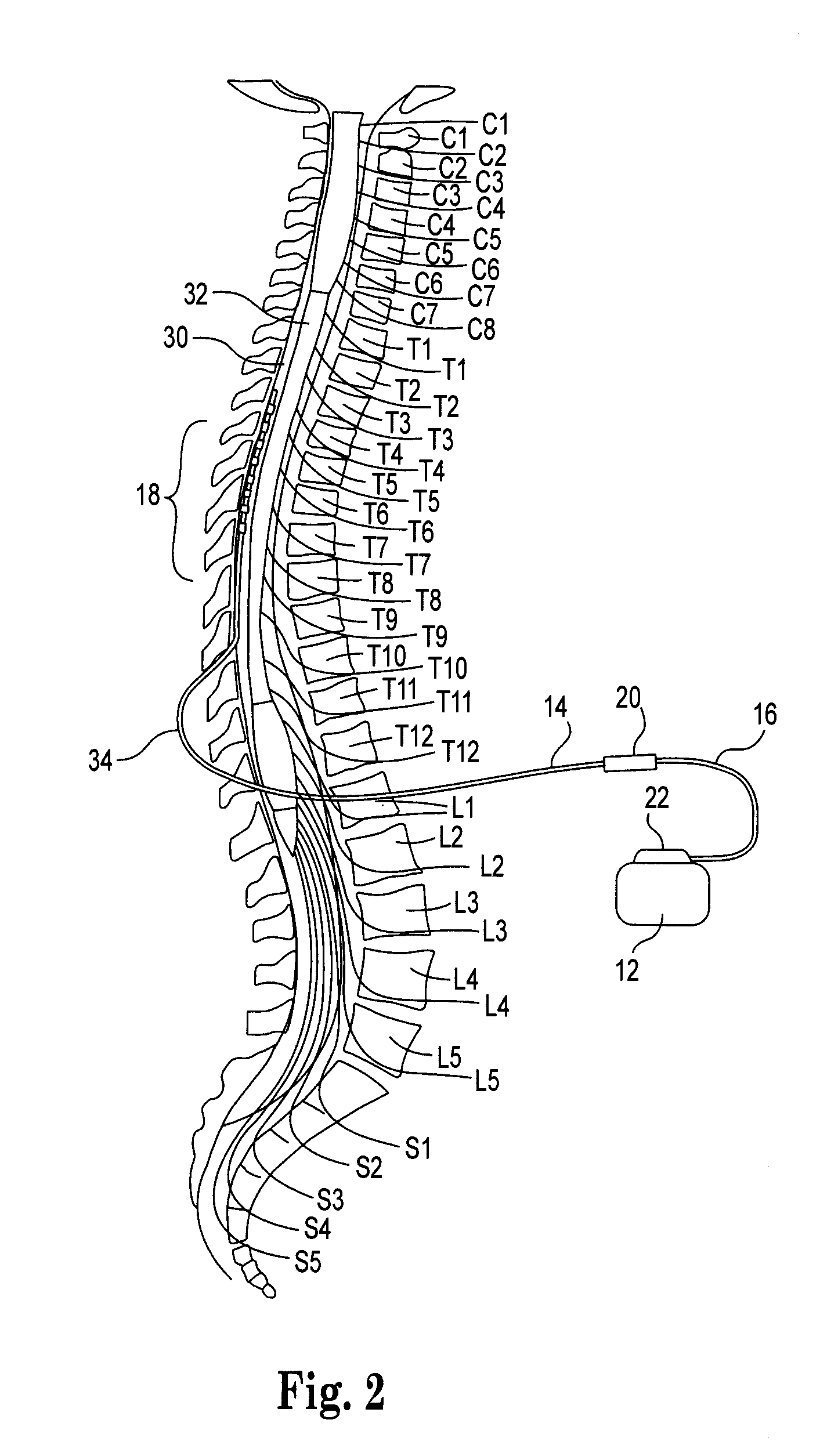
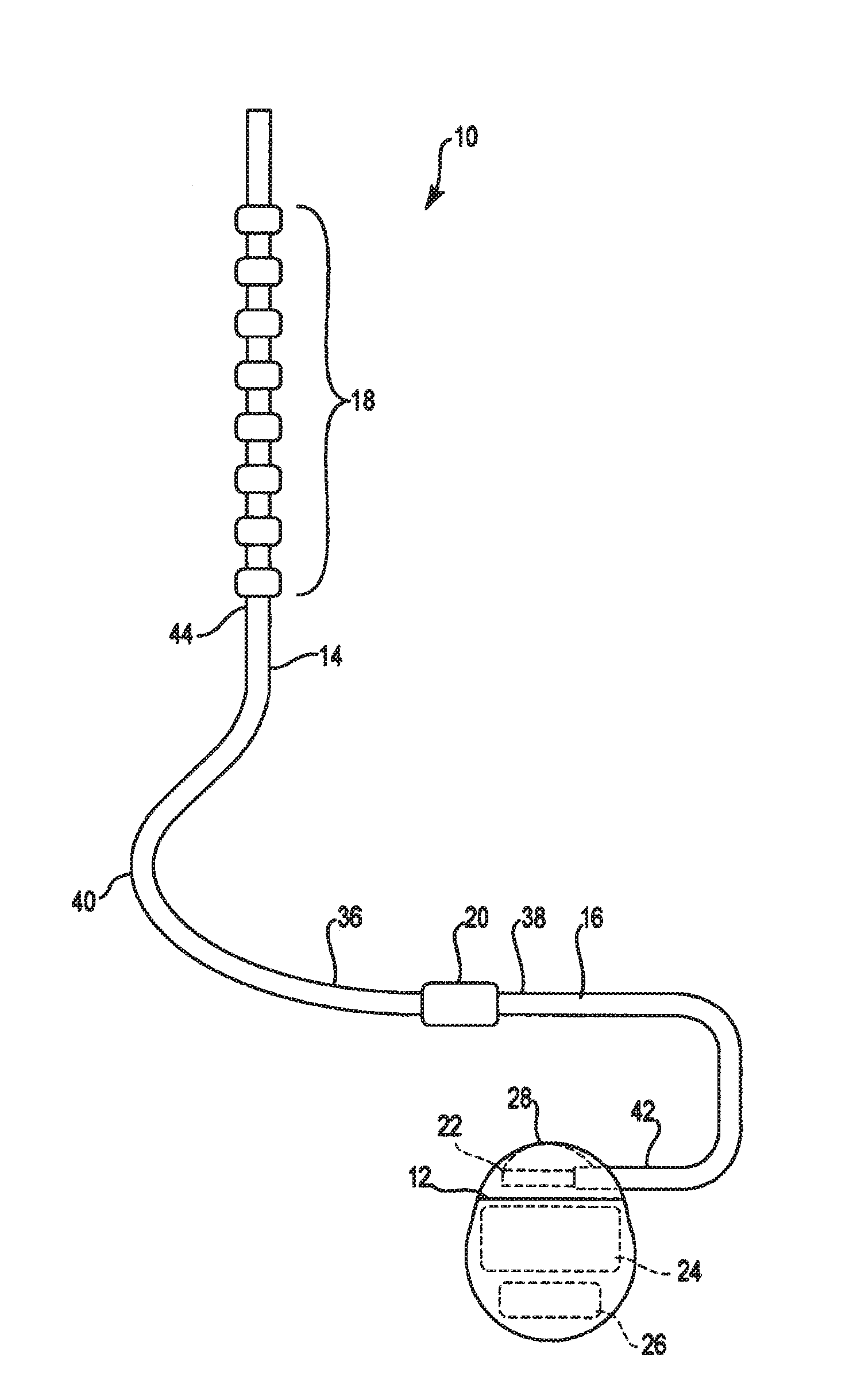
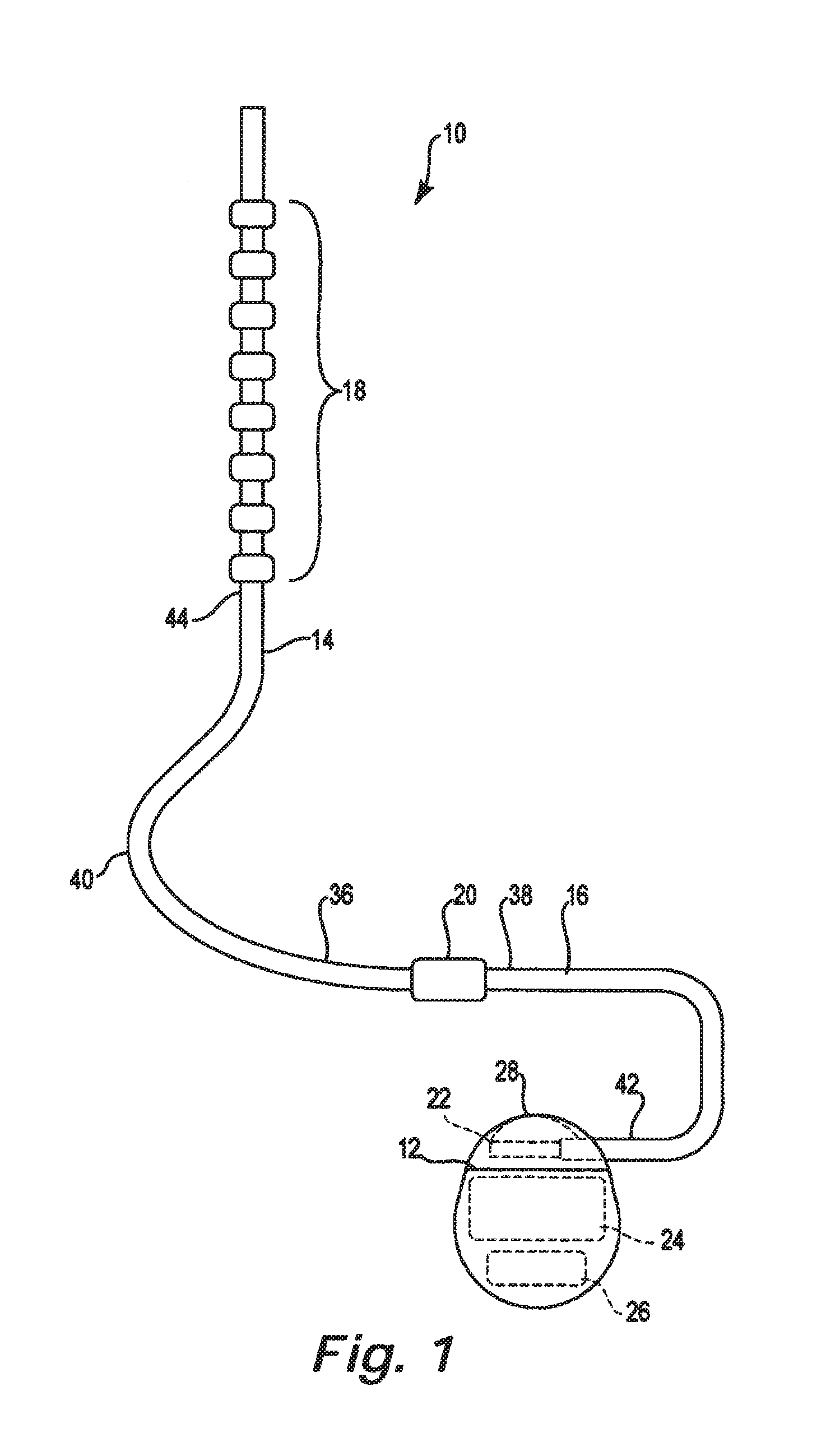
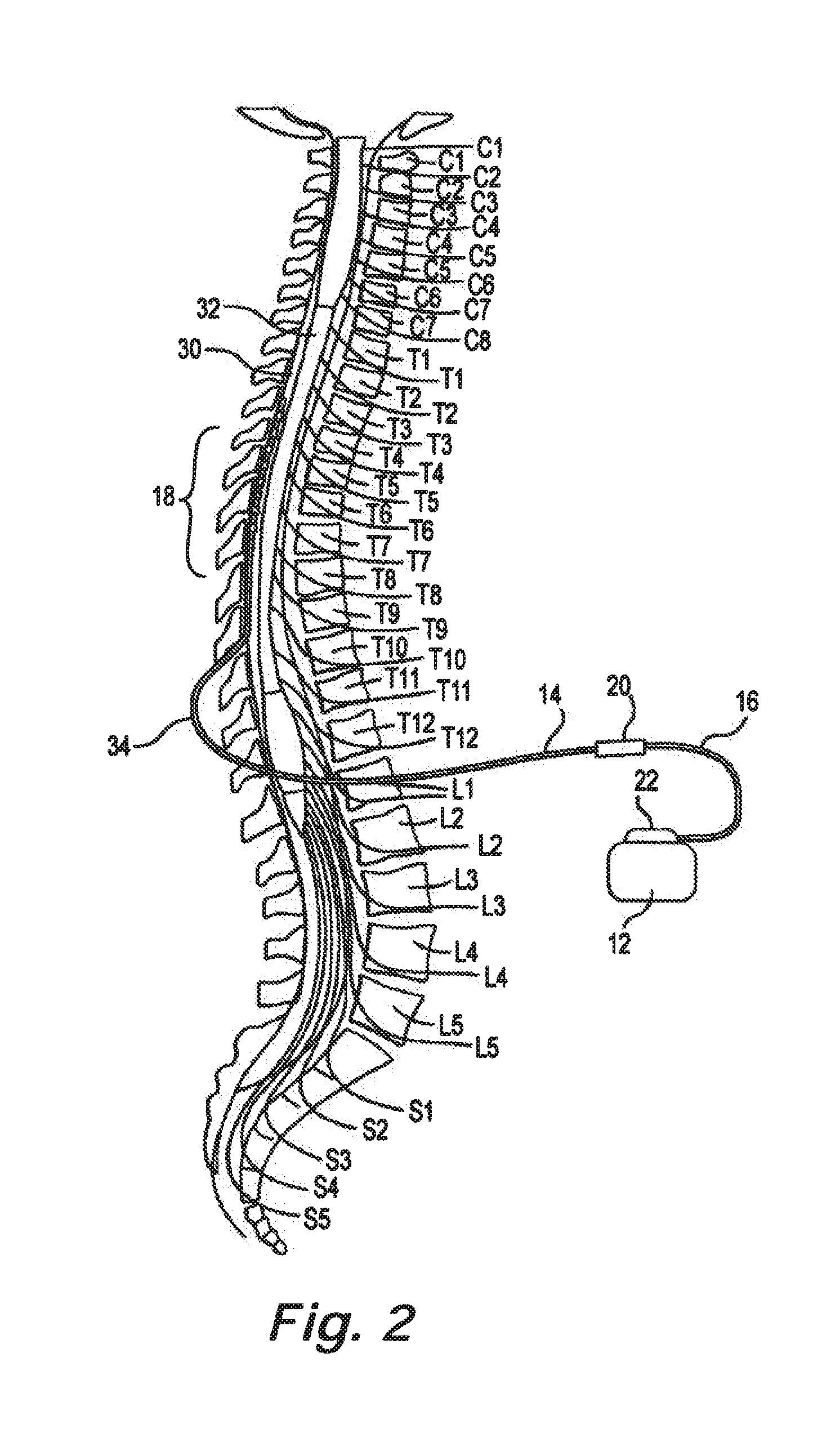
Catheter system and method for administering regional anesthesia to a patient
ActiveUS7120487B2Easy to controlMinimize movementSpinal electrodesAnaesthesiaEngineeringCatheter introducer
Owner:NELSON DAVID A
Safety needle assembly with correct medication connection
InactiveUS20070179454A1Smooth connectionReadily matableSurgical needlesFiltering accessoriesLocking mechanismEpidural needles
To ensure that there is no misconnection between a needle, such as for example a spinal or epidural needle, and the appropriate medication, the needle hub the needle is formed, by molding or extrusion, to have a given configuration. An adapter is placed between the needle and a conventional fluid store, or medical line. The end of the adapter that connects to the needle assembly is formed to have a configuration that is complementary to that of the hub of the needle assembly, so that the adapter and the needle hub may be readily mated to each other. The other end of the adapter is formed to have another configuration, for example a conventional luer, that is readily connectable to a conventional fluid store, such as a syringe that has a conventional luer. The adapter is formed by inter-fitting two elements to effect an integral locking mechanism to prevent the uncoupling of the fluid store and the adapter, once they are securely coupled. By thus configuring the hub of the needle to have a given configuration that is matable only to the end of an adapter that has a complementary configuration, and further providing an adapter that prevents the removal of the fluid store connected thereto, the potential for mis-connecting the needle to a fluid store that contains a different medicament is greatly reduced, if not totally eliminated.
Owner:SMITHS MEDICAL ASD INC
Ultrasonic positioning device for epidural space and method using the same
InactiveUS20110106052A1Ultrasonic/sonic/infrasonic diagnosticsAnaesthesiaLoss of resistanceTransducer
An ultrasonic positioning device for real time measuring the distance between the epidural needle and the epidural space is provided. The ultrasonic positioning device at least includes an epidural needle having a hollow interior; an ultrasound needle transducer disposed in the hollow interior of the epidural needle and connected to an ultrasonic driving device; and a loss-of-resistance checking syringe connected to the epidural needle so as to confirm whether the epidural needle is inserted into the epidural space.
Owner:NATIONAL YANG MING UNIVERSITY
Apparatus and method for safely inserting an introducer needle into epidural space
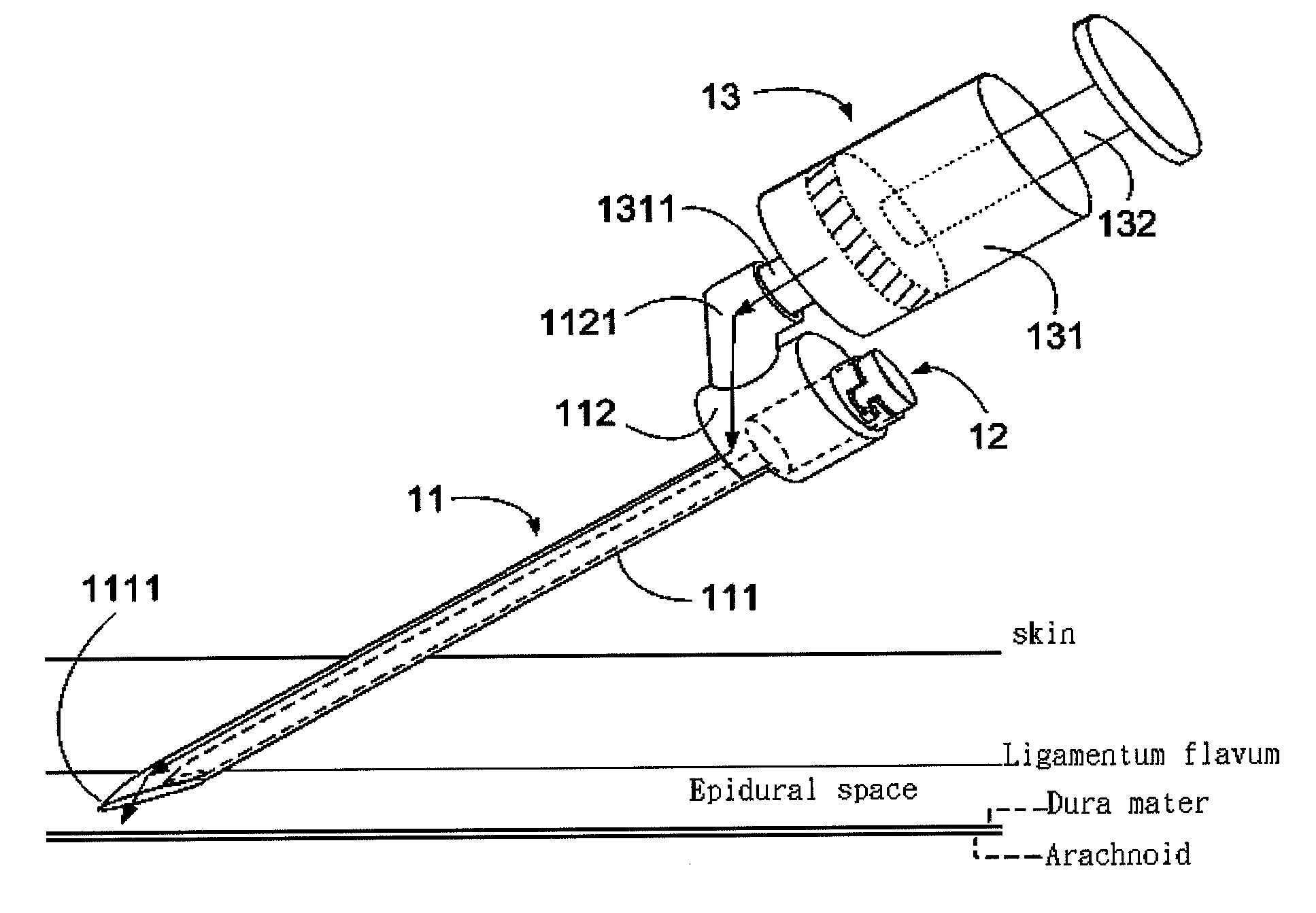
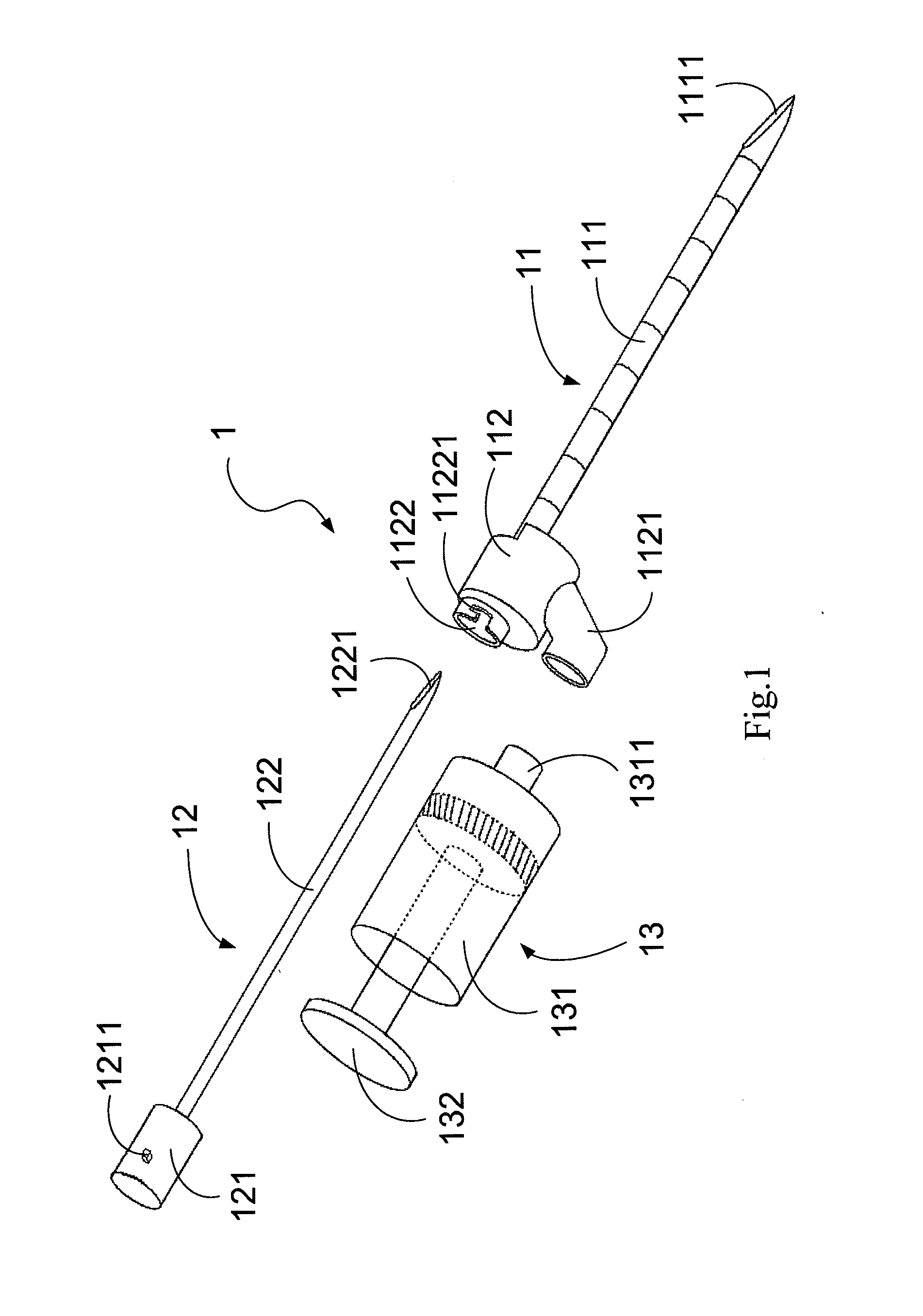
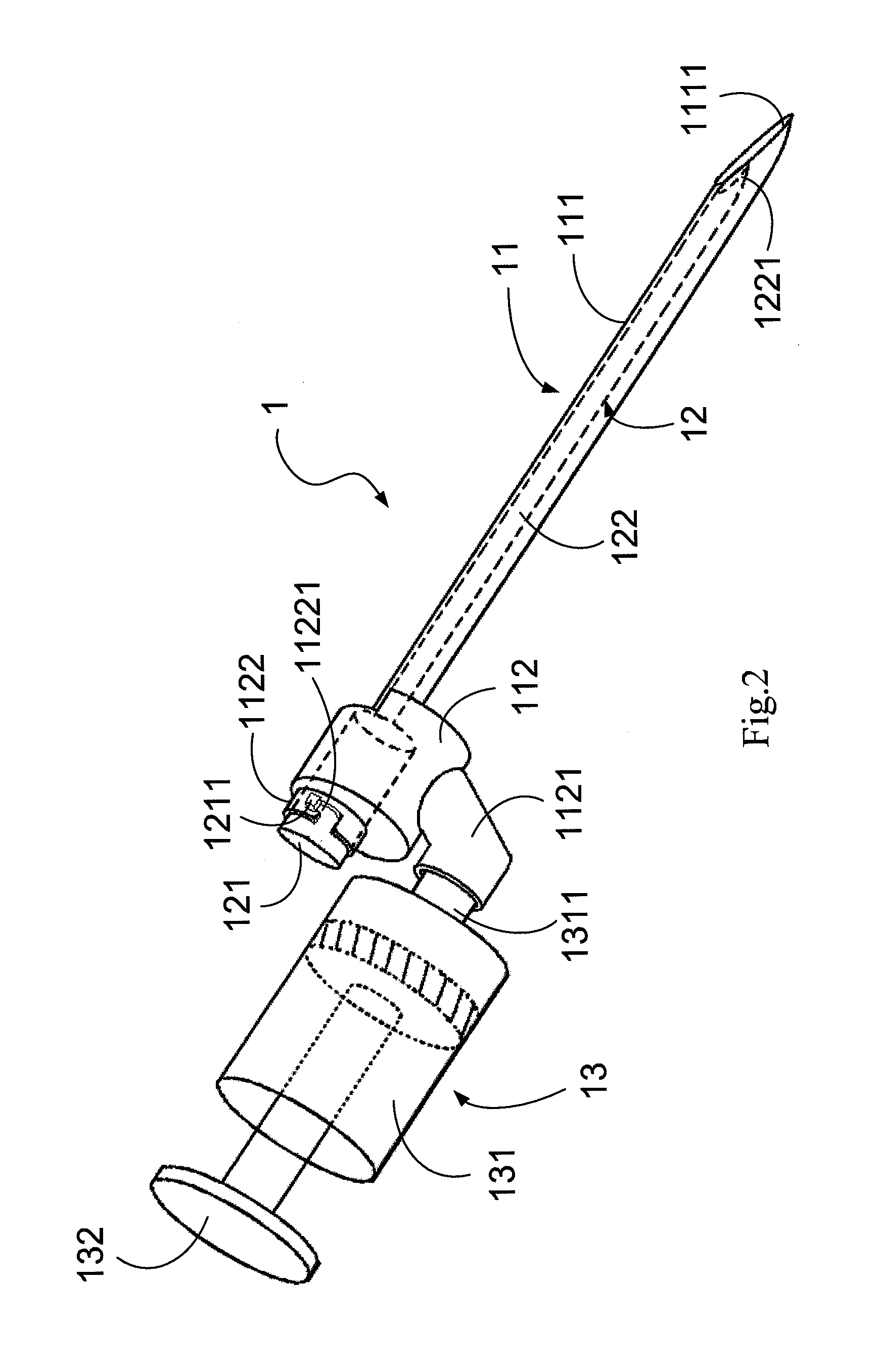
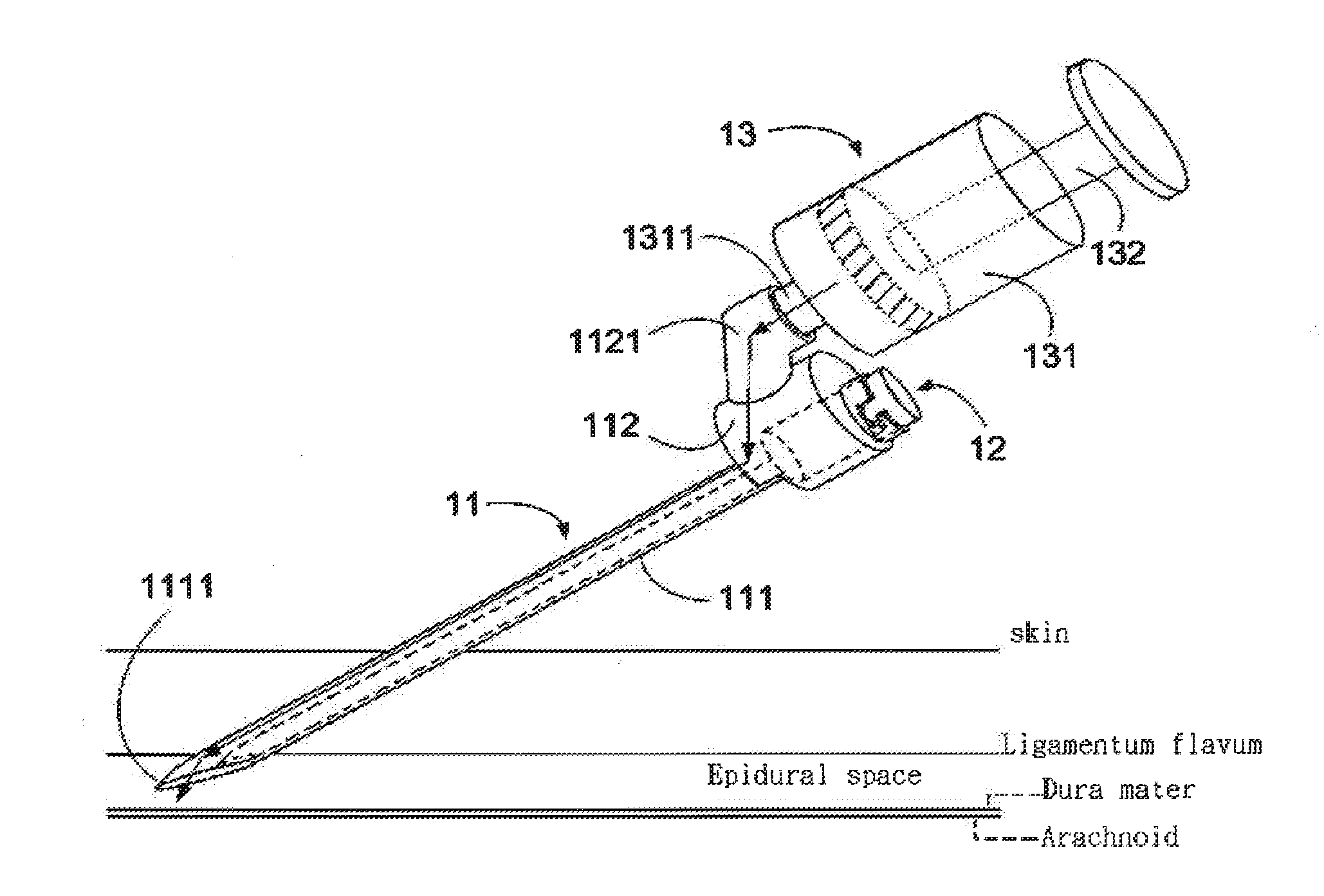
ActiveUS20130072900A1Achieve accessLoss of resistanceInfusion syringesSurgical needlesSpinal columnLoss of resistance
An epidural needle assembly is provided, permitting the accurate introduction of a large bore introducer sleeve needle into the epidural space in the spinal column of a patient using the loss of resistance technique. The large bore introducer needle has a beveled side opening at its tip and with a tissue piercing point at the distal extension of the needle. A syringe needle is received within the introducer needle with a distal head of the syringe needle filling the beveled side opening at the distal end of the introducer needle when the syringe hub is fully seated. The lumen of the syringe needle exits the syringe needle head at the side opening of the introducer needle immediately adjacent the tissue piercing point of the introducing needle. The needle assembly may be inserted and advanced, with precise tactile feedback, into a patient, thus accurately detecting the introduction of the introducer needle into epidural space.
Owner:COLANTONIO ANTHONY J
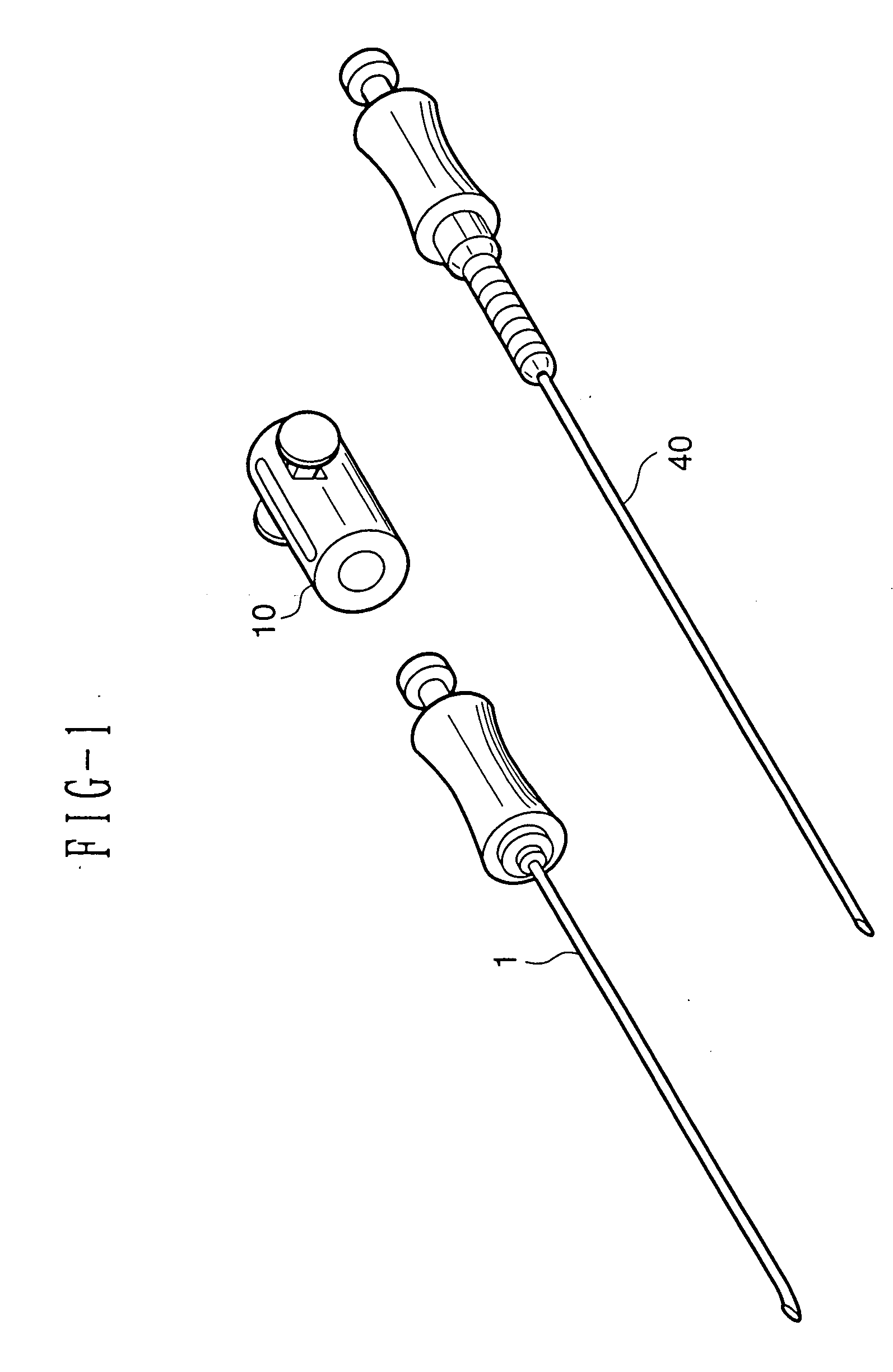
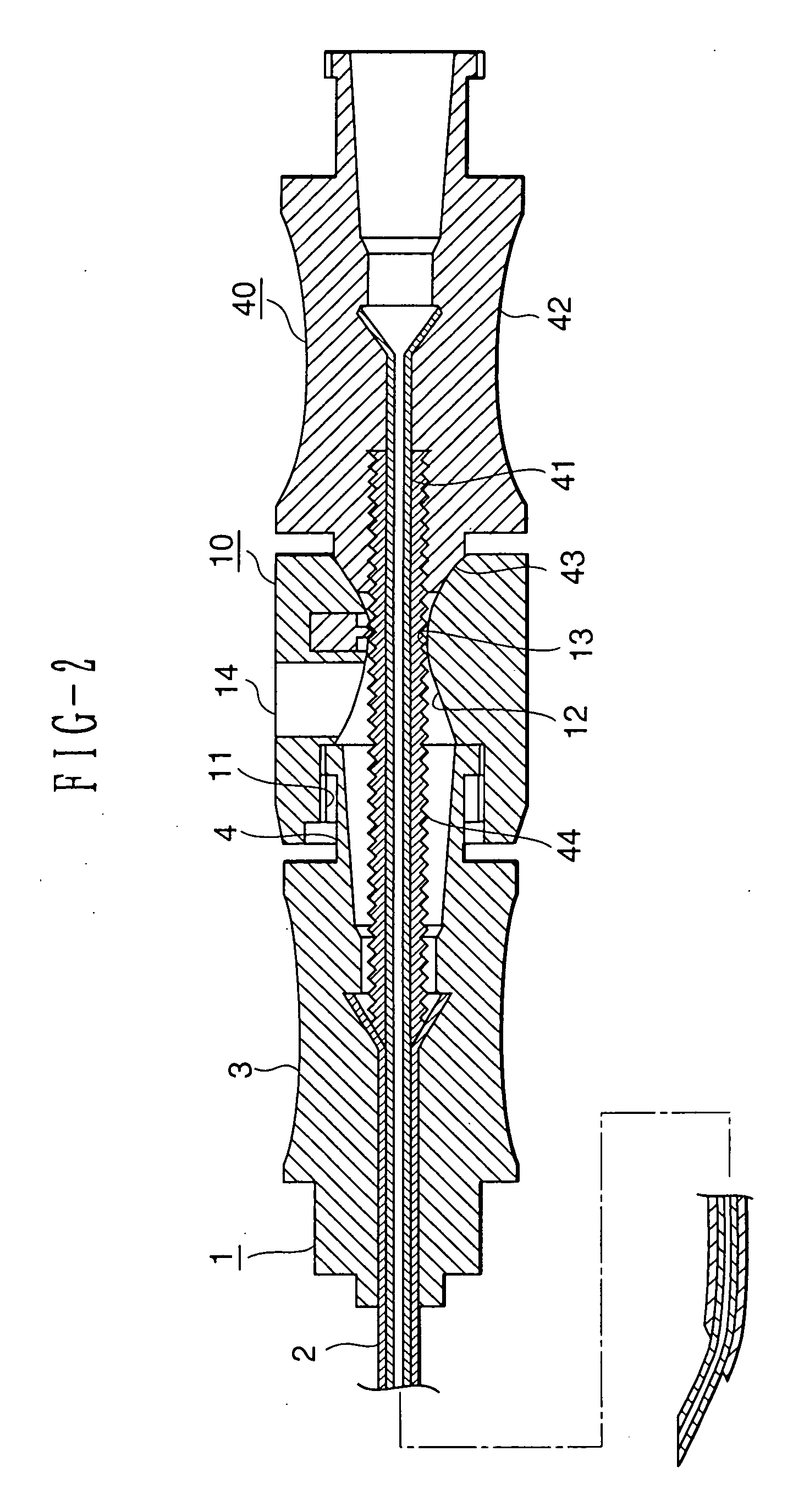
Medical anesthetic needle
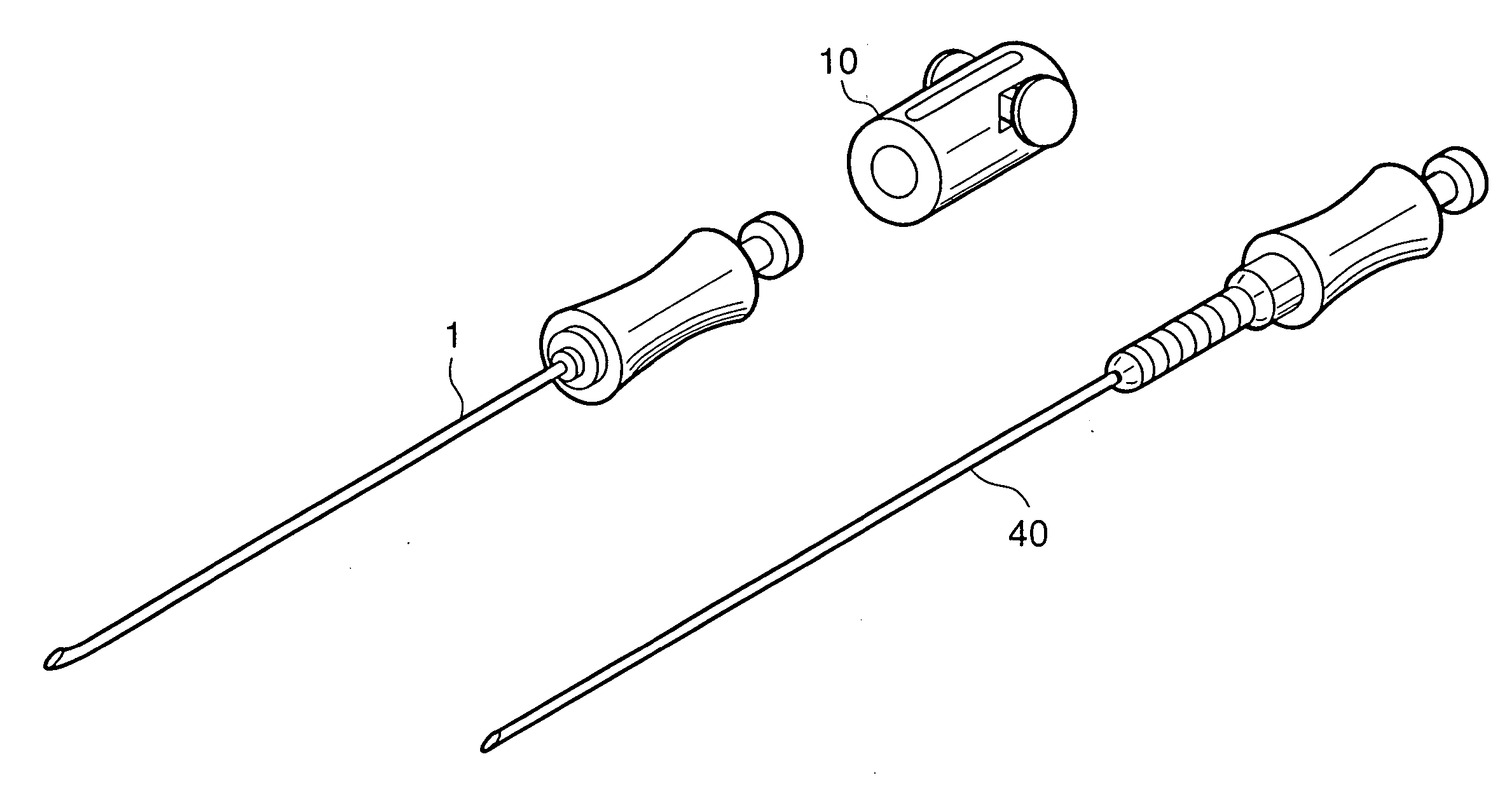
InactiveUS20060155246A1Delicate touchSmoothly rotate the anesthetic needleDiagnosticsInfusion syringesAnesthesia needleEpidural needles
An anesthetic needle for medical use that is extremely easy to use is provided. The anesthetic needle includes an epidural needle (1) and an anesthetic needle (40) that is rotatably inserted into the epidural needle from a proximal end portion. The epidural needle is provided, at the proximal end portion, with a length adjusting mechanism (10) of a tube shape extending in an axial direction. The anesthetic needle is provided, at a proximal end portion, with an engaging portion (44) of a circular tube shape loosely fitted within the length adjusting mechanism of the epidural needle. A needle is inserted through a center portion of the engaging portion. A plurality of circumferential grooves (45) are juxtaposed axially on an outer peripheral surface of the engaging portion. The length adjusting mechanism is provided therein with an engaging projection (17). The engaging projection engages with the circumferential grooves to inhibit the axial movement of the anesthetic needle, while being slidable circumferentially with the circumferential grooves.
Owner:DR JAPAN
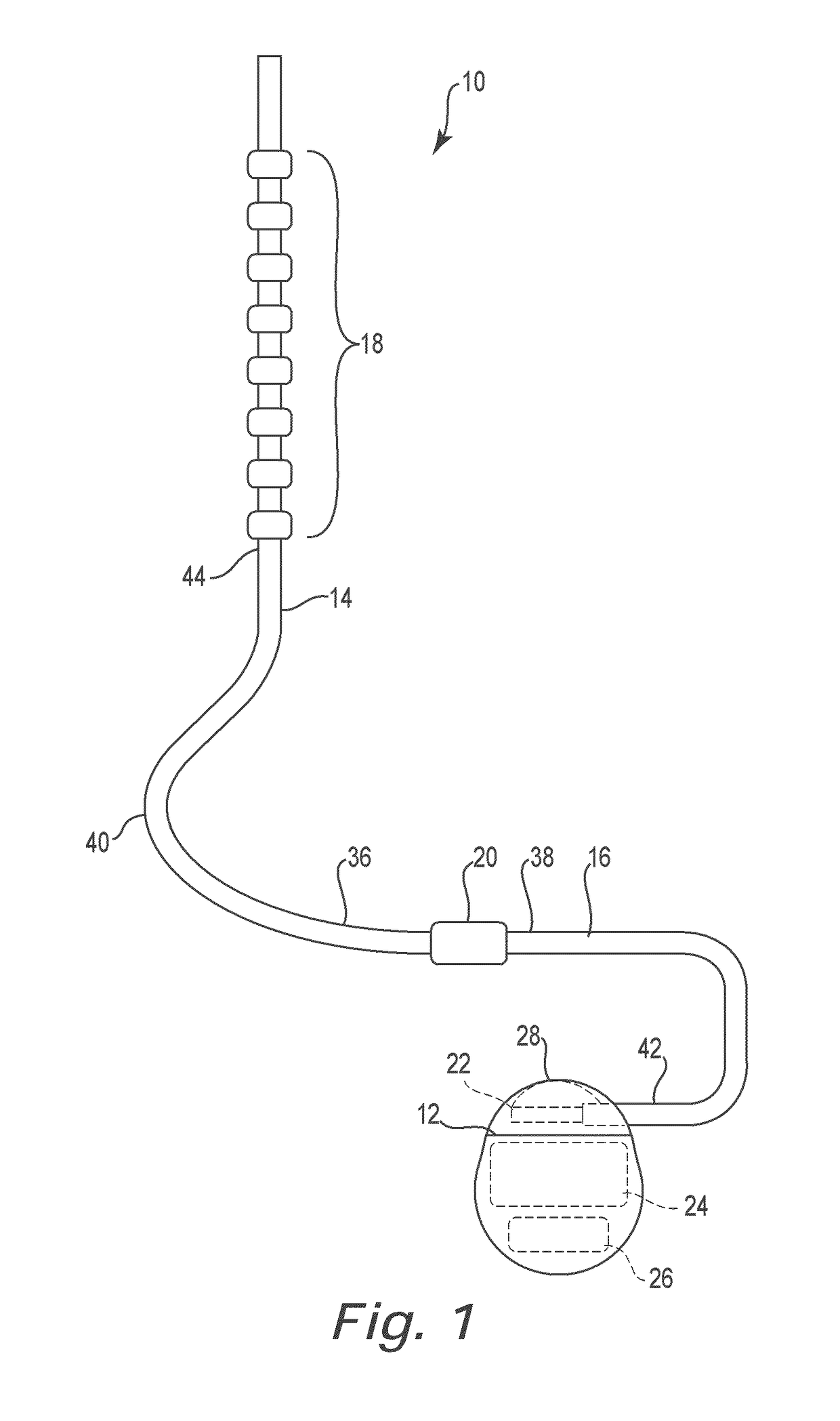
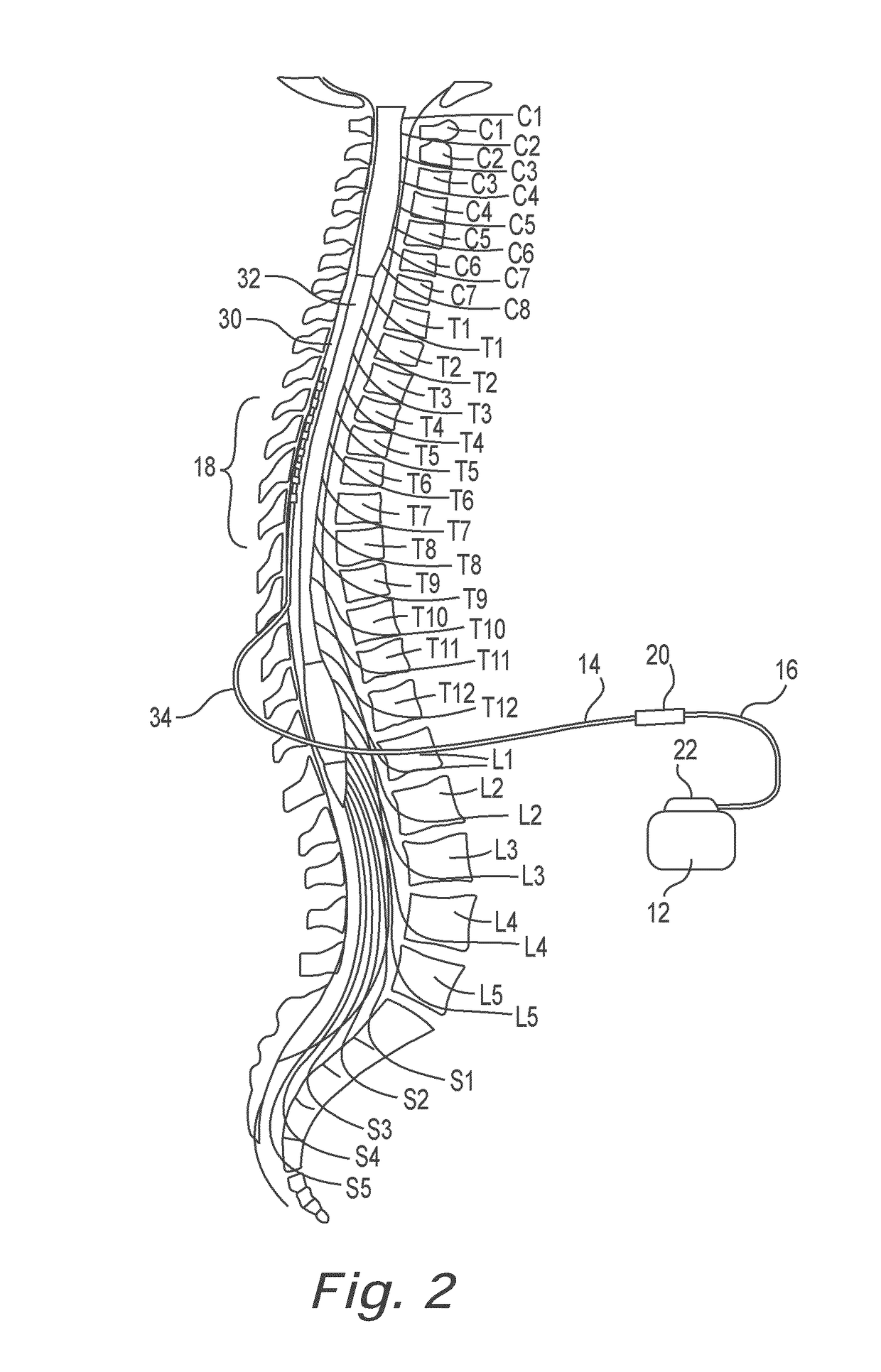
Lead identification system
A lead identification system for tracking a plurality of neurostimulation leads during implantation in a patient, and a method of using the same. The lead identification system includes a plurality of lead indicators each adapted to attach to one of a plurality of epidural needles to identify the leads. At least one clip adapted to releasably attach to proximal ends of the leads is provided with corresponding lead indicators. The trial cable for conducting trial stimulation includes connectors with corresponding lead indicators. A plurality of lead indicator stylets are provided for insertion into lumens at the proximal ends of the leads. The pulse generator also has connectors with corresponding lead indicators. The various lead indicators permit a surgeon to track a particular lead to the corresponding connectors on the pulse generator.
Owner:CIRTEC MEDICAL CORP
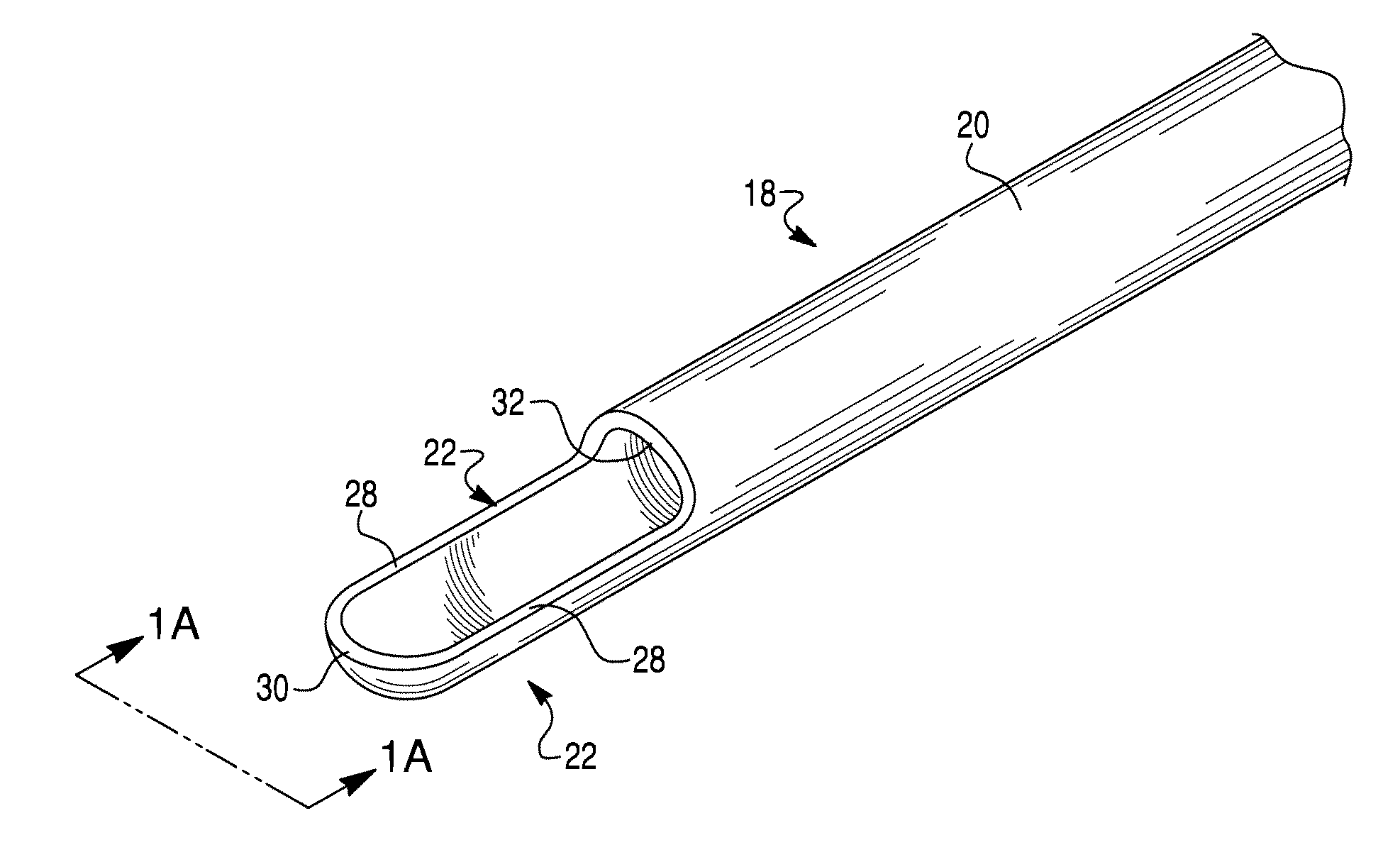
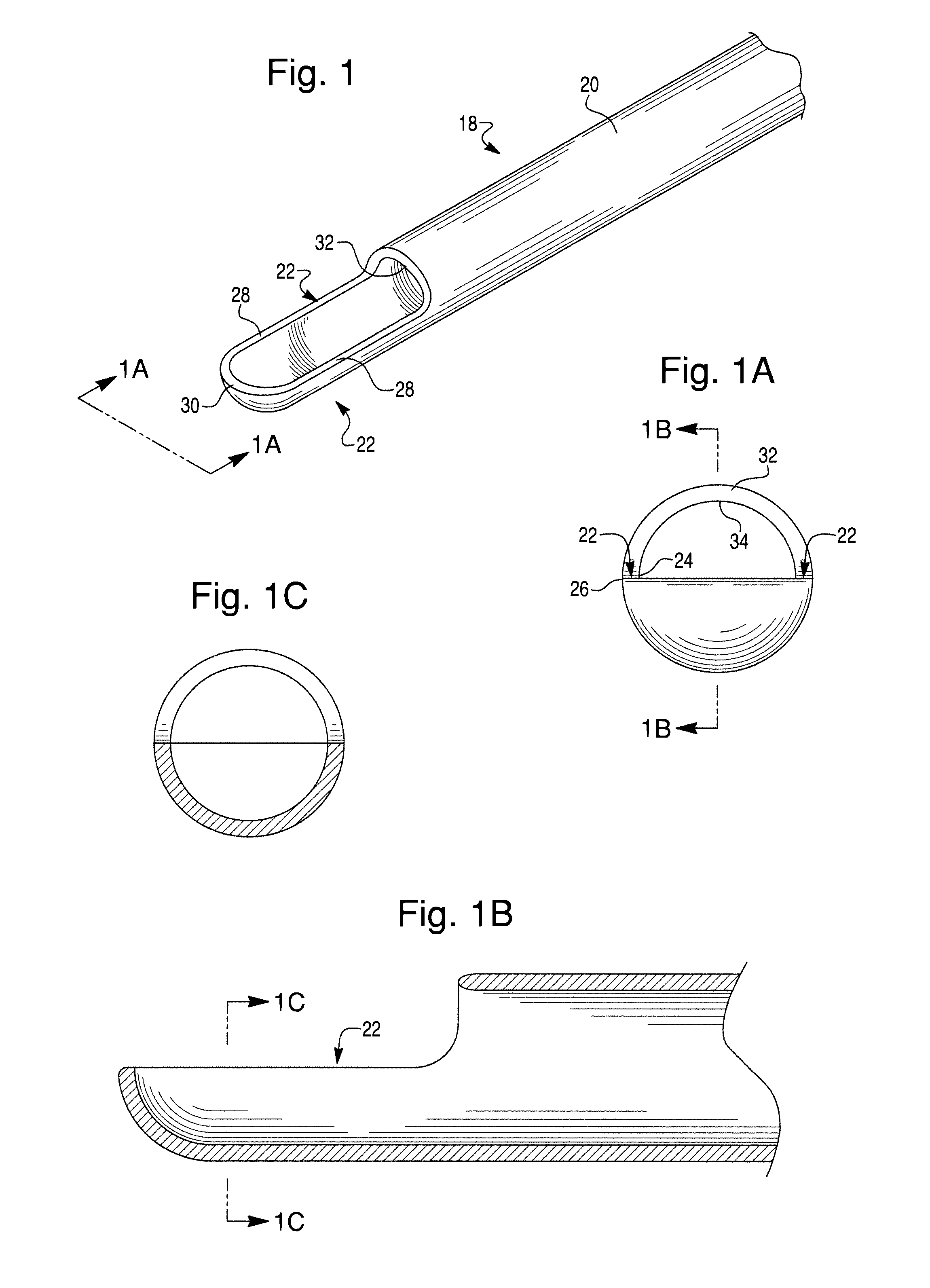
Epidural needle for spinal cord stimulation
InactiveUS20120232564A1Great riskHelp shapeSpinal electrodesDiagnosticsLoss of resistanceOuter Cannula
An epidural needle for implanting therapy delivery elements in an epidural space. The epidural needle includes at least an outer cannula containing an inner cannula, and a stylet located within the lumen of the inner cannula. The inner cannula substantially extends across the elongated opening at the distal end of the outer cannula to form a seal with the ligament during “loss of resistance” testing. Loss of resistance testing is performed by removing the stylet from the inner cannula. Once the epidural needle is positioned in the epidural space, the inner cannula is removed to facilitate implantation of a therapy delivery element in the epidural space.
Owner:WILSON GREATBATCH LTD
Epidural catheter system and methods of use
InactiveUS20070083184A1Elasticity can be extremely flexibleEasy to useSurgical needlesMedical devicesSterile environmentSTERILE FIELD
An epidural injection is used in medical procedure to administer medication to a patient's epidural space in the spine, usually to alleviate pain. Although effective in purpose, current medical procedure to administer an epidural injection does contain a flaw that exposes the patient to possible infection, usually manifested as an epidural abscess or bacterial meningitis. A source for infection stems from the manner the epidural catheter, specifically the proximal end not being inserted into the patient, is traditionally handled throughout the procedure—usually freely hanging, susceptible to breaking the sterile field and becoming contaminated. The current invention, an epidural catheter dispenser system, seeks to eliminate this risk of epidural catheter contamination by maintaining the epidural catheter, especially the proximal catheter end, in a sterile dispenser that can be easily manipulated by a physician. The epidural catheter dispenser system defines an inner cavity in which an epidural catheter may be loaded. When ready for use, a distal catheter end is extracted from the dispenser's inner cavity through a dispenser aperture on the dispenser's distal end piece, or top, allowing the physician to direct the epidural catheter into an epidural needle bore and into a patient's epidural space. Because the epidural catheter dispenser system and its epidural catheter contents fit easily into the palm of a physician's hand, the proximal catheter end is permanently in a controlled, contained sterile environment throughout the entire catheter placement procedure until extracted from the dispenser. The current invention minimizes and virtually eliminates the risk of epidural catheter contamination. Thus, the epidural catheter dispenser system provides benefits beyond existing epidural injection procedures including: (1) reduced risk of infection of the patient receiving an epidural injection; (2) easier catheter management for the physician; (3) better control of the medical microenvironment for the physician; and (4) improved medical efficiencies.
Owner:SIMPSON ROBERT C
Ultrasonic positioning device for epidural space and method using the same
An ultrasonic positioning method for real time measuring the distance between the epidural needle and the epidural space is provided. The ultrasonic positioning method at least includes a puncturing step; an advancing step; a positioning step for simultaneous detecting reflected ultrasonic signals from the ligamentum flavum (LF) and dura mater (DM); a replacement step for removing the ultrasound needle transducer and putting an injecting catheter; and an injection step for injecting an anesthetic into the epidural space via the injecting catheter.
Owner:NATIONAL YANG MING UNIVERSITY
Variable Extension Combined Spinal/Epidural Needle Set and Method For Its Use
InactiveUS20080183151A1Low inner diameter requirementEasy to operateCannulasInfusion syringesEpidural needlesBiomedical engineering
Owner:MARSH RONALD W +1
Lead identification system
In some examples, a lead identification system includes a first set of first lead indicators and a second set of second lead indicators. Each of the first lead indicators is configured to removably attach to at least one of a first therapy delivery element, a first epidural needle, or a first connector to uniquely identify at least one of the first therapy delivery element, the first epidural needle, or the first connector during implantation of the first therapy delivery element in the patient. Each of the second lead indicators is configured to removably attach to at least one of a second therapy delivery element, a second epidural needle, or a second connector to uniquely identify at least one of the second therapy delivery element, the second epidural needle, or the second connector during implantation of the second therapy delivery element in the patient.
Owner:CIRTEC MEDICAL CORP
Stylet free flexible-tip epidural catheter and method of making
InactiveUS20080015547A1Effectively sectionConveniently madeLaminationLamination apparatusEpidural needlesCatheter device
The distal end of a flexible tip epidural catheter is stiffened by the insertion of a stress oriented plastic tubular section into the end of the interior of the catheter removed from a terminal flexible tip and then expanded into contact with the wall of the interior of the catheter interior to stiffen a section approximately the length of an epidural needle in which it is to be used.
Owner:BEISEL ROBERT F
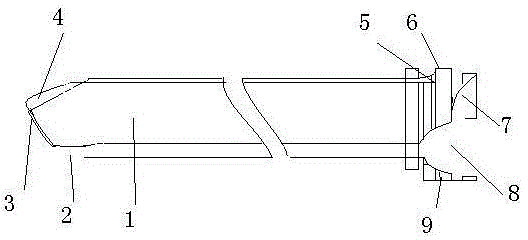
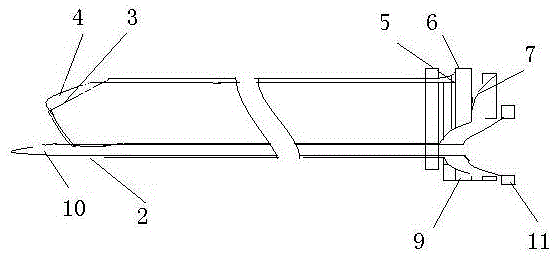
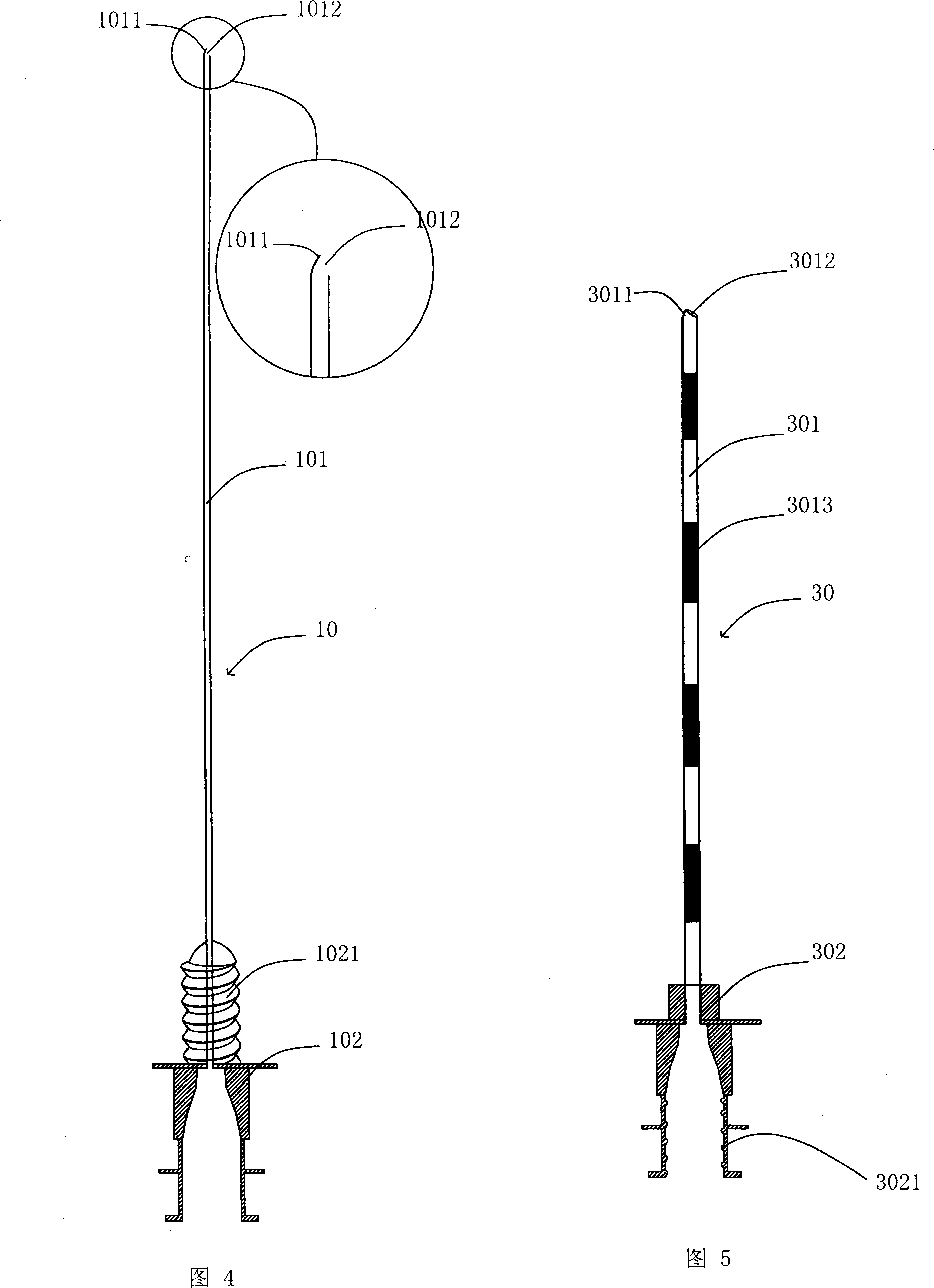
Safe combined spinal-epidural puncture needle
A safe combined puncture epidural anesthesia puncture needle is mainly composed of an epidural puncture needle, an epidural puncture needle core, a lumbar puncture needle and a lumbar puncture needle core. The arc-shaped crook of the epidural puncture needle is provided with a lumbar puncture dorsal pore. The epidural puncture needle core is composed of a safety protection device at the front part, a negative pressure conduction device in the middle, a negative pressure display device at the rear part, an elastic forward device at the tail part and a needle core base, and an epidural puncture needle core is connected with an lumbar puncture needle rear base in a detachable fixing manner. Apart from achieving the purpose of identifying the epidural space doubly, the puncture needle can avoid the needle point of the epidural puncture needle and the epidural conduit entering the subarachnoid space by the needle hole of the lumbar puncture needle, the lumbar puncture needle is prevented from being broken, and the lumbar puncture needle is conveniently fixed in the epidural puncture needle.
Owner:王家松
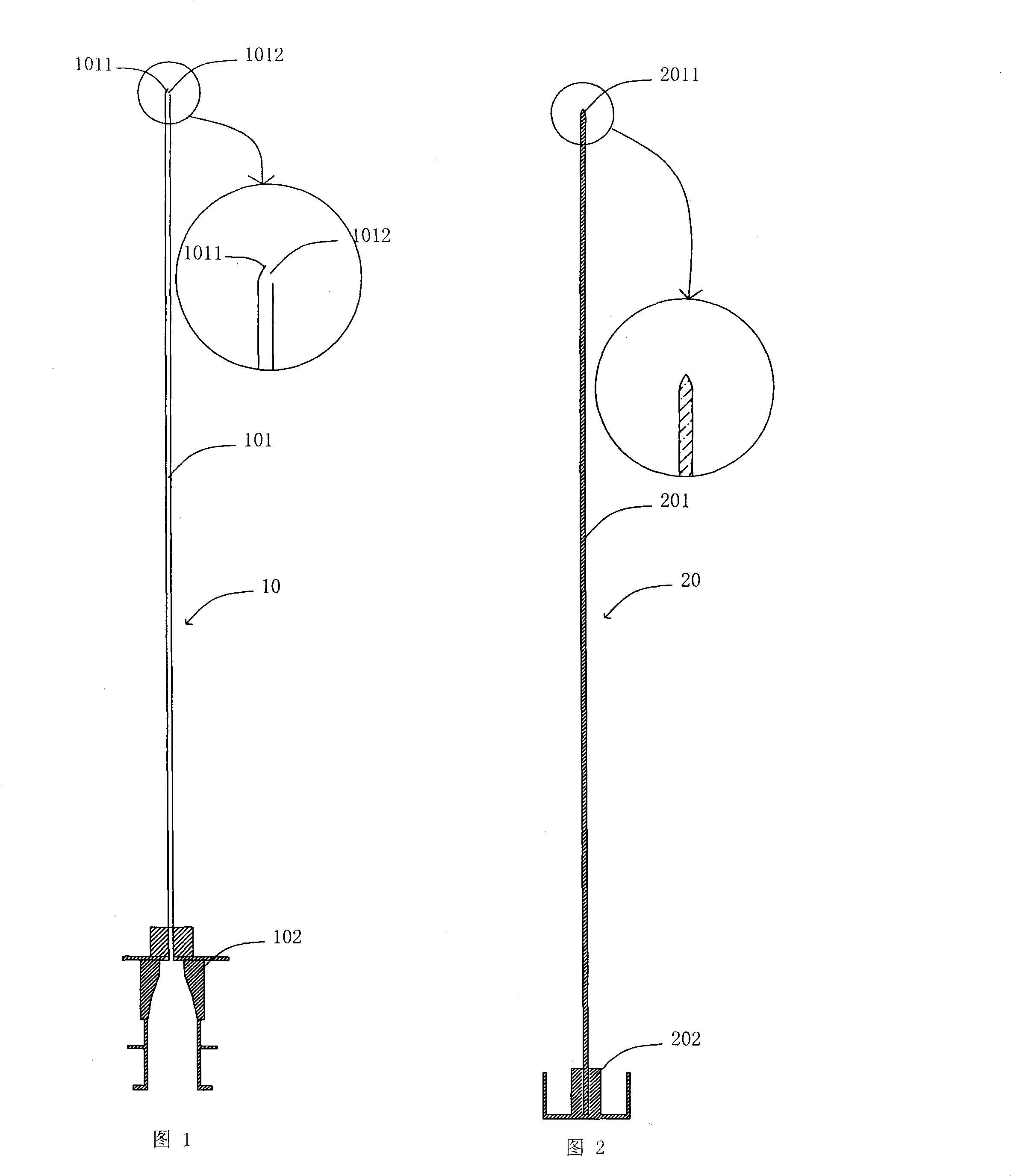
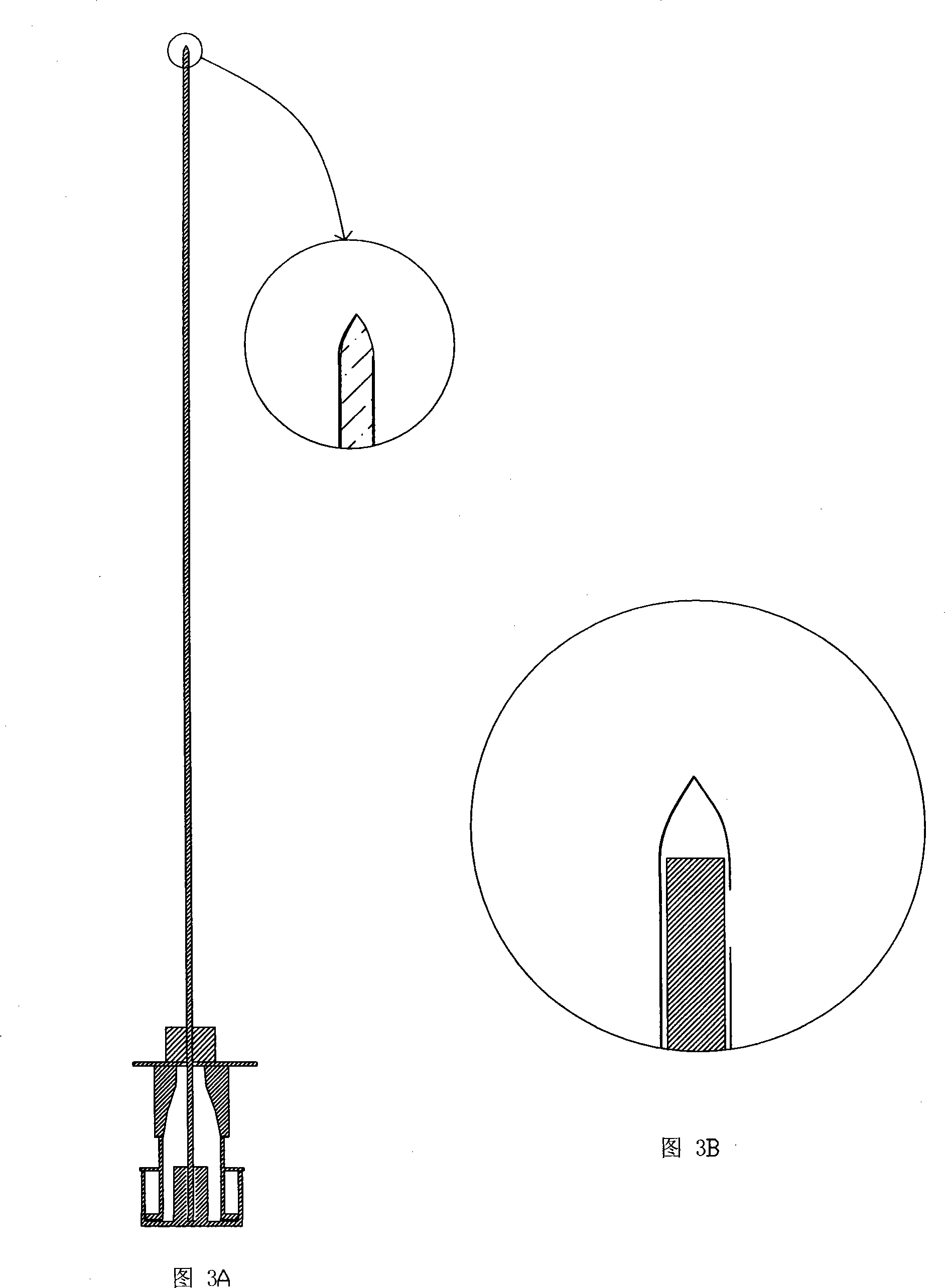
Spinal needle and the intraspinal anaesthetic puncture device compromising the same
InactiveCN101204334ASmoothly fixedEasy to fixAnaesthesiaSurgical needlesSpinal needlesEpidural needles
The invention relates to a medical appliance, in particular relating to a puncture needle for lumbar anesthesia and the intraspinal anesthesia puncture device with the puncture needle for lumbar anesthesia. The puncture needle for lumbar anesthesia comprises a needle and a stylet, wherein, the needle comprises a first needle and a first neilsbed which is permanently connected with the first needle; the infusion hole of the needle is arranged on the sidewall of the first needlepoint; as the opening of the infusion hole is more closely to the front end of the first needlepoint, the leakage of liquid medicine out of the subarachnoid cavity is easy to be prevented when the lumbar anesthesia is injected. The intraspinal anesthesia puncture device of the invention comprises an extradural puncture needle and a puncture needle for lumbar anesthesia, wherein, at the matching place of the first neilsbed and the third neilsbed of the puncture needle for lumbar anesthesia a screw thread is arranged With cooperation of the screw thread, the needle for lumbar anesthesia is easy to be fixed and the length of the needle for lumbar anesthesia exceeding the epidural can be accurately adjusted, thus the occurrence of a phenomenon that the needle for lumbar anesthesia can not reach the subarachnoid cavity due to a short length of the needle for lumbar anesthesia is decreased.
Owner:赵阳 +1
Epidural needle for electrode epidural catheter and method of manufacture
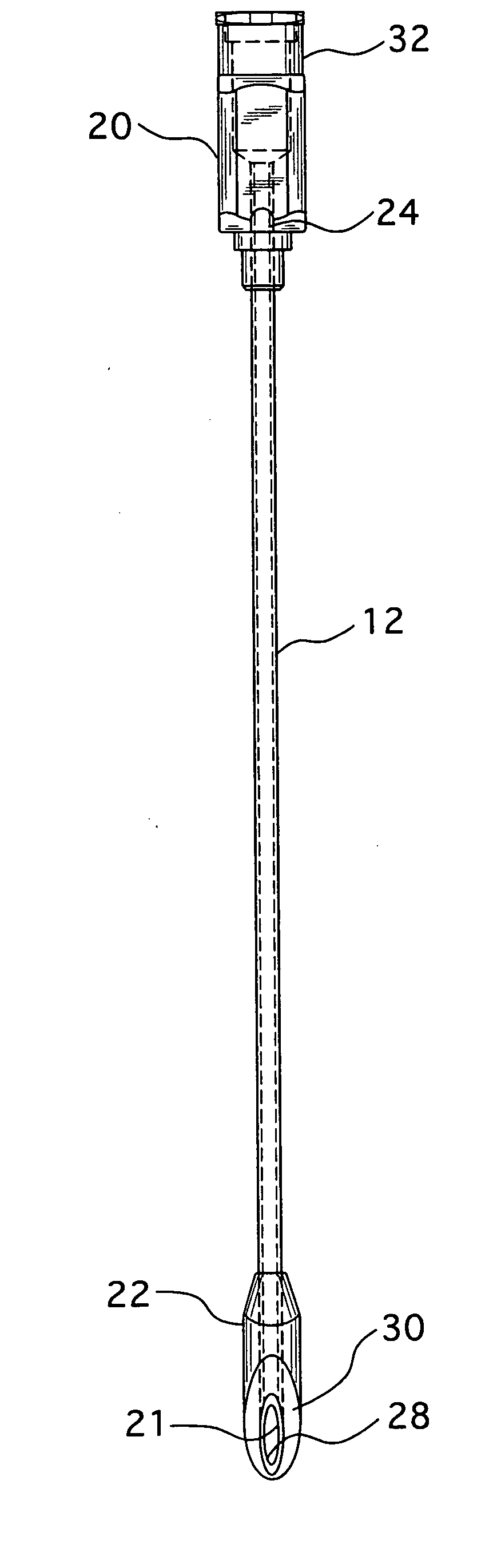
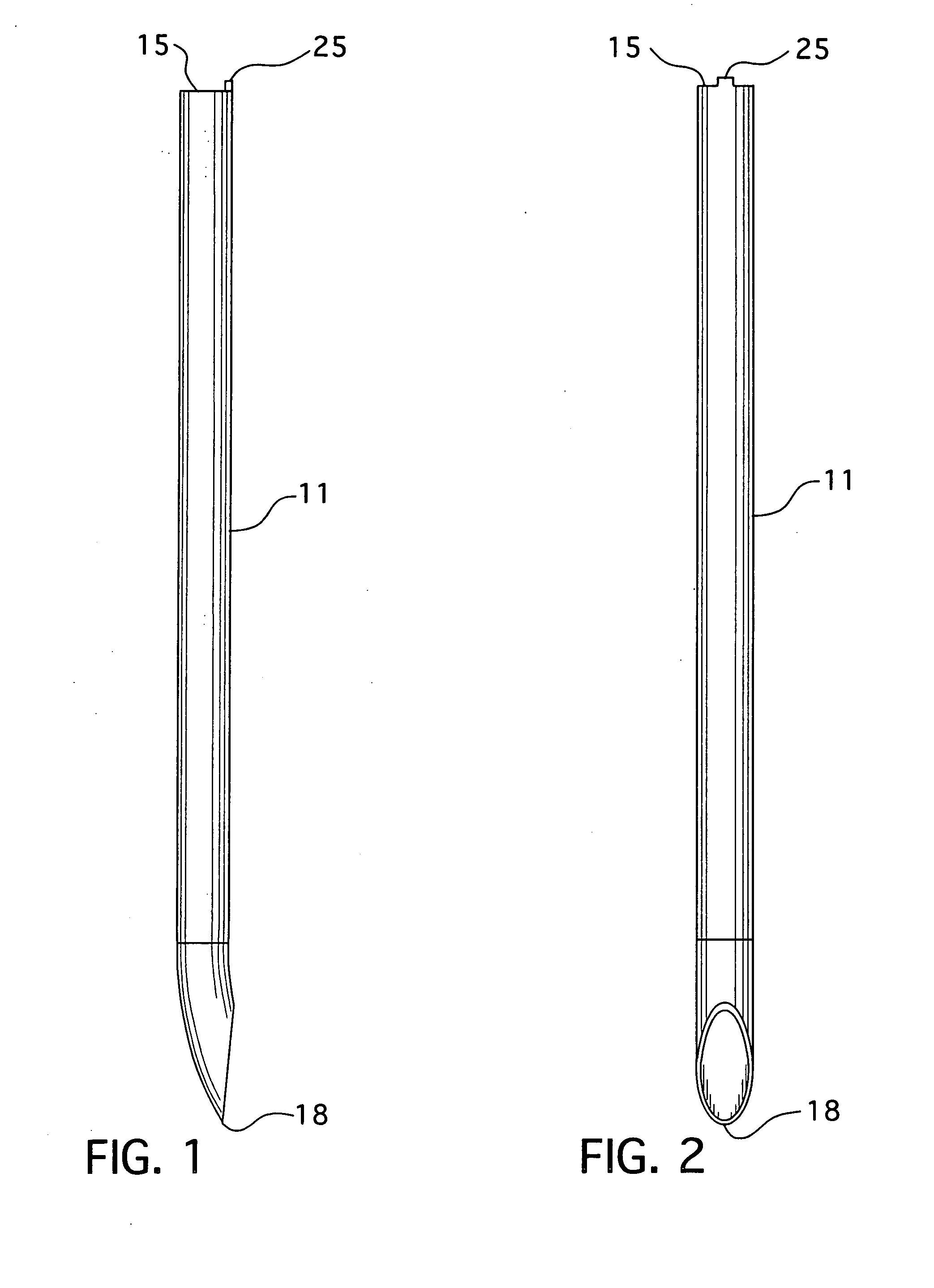
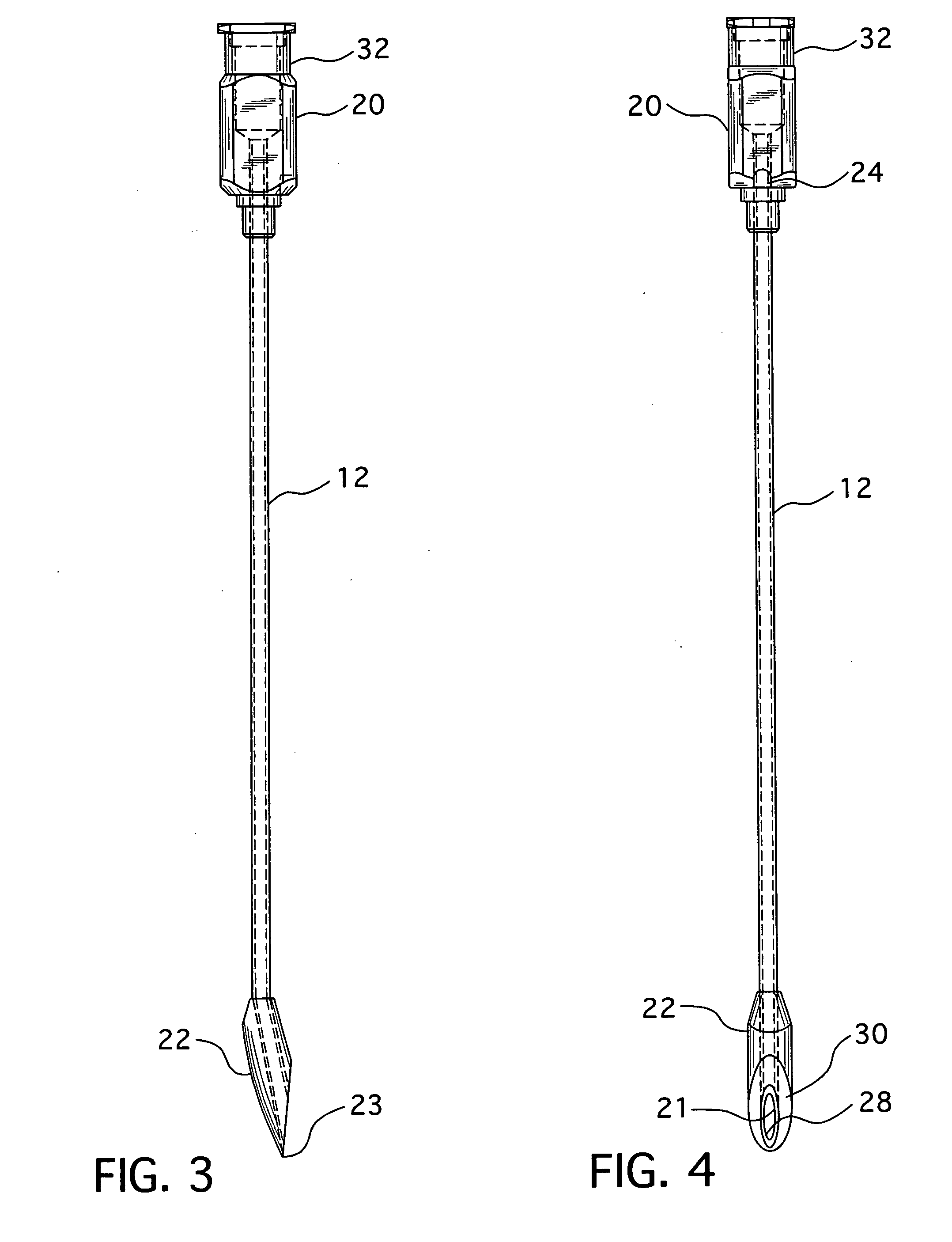
An epidural needle for placement of a pain management electrode includes an elongated bevel having a heel segment defined by a proximal rounded inner edge in which the heel has an electropolished radius of at least about 0.002 inch extending substantially continuously along the inner edge that defines the heel; a method for fabricating the needle.
Owner:SPECTRA MEDICAL DEVICES
Dual-channel acoustic-guided epidural puncture needle
InactiveCN106037895AGuaranteed successGuaranteed success rateSurgical needlesTrocarSpinal needlesOperation mode
A dual-channel acoustic-guided epidural puncture needle is composed of a pressure-taking device, an epidural puncture needle tube, a spinal needle assembly, a needle seat, and a syringe pump connector. An anesthetic catheter channel is arranged inside a needle wall of the epidural puncture needle tube, the end of the anesthetic catheter channel is provided with a bend needle end, the top of the needle wall is provided with a spinal needle tube channel, the needle seat has a double-channel structure, a spinal needle inlet and an anesthetic catheter inlet at one end of the needle seat respectively communicate with the spinal needle tube channel and the anesthetic catheter channel of the epidural puncture needle tube, the spinal needle assembly is composed of a spinal needle, a positioning sleeve and a needle handle, the spinal needle comprises a spinal needle tube wall and a spinal needle tube cavity, and a connecting tube at an outlet end of the pressure-taking device is connected to the needle seat through a connecting pipe connector. According to the puncture needle, a double-channel entry structure of the anesthetic catheter and the spinal needle is adopted, the operation mode of combined spinal-epidural anesthesia is changed, and the success of the puncture and anesthesia is guaranteed; and the structure of the spinal needle with the positioning sleeve and the structure of the epidural needle tube with a back hole are provided so that a guarantee is provided for correct implementation of spinal anesthesia.
Owner:王刚
Stylet free flexible-tip epidural catheter and method of making
The distal end of a flexible tip epidural catheter is stiffened by the insertion of a stress oriented plastic tubular section into the end of the interior of the catheter removed from a terminal flexible tip and then expanded into contact with the wall of the interior of the catheter interior to stiffen a section approximately the length of an epidural needle in which it is to be used.
Owner:BEISEL ROBERT F
Lead identification system
ActiveUS20140350655A1Permit trackDecrease stockSpinal electrodesDiagnosticsEngineeringEpidural needles
In some examples, a lead identification system includes a first set of first lead indicators and a second set of second lead indicators. Each of the first lead indicators is configured to removably attach to at least one of a first therapy delivery element, a first epidural needle, or a first connector to uniquely identify at least one of the first therapy delivery element, the first epidural needle, or the first connector during implantation of the first therapy delivery element in the patient. Each of the second lead indicators is configured to removably attach to at least one of a second therapy delivery element, a second epidural needle, or a second connector to uniquely identify at least one of the second therapy delivery element, the second epidural needle, or the second connector during implantation of the second therapy delivery element in the patient.
Owner:CIRTEC MEDICAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com