Kit and process for PCR amplification detecting type 2 pig streptococcus virulence gene
A technology for detecting kits and virulence genes, which is applied in biochemical equipment and methods, measurement/testing of microorganisms, DNA/RNA fragments, etc. It can solve problems such as difficult emergency detection, PCR reaction and cycle conditions are not the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] SS2 related virulence gene detection kit, including the following components:
[0117] (1) Qiagen DNA extraction reagent
[0118] (2) Multiplex PCR amplification detection reagent: It is made by mixing 10×PCR buffer, deoxynucleoside triphosphate (dNTP) mixture, DNA polymerase and specific amplification primers, etc., with distilled water as a solvent.
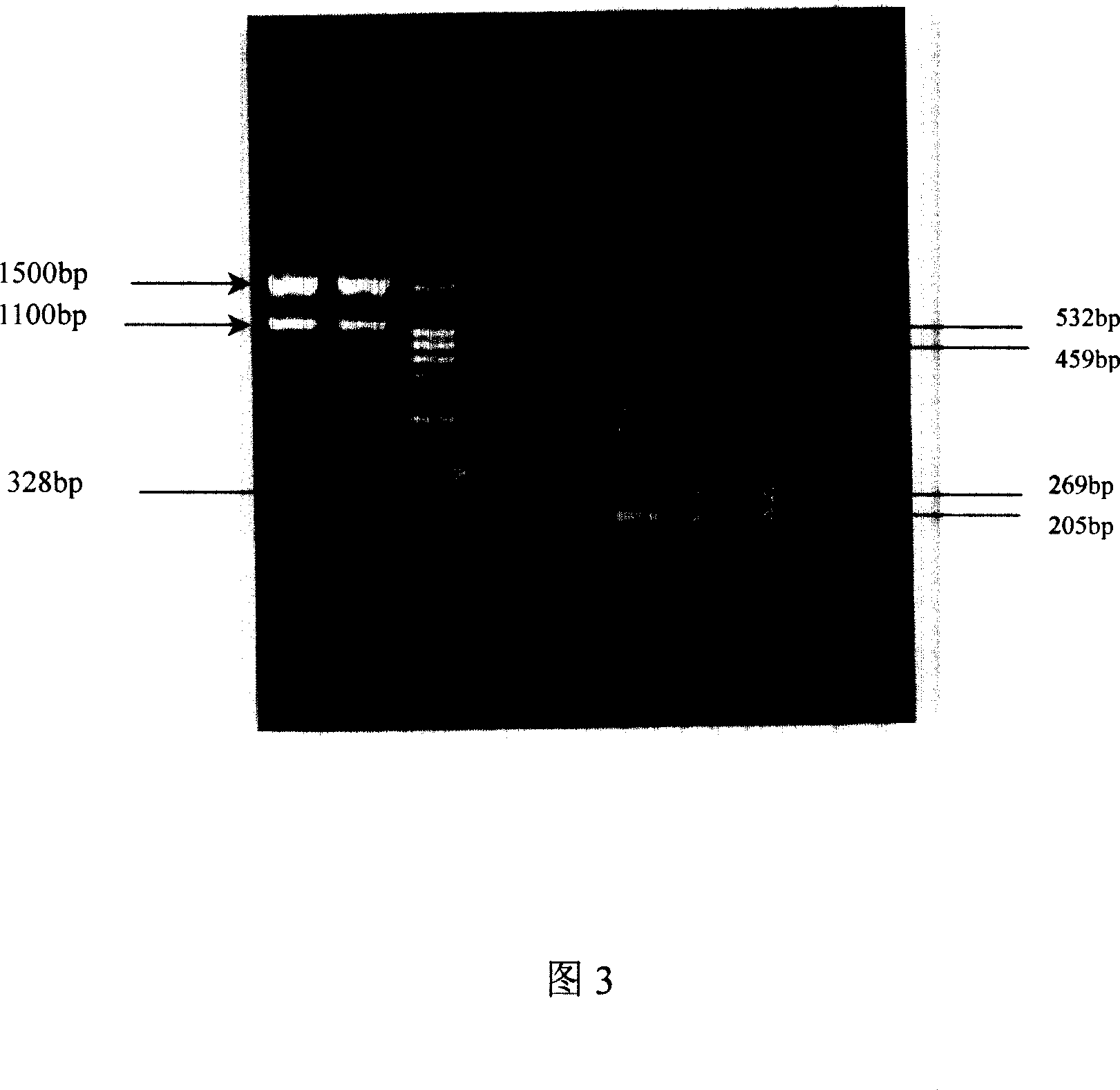
[0119] There are 8 pairs of specific amplification primers, and the names of the primers, the size of the sequence amplification products and their management are shown in Table 1.
[0120] Table 1: Primer sequences and groups
[0121]
[0122] The multiplex PCR amplification detection reagent is mixed with distilled water as a solvent by the following components:
[0123] 10×PCR buffer content volume percentage 10%
[0124] Deoxynucleoside triphosphates:
[0125] dATP 200 μM, dCTP 200 μM, dGTP 200 μM, dTTP 200 μM, dUTP 50 μM
[0126] Specific primer tube 1 (100×) Content of each primer 50μM
[0127] Specif...
Embodiment 2
[0152] Relevant virulence gene detection kit, including components such as Example 1, also includes: SS2 DNA control template (DNA ≥ 100ng / μL), source: Streptococcus suis serotype 2 (Streptococcus suis serotype 2) Danish reference strain (Zhejiang University Professor Fang Weihuan) was obtained by extracting DNA by conventional methods.
[0153] PCR specificity test: positive reference strain adopts Streptococcus suisserotype 2 (Streptococcus suisserotype 2) Danish reference strain (i.e. the SS2 DNA control template used in this preferred SS2 virulence gene detection kit); negative reference strain adopts: A) Streptococcus : Streptococcus pneumoniae (Streptococcus pneumoniae, S.pne), beta-hemolytic streptococcus (β-hemolytic streptococcus, S.β-hemo), viridans streptococcus (Streptococcus viridans, S.vi); B) other genera : Neisseria meningitides (Neisseriameningitides, N.m), Staphylococcus epidermidis (S.epi) and Vibrio cholerae (Vibrio cholerae, V.cho), etc., the source of the...
Embodiment 3~7
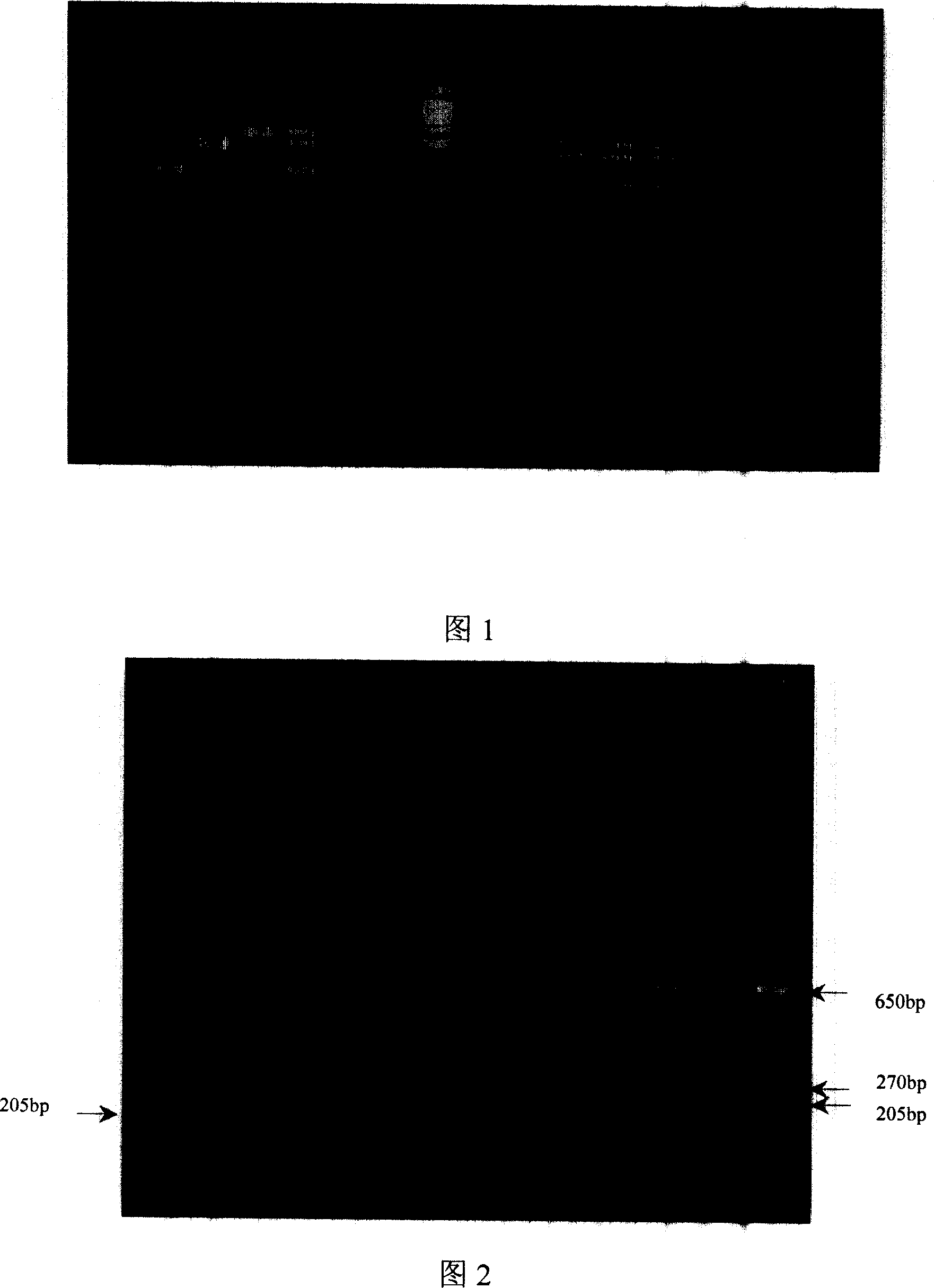
[0155] Embodiment 3~7 adopts live cell counting method to carry out colony counting to SS2 positive reference strain: get the bacterium suspension of 0.5 McFarland unit, do a series of 10 times of dilutions, then quantitative dilutions are carried out plate culture, according to Count the number of colonies cultured and calculate the number of viable bacteria in the culture. Starting from 10 million, serially dilute 10 times to 1 bacterium, apply the above-mentioned kit, and carry out PCR amplification according to the preferred method of the present invention, to detect the detection ability of the kit used for the sample. The results showed that when specific bands appeared in all 8 target gene fragments, the PCR sensitivity was 78 CFU / reaction. When the DNA template was lower than 7.8 CFU, some bands were not obvious (see Table 4, Figure 1).
[0156] Table 4: PCR Sensitivity Test ×
[0157] Target gene (bp)
[0158] × Note: The strain uses SS2 positive referen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com