Method for measuring nitrofuran antibiotics in cosmetics
A technology of nitrofuran and determination method, which is applied to measurement devices, instruments, scientific instruments and other directions, can solve the problems of auditory hallucinations, hallucinations, irritability, protracted and difficult to heal, etc., achieves accurate determination method, high specificity and sensitivity, avoids The effect of false positive results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Instruments and reagents
[0037] Agilent1200 liquid chromatograph (with diode array detector) (Agilent, USA); ultraviolet-visible spectrophotometer (SHIMADZU UV-3600); electronic balance (AB204-S, METTLER TOLEDO, USA); ultrapure water device (Milli- Q, Millipore Company of the United States); high-speed refrigerated centrifuge (CR21G, Hitachi Company of Japan); MS2 vortex oscillator (IKA Company of Germany); nitrogen blowing instrument (N-EVP112, Organomation Associates Inc of the United States); fume hood (FC- 150, Dalian Labtech Laboratory Equipment Co., Ltd.); microporous membrane (0.22 μm, organic system, Pall Company, USA); methanol and acetonitrile were chromatographically pure (Fisher Company); ammonium acetate was analytically pure (content ≥ 98.0% , Shantou Xilong Chemical Co., Ltd., Guangdong Province)
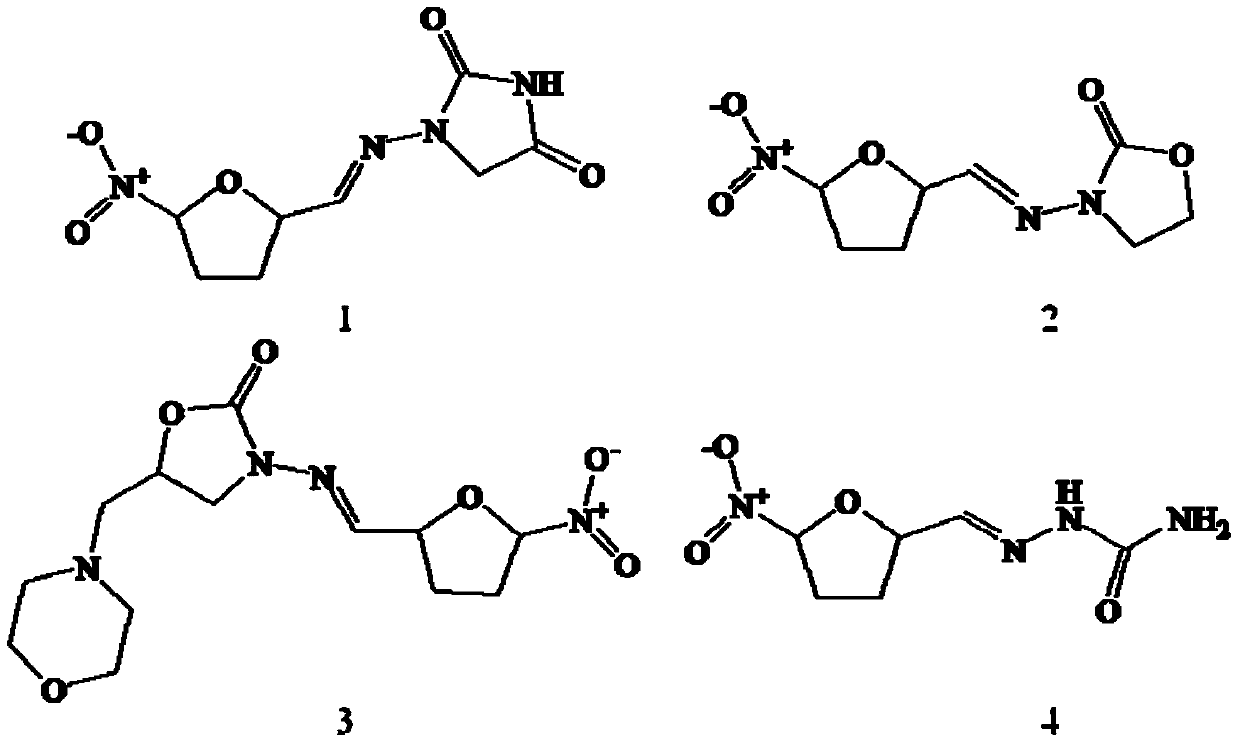
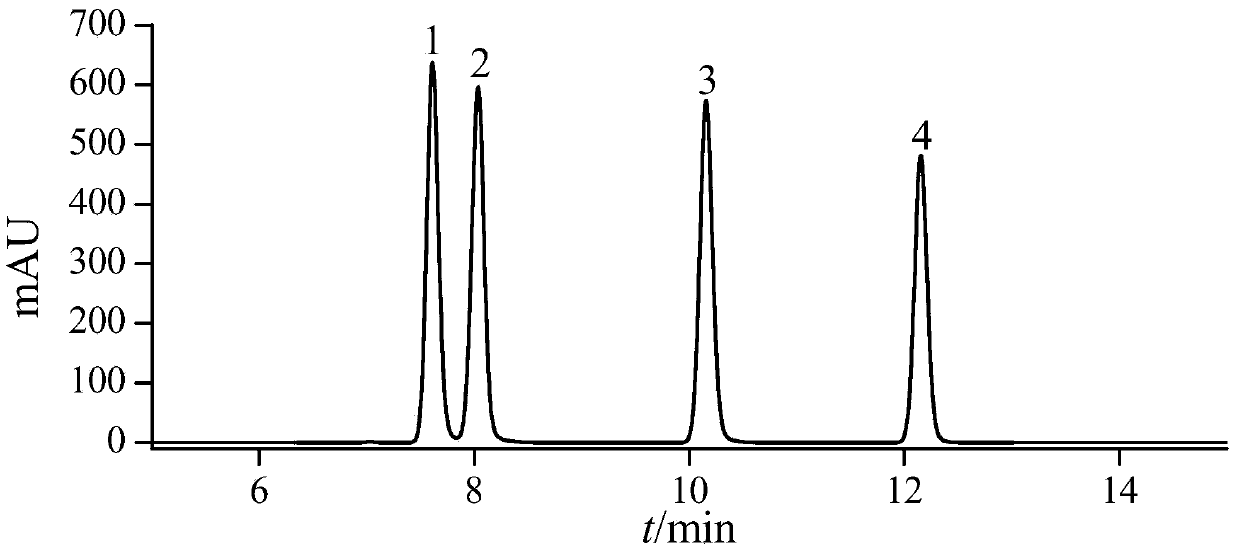
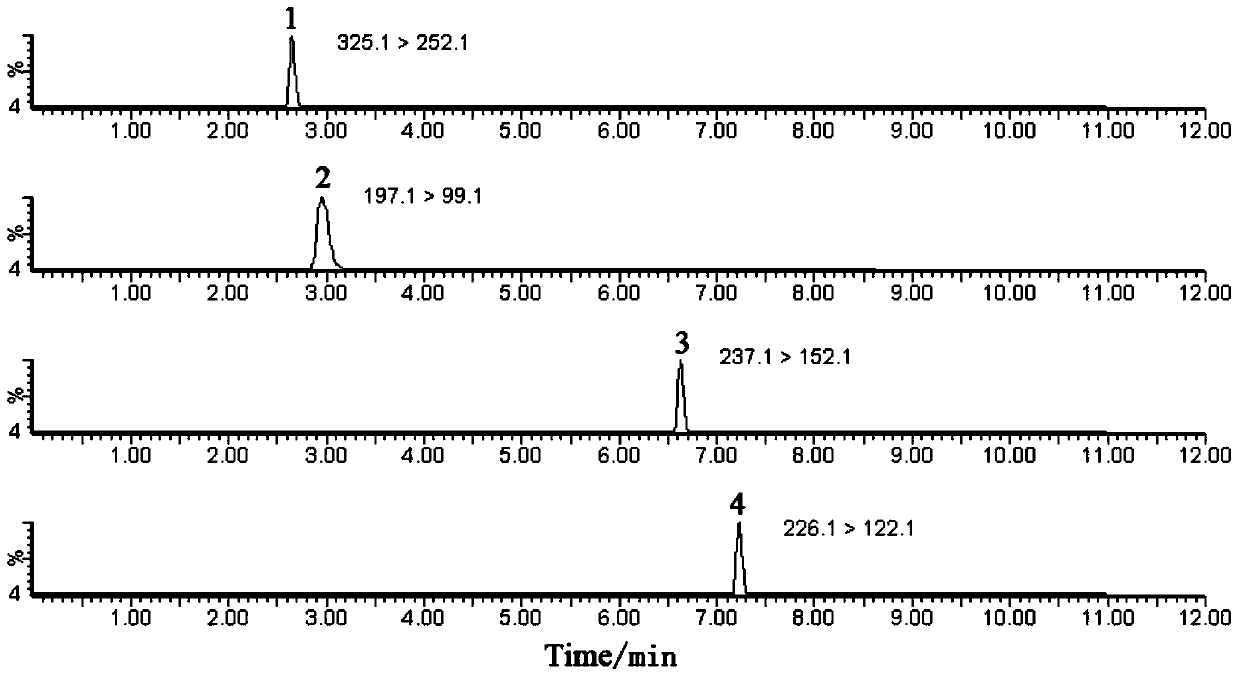
[0038]See Table 4 for detailed information on the four substances of nitrofurantoin, furazolidone, furaltadone and nitrofurazone, and see the structural ...
Embodiment 2
[0079] Preparation of standard solution and investigation of its stability
[0080] During the preparation of the standard solution, according to the operating requirements in the literature, when the 1mg / mL storage solution was prepared, it was found that it was difficult to completely dissolve the nitrofurazone standard with a common single organic solvent (acetonitrile and methanol). In the experiment, acetonitrile and methanol were used. The mixed solution in the ratio between was used as the solvent to dissolve the substance, and it was found that when 20% methanol-acetonitrile was used, the compound could be completely dissolved.
[0081] Since the nitrofuran itself is unstable, it will be partially degraded and lost under the conditions of high temperature, air exposure and direct sunlight. Therefore, this experiment investigated the chromatogram changes of the four compounds under the conditions of direct sunlight for 1h, 2h and 3h. ,Such as Figure 4 As shown, the ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com