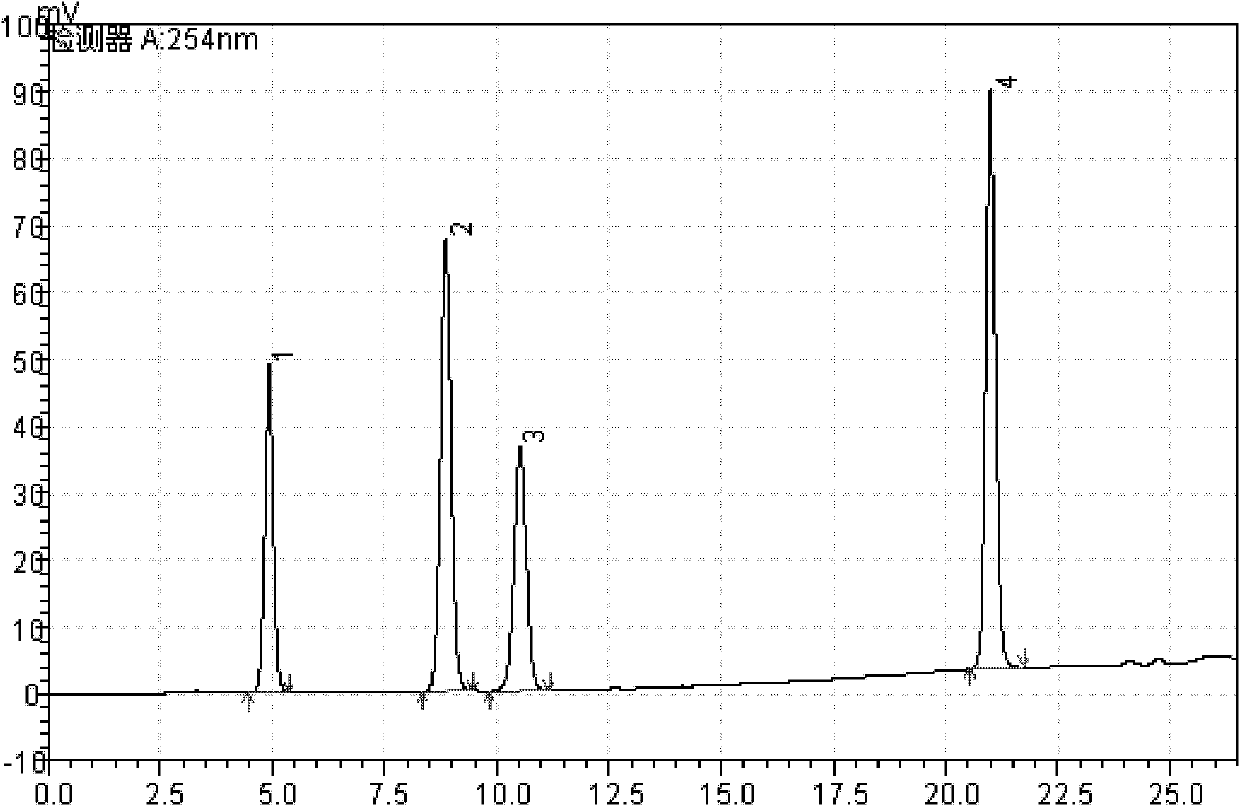
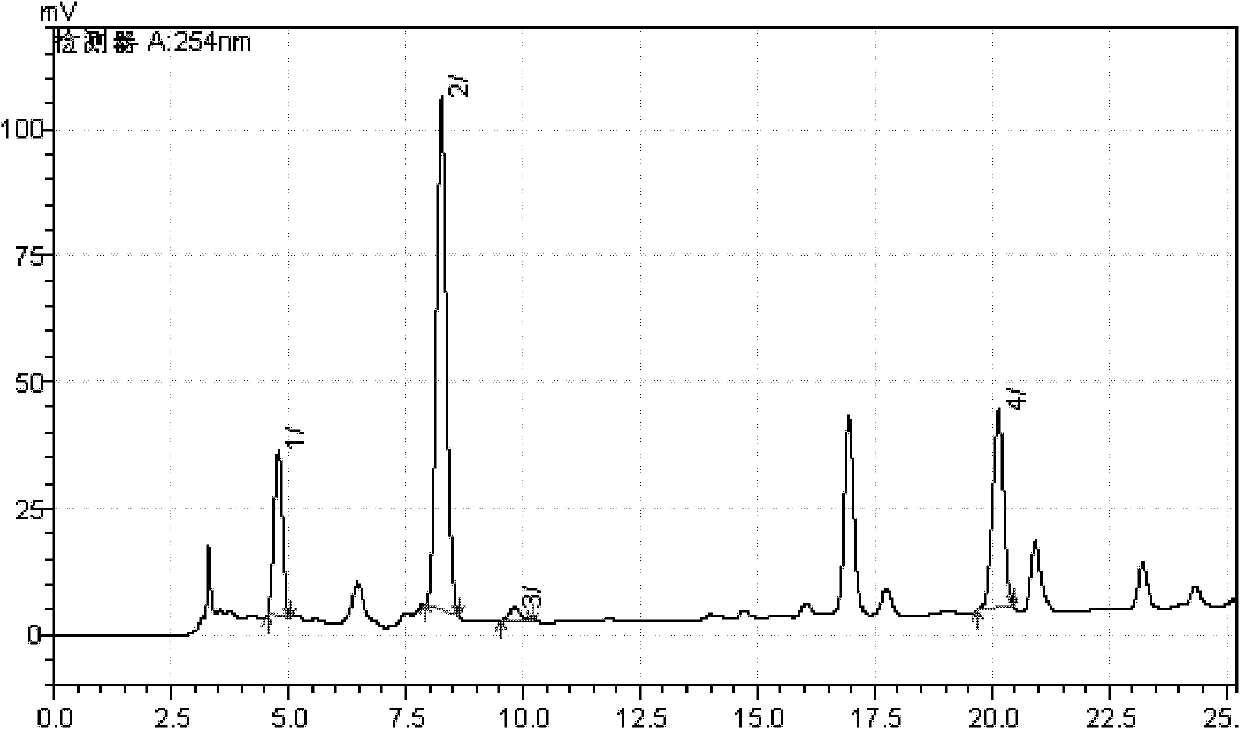
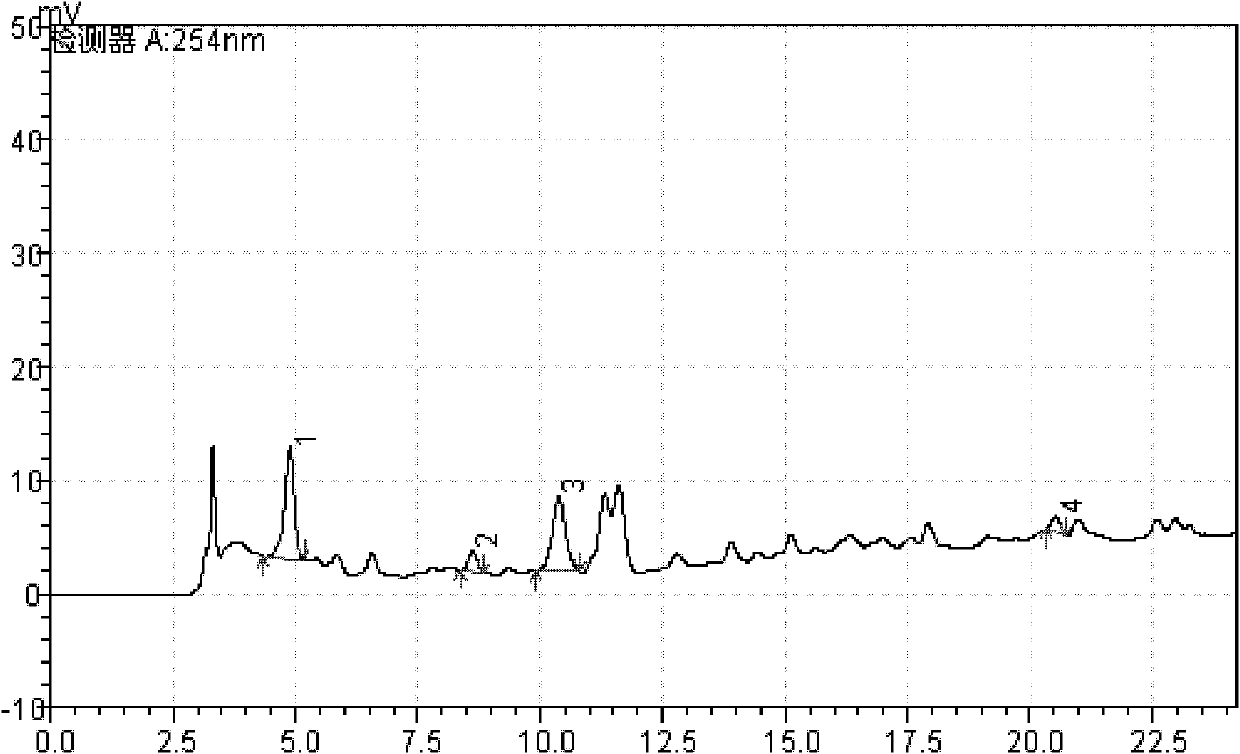
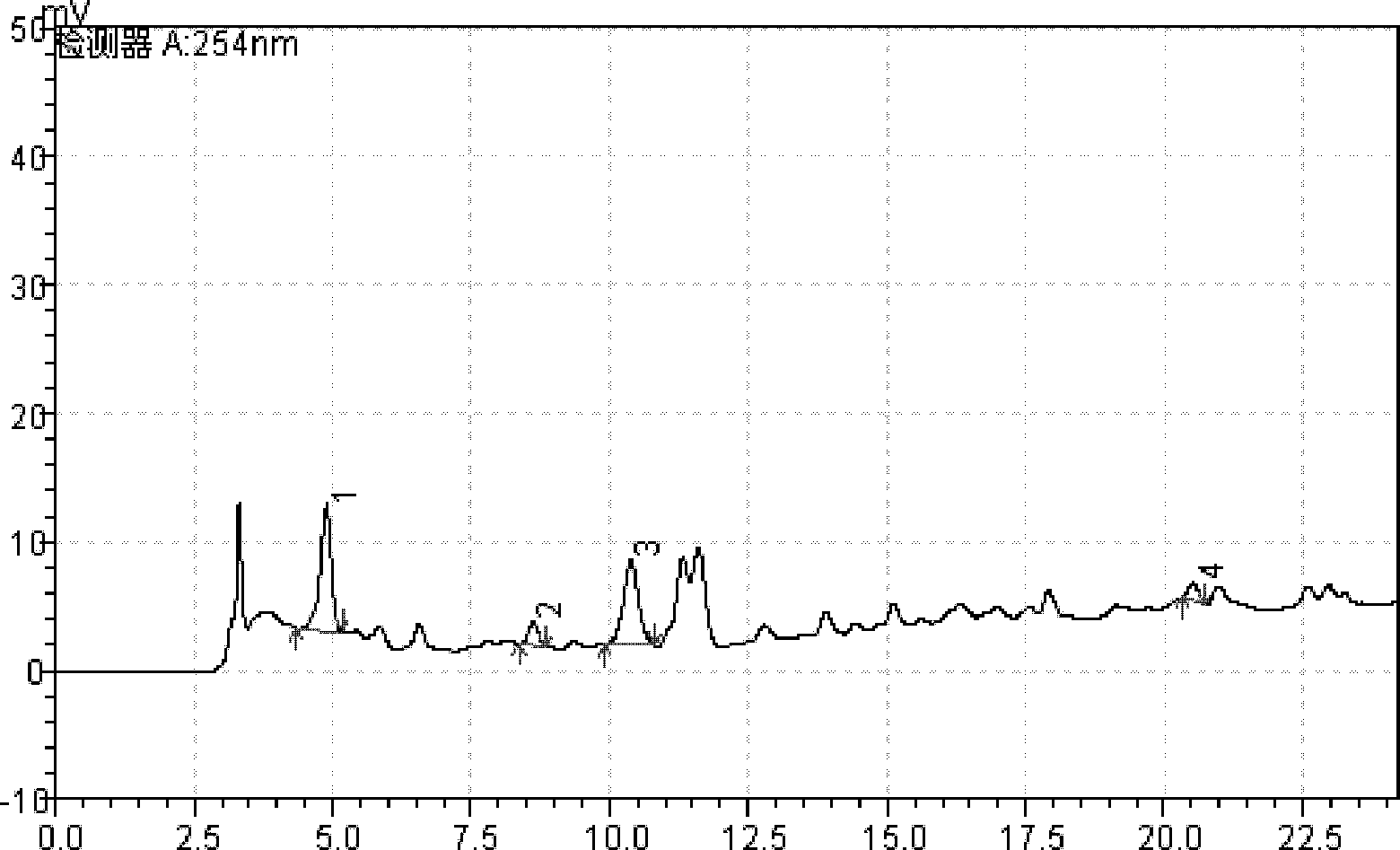
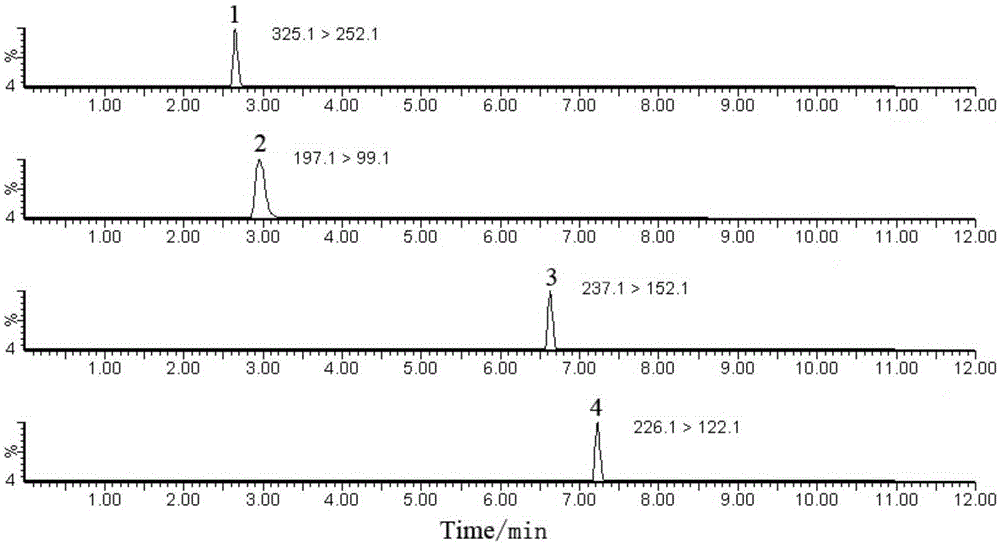
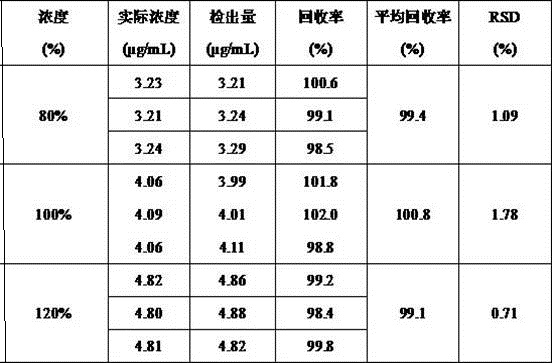
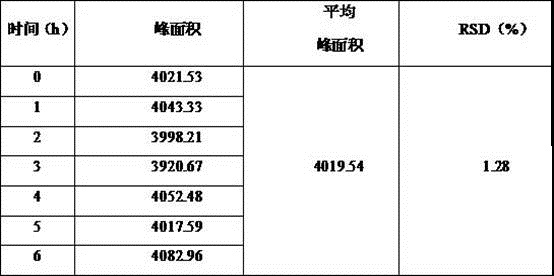
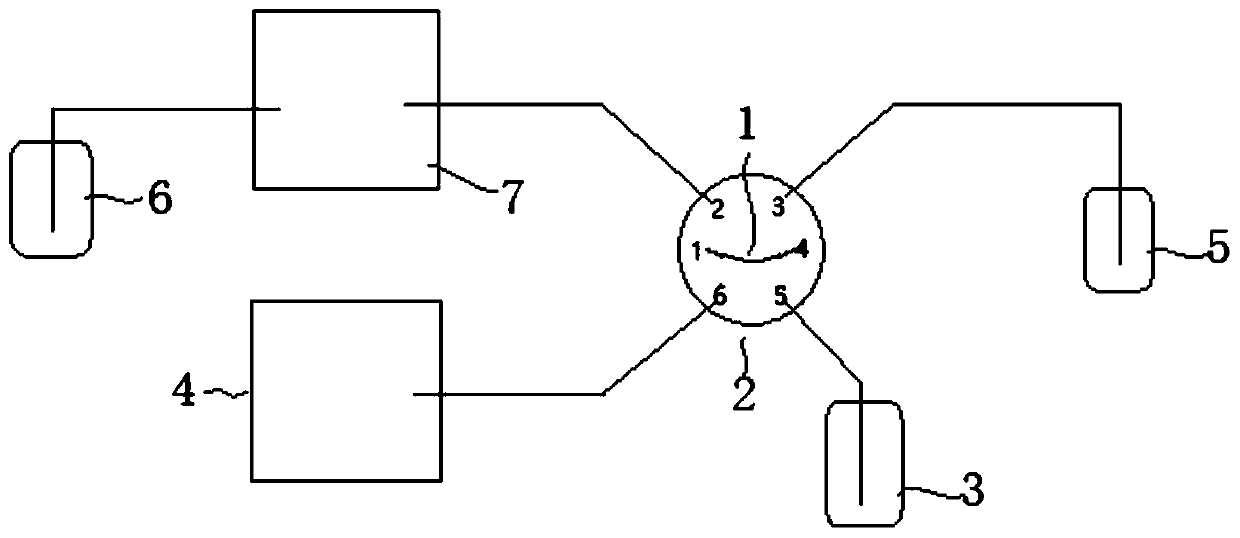
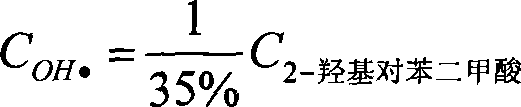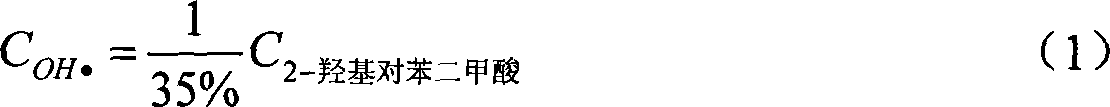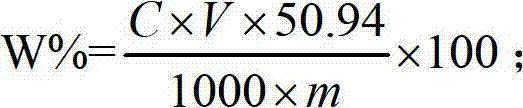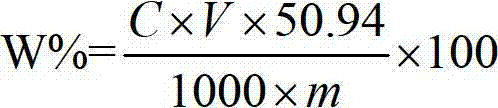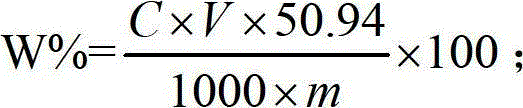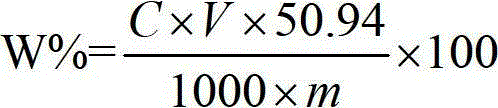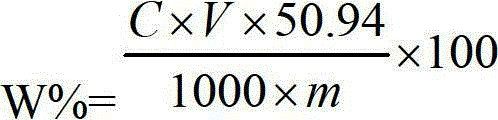Patents
Literature
32results about How to "The determination method is accurate and reliable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydroxy free radical concentration determination method
InactiveCN101241076AOvercome the limitations of dissolutionThe determination method is simpleFluorescence/phosphorescenceSpecial data processing applicationsSpectrofluorometerHydroxyl radical
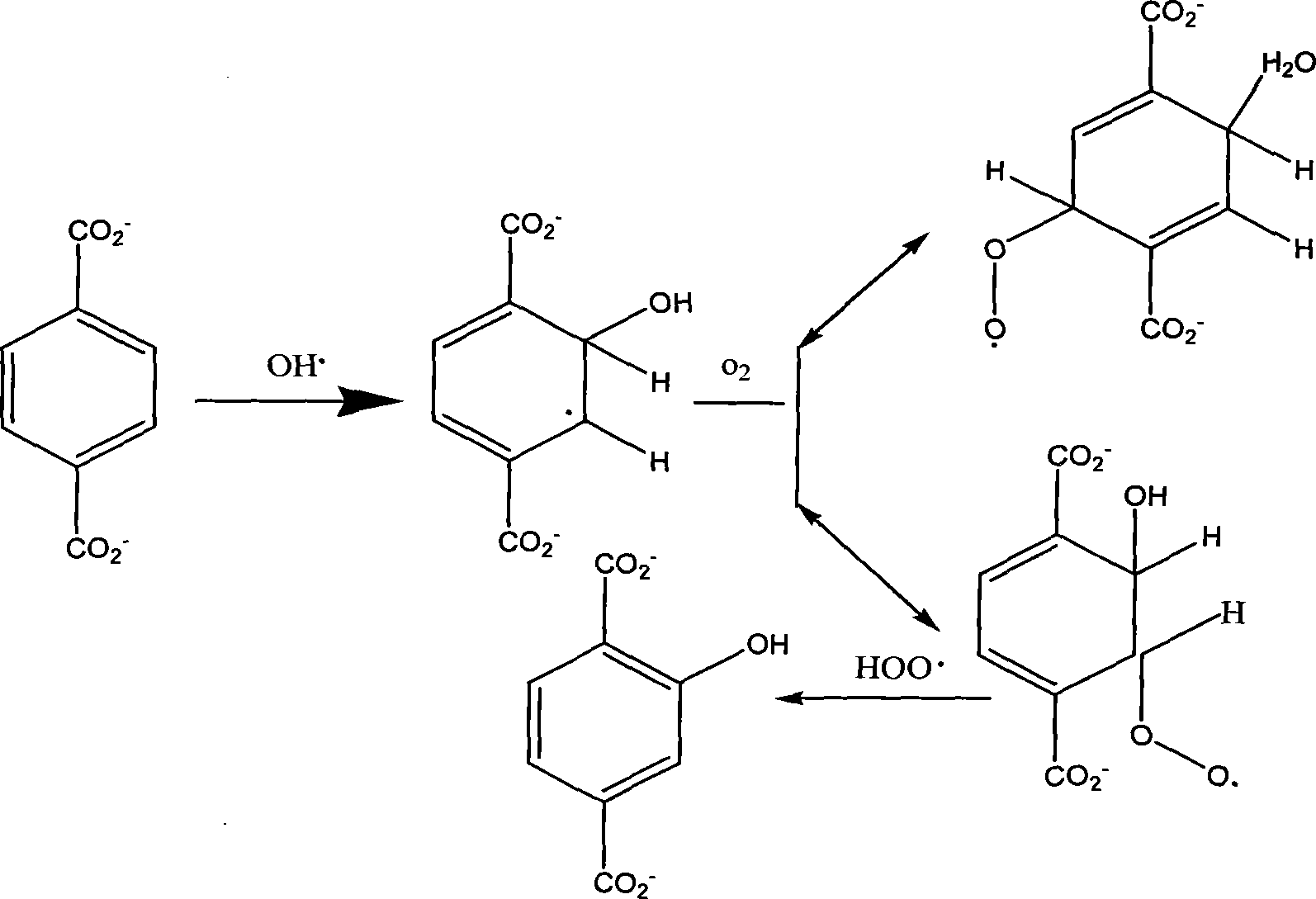
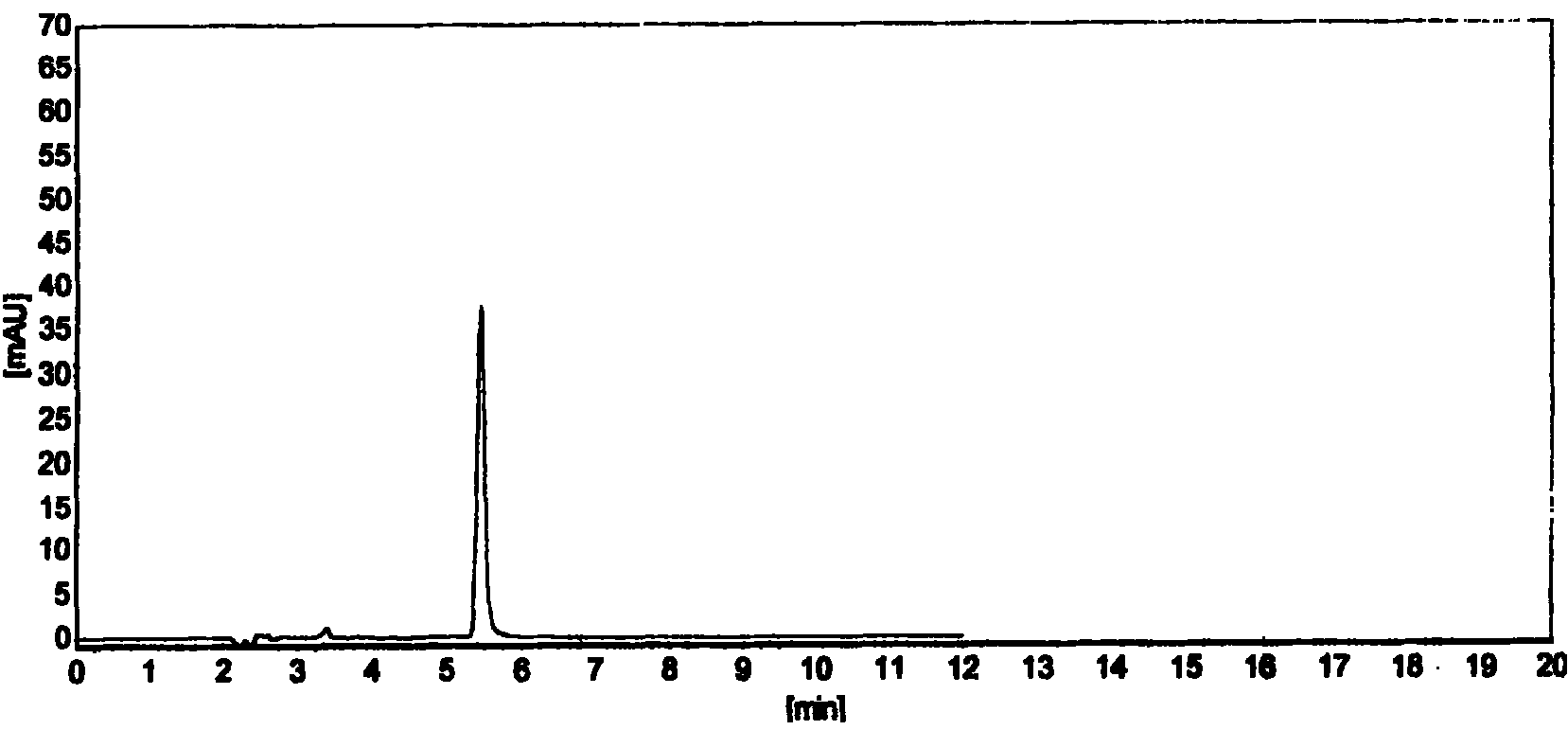
The present invention provides a method for detecting concentration of hydroxyl radical. In the system produced by adding capture agent terephthalic acid sodium salt (NaTA) into hydroxyl radical, 2-hydroxyl terephthalic acid (HTA) is produced by reaction of terephthalic acid sodium salt and hydroxyl radical, which has stable fluorescence and is detected by fluorescence spectrophotometer (excited wavelength lambada ex=315 nm, projecting wavelength lambada em=425 nm), and then the concentration of 2-hydroxyl terephthalic acid is conversed to the concentration of hydroxyl radical. The detecting method of present invention is easy to do, credibility and nicety, especial for used in the conditions of circumstance produced hydroxyl radical at the value of pH<12, or the solution of the hydroxyl radical produced system required the conductivity.
Owner:JIANGSU UNIV
Method for detecting free fatty acid content in compound traditional Chinese medicine
The invention discloses a method for detecting the free fatty acid content in a compound traditional Chinese medicine. By the method, the free fatty acid content in the compound traditional Chinese medicine is measured by high performance liquid chromatography; and a chromatographic signal is detected by an ultraviolet detector. Furthermore, the invention relates to a quality detection method for any one or more than one of linoleic acid, linolenic acid, palmitoleic acid, oleic acid, palmitic acid, stearic acid and palmitic acid. The method has the characteristics of high stability, high precision, wide linear range, wide application range and the like.
Owner:SHINEWAY PHARMA GRP LTD
Method for measuring nitrofuran antibiotics in cosmetics
ActiveCN103926340AThe determination method is accurateHigh sensitivity and specificityComponent separationChemistryMass spectrum analysis
The invention discloses a method for measuring nitrofuran antibiotics in cosmetics. The method comprises the following steps: (1) pretreating a sample, namely adding the sample into a mixed solution of acetonitrile and methanol in a volume ratio of 70:30, uniformly mixing, performing ultrasonic extraction, centrifuging, airing supernatant till the supernatant is nearly dry, dissolving residues in a mixed solution of a 15mmol / L ammonium acetate solution and acetonitrile according to a volume ratio of 85:15, and sieving the solution through a micro-porous filter membrane with the size of 0.22 micron, thereby obtaining filtrate for later use; (2) performing high performance liquid chromatography separation on the filtrate, eluting a mobile phase by a gradient elution program, and quantifying according to an external standard method; and (3) confirming a detected positive sample by high performance liquid chromatography-mass spectrometry / mass spectrometry. According to the method, four nitrofuran antibiotics such as macrodantin, furazolidone, furaltadone and nitrofurazone are simultaneously detected. The method is accurate, reliable and high in sensitivity.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
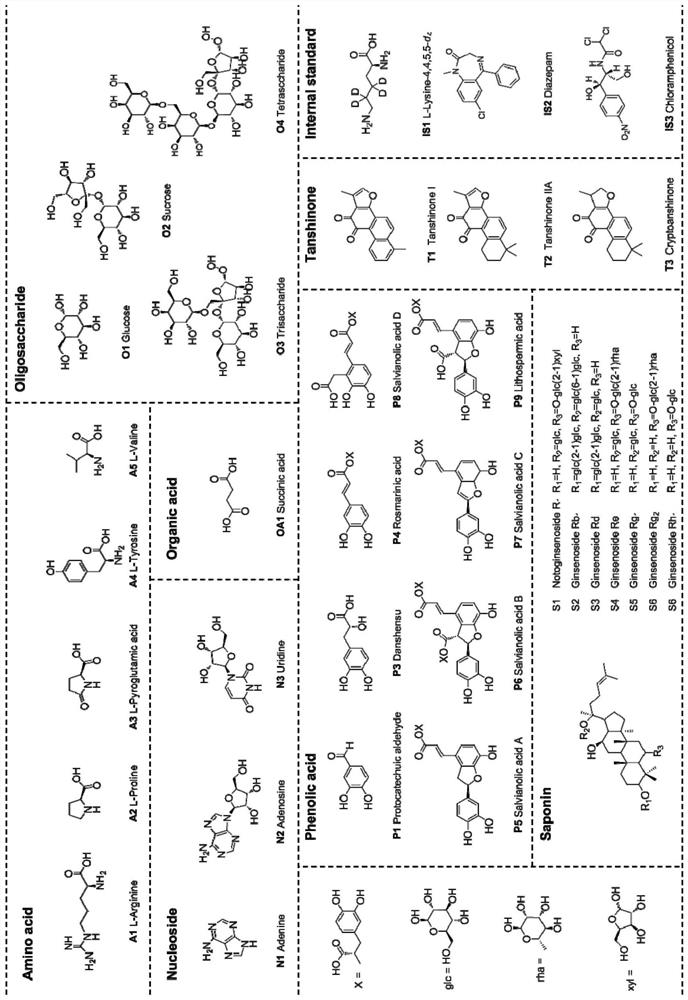
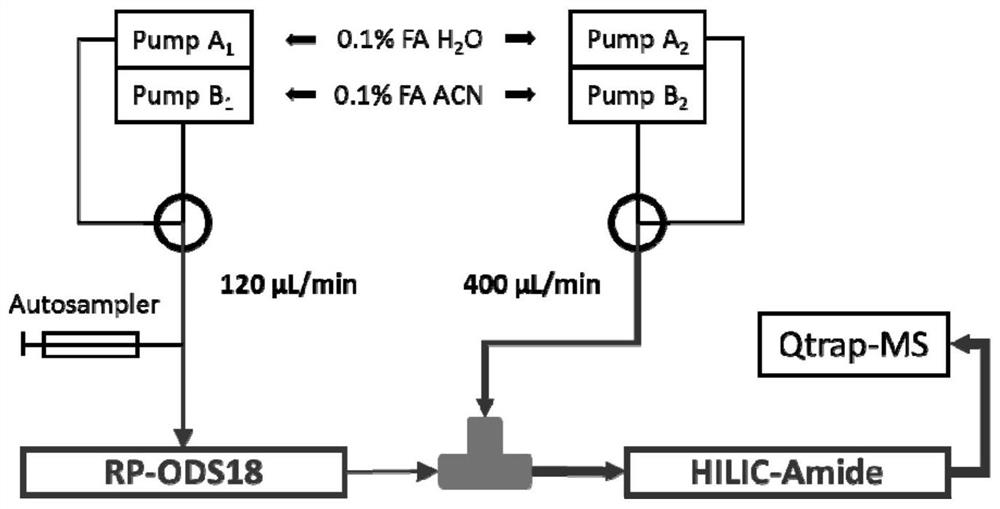
Method for measuring 31 components in compound radix salviae miltiorrhizae extract or related medicinal materials simultaneously
ActiveCN107991399AReliable methodThe determination method is accurate and reliableComponent separationMedicinal herbsAnalyte
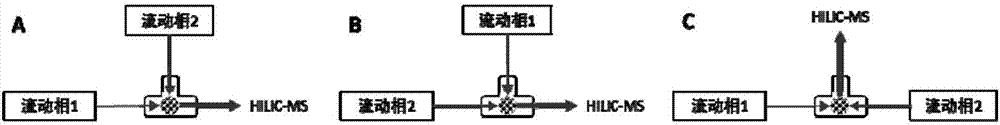
The invention relates to a method for measuring 31 components in compound radix salviae miltiorrhizae extract or related medicinal materials simultaneously. The method adopts a direct series-reverse phase-hydrophilic interaction chromatography mass spectrometer combination method and comprises steps as follows: step one, preparing a sample solution; step two, preparing series of standard solutions; step three, preparing an internal standard solution; step four, preparing a solution for test products; step five, injecting the solution obtained in the step four into a direct series-reverse phase-hydrophilic interaction liquid chromatography mass spectrometer combination system so as to obtain an extracted ion flow diagram of 31 analytes, and calculating content of the 31 components in the compound radix salviae miltiorrhizae extract or the related medicinal materials according to the extracted ion flow diagram.
Owner:TIANJIN TASLY PHARMA CO LTD
Method for determining vanadium in silicon-vanadium alloy
ActiveCN102928425ASolve technical problemsSolve the defect of low measurement resultsMaterial analysis by observing effect on chemical indicatorHydrofluoric acidAmmonium ferrous sulfate
Owner:RUI STEEL INDAL OF PANZHIHUA GANGCHENG GROUP
Method for simultaneously measuring content of multiple active ingredients in eucommia ulmoides
InactiveCN102890126AImprove detection efficiencyThe determination method is accurateComponent separationInjection volumeChlorogenic acid
The invention discloses a method for simultaneously measuring the content of multiple active ingredients in eucommia ulmoides. The method adopts high performance liquid chromatography for analysis; the chromatographic condition is taking octadecylsilane bonded silica as a filler; the mobile-phase gradient elution process is: the mobile phase A is methanol, and the mobile phase B is an aqueous solution of phosphoric acid with mass concentration of 0.1%; the content of the mobile phase B is increased from 30% to 70% by mass according to the first-order linear gradient in 0.01-30.00min, reduced to 30% in 30.00-30.01min and kept at 30% in 30.01-40.00min; the flow rate is 0.8ml / min; the column temperature is 30 DEG C; the sample injection volume is 5mul; the detection wavelength detection process is: the 254nm wavelength is selected in 0.01-7.00min, the 277nm wavelength is selected in 7.00-12.50min, and the 254nm wavelength is selected in 12.50-40.00min; and multiple active ingredients include geniposidic acid, chlorogenic acid, pinoresinol diglucoside and rutin. According to the invention, the method is verified by linear relation survey, stability test, precision test, reproducibility test and sample injection recycling test, and the analysis method is proved to be accurate and reliable.
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
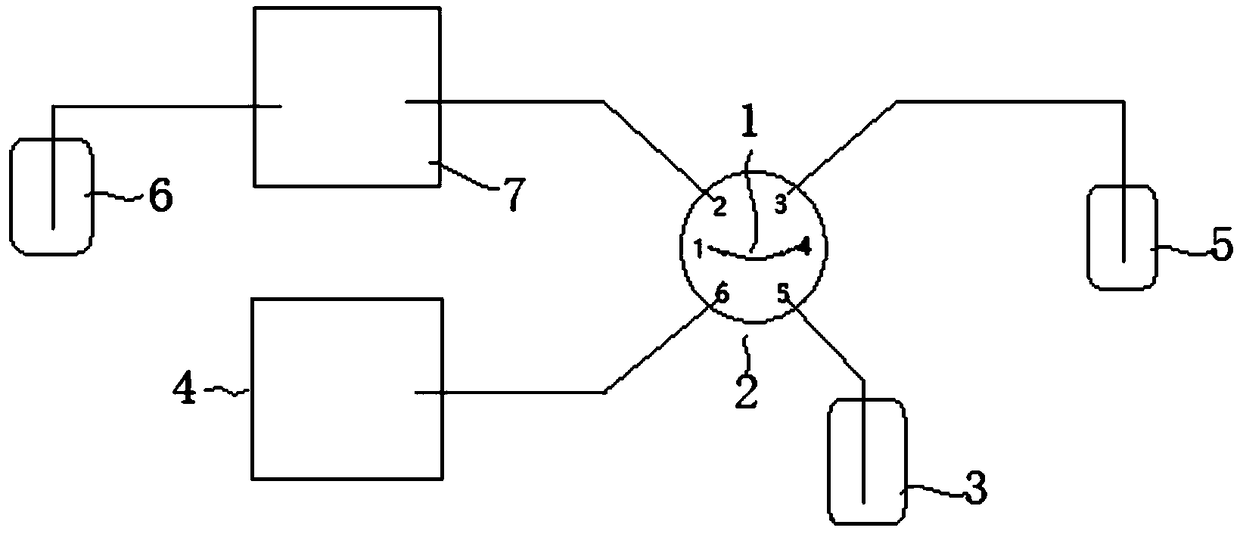
Ion chromatograph sample injection volume measurement device and sample injection volume measurement method
ActiveCN109030704AThe determination method is accurate and reliableHigh measurement accuracyVolume measurement apparatus/methodsComponent separationPhysicsIon
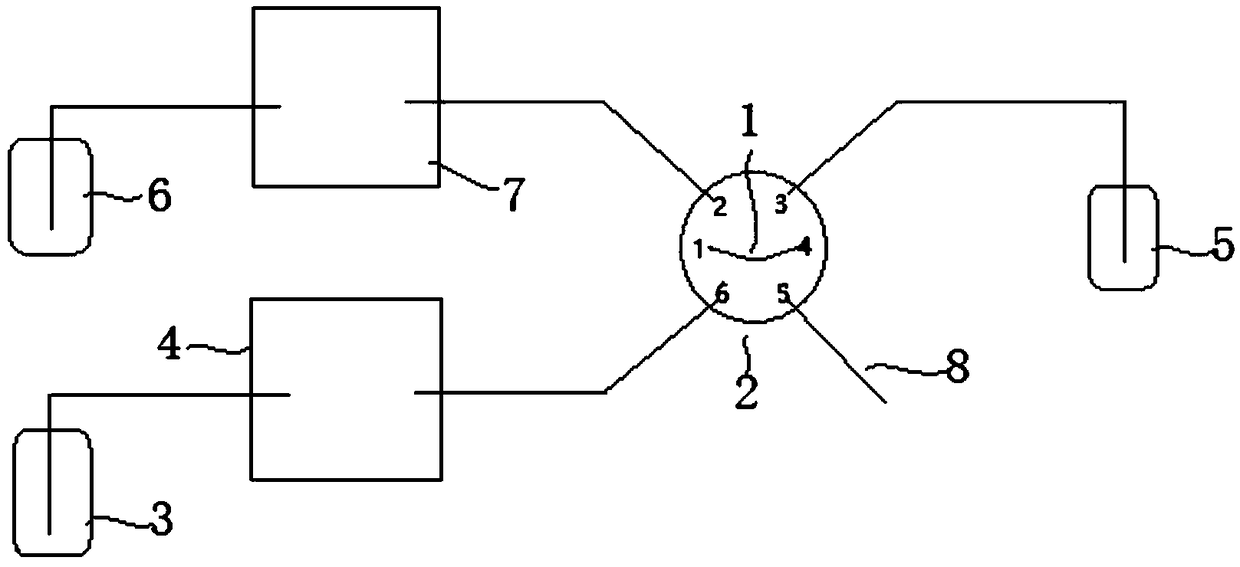
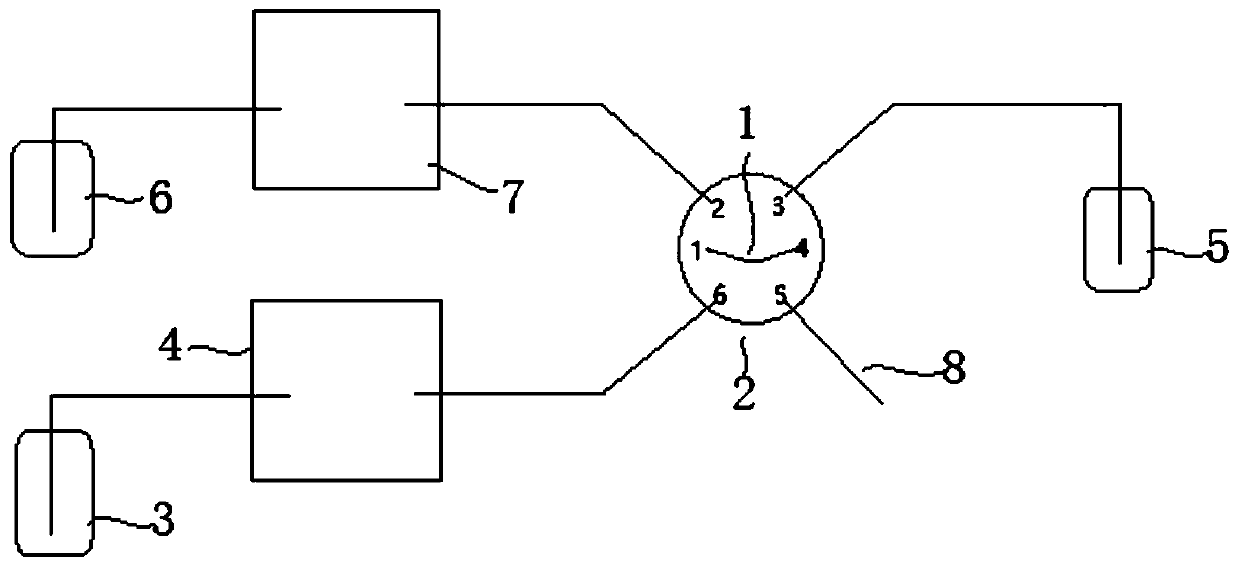
The invention belongs to an ion chromatograph sample injection volume measurement device and a sample injection volume measurement method, and aims at accurately measuring sampling volumes in full quantitative loop sample injection and partial quantitative loop sample injection of ion chromatographs. According to the ion chromatograph sample injection volume measurement device and the sample injection volume measurement method, a sample injection valve is utilized to ensure that inorganic salt solution and a quantitative loop, and flushing fluid and the quantitative loop are communicated withan ion chromatograph of a measurement solution passage or a flushing channel technical scheme in turn to carry out sample injection volume measurement; and by using the sample injection volume measurement method of the device, the technical problems that in existing ion chromatograph sample injection volume measurement devices and sample injection volume measurement methods, the gas flow rate is low to cause the fact that residual liquid exists in quantitative loops, the gas flow rate is high to cause the fact that certain liquid is brought away, and the measured liquid is easy to evaporate tocause loss and then influence the measurement correctness.
Owner:QINGDAO SHENGHAN CHROMATOGRAPH TECH CO LTD
Method for detecting newly increased detection components in traditional Chinese medicinal Tang Herb for treating AIDS
The invention provides a method for detecting newly increased detection components in a traditional Chinese medicinal Tang Herb for treating AIDS. The method for detecting newly increased detection components comprises the following steps: 1, discriminating Hedyotis diffusa and Medicine Terminalia Fruit components in the Tang Herb through adopting thin-layer chromatography; and 2, simultaneously detecting gallic acid, chlorogenic acid, liquiritin and picroside II through gradient elution of a mobile phase under same high performance liquid chromatographic conditions. The content of gallic acid in every tablet of Tang Herb is not less than 0.9 mg, the content of chlorogenic acid in every tablet of the Tang Herb is not less than 0.5 mg, the content of liquiritin in every tablet of the Tang Herb is not less than 0.25 mg, and the content of the picroside II in every tablet of the Tang Herb is not less than 0.45 mg. Full-inspection projects confirm that the newly increased detection component detection method can be added to an original detection method in order to further improve the detection kind and the detection method of a medicine and facilitate the batch production determination of the traditional Chinese medicine Tang Herb and the improvement of the product quality. The detection method provided by the invention has the advantages of good stability and reliability, high specificity and large application values.
Owner:SHANGHAI HUNDREDS HERBAL PHARMA
System for automatically monitoring summarized information of underground fluid
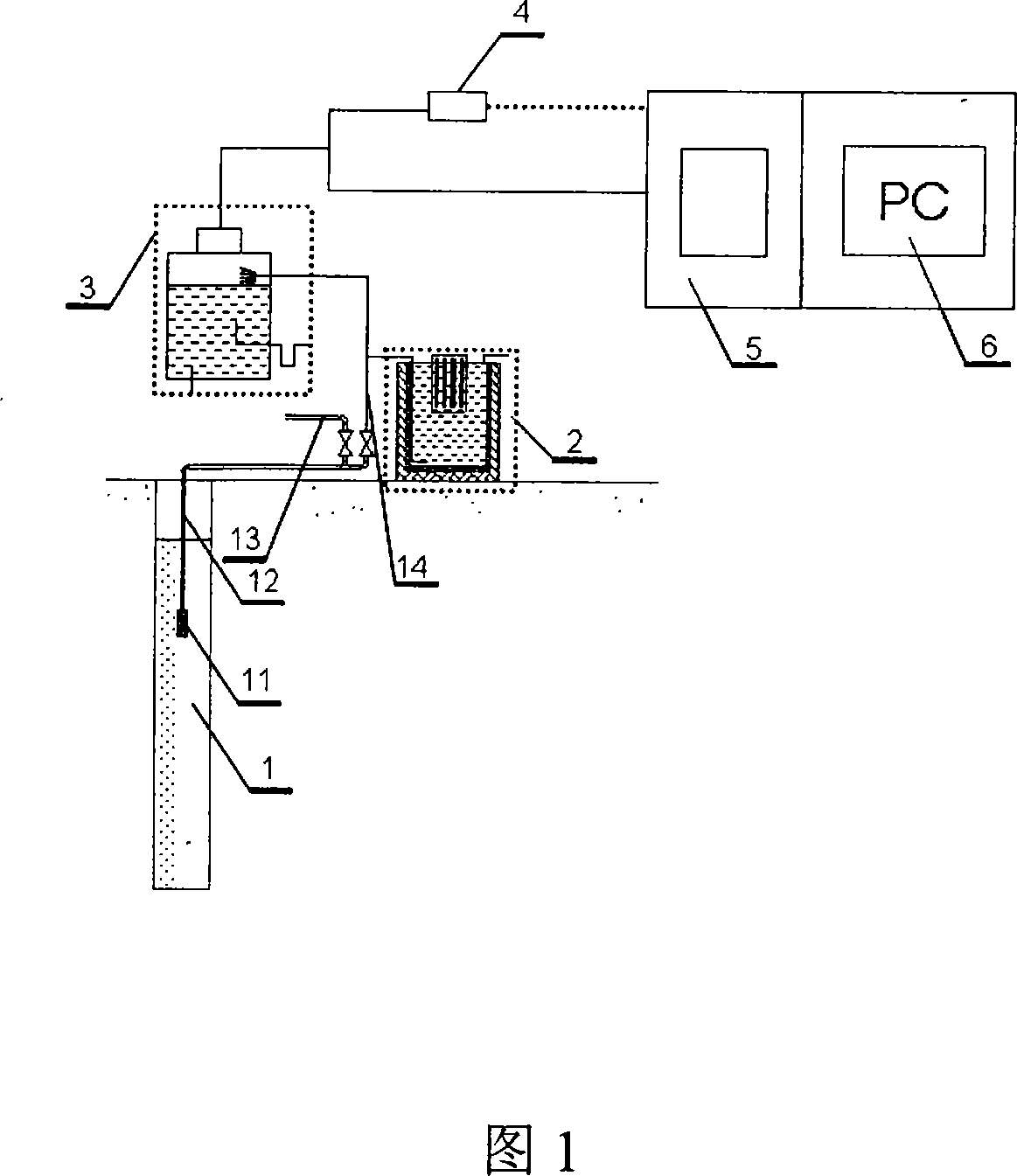
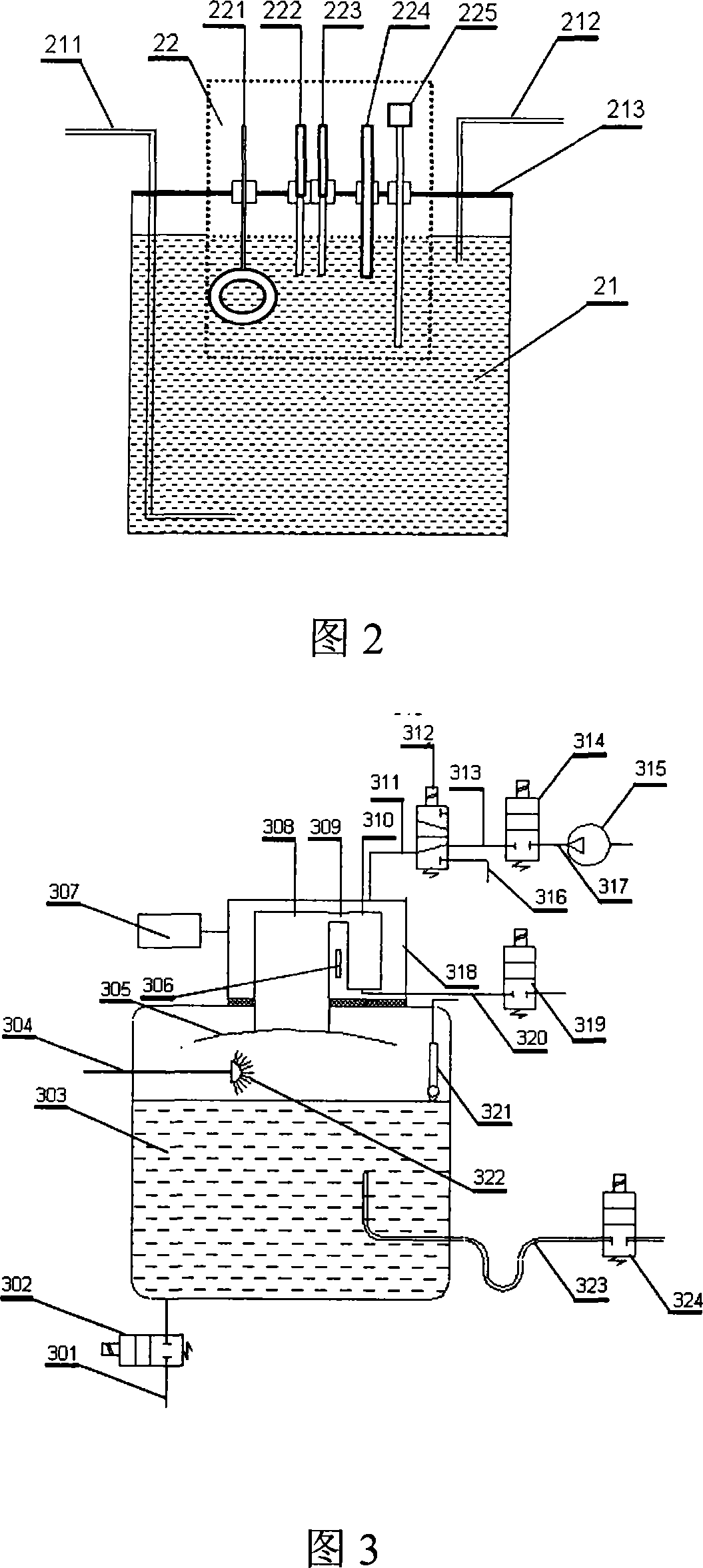
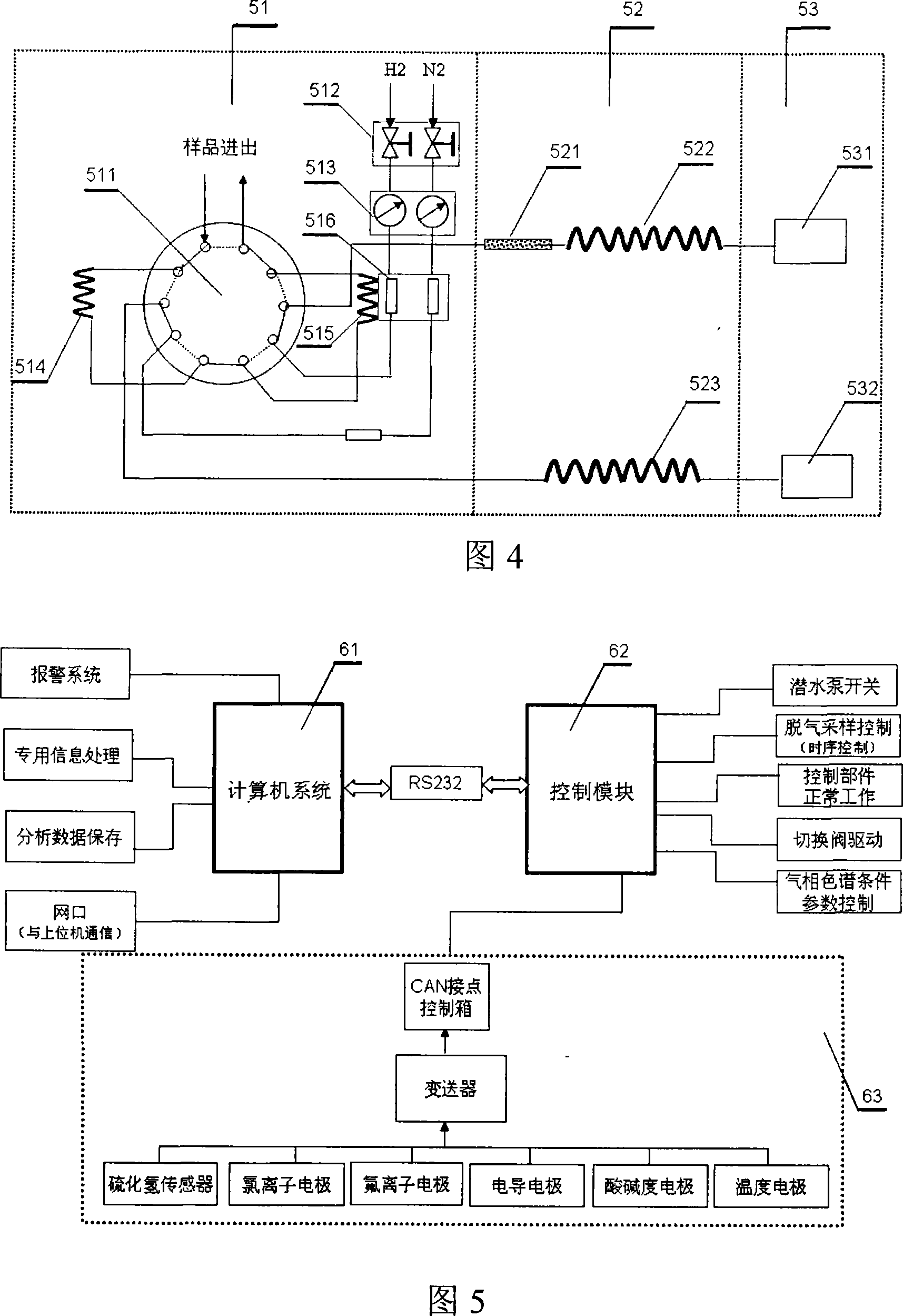
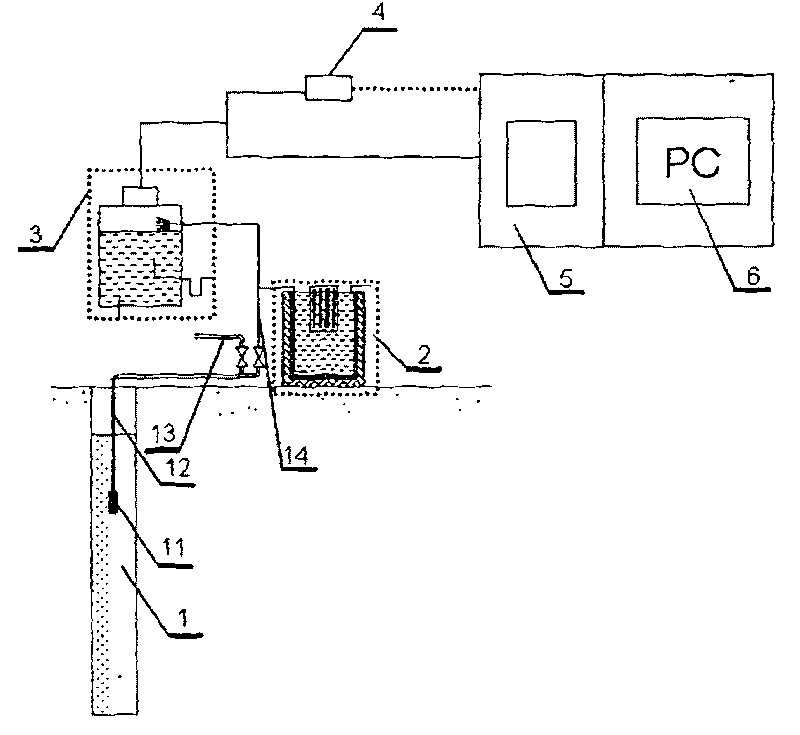
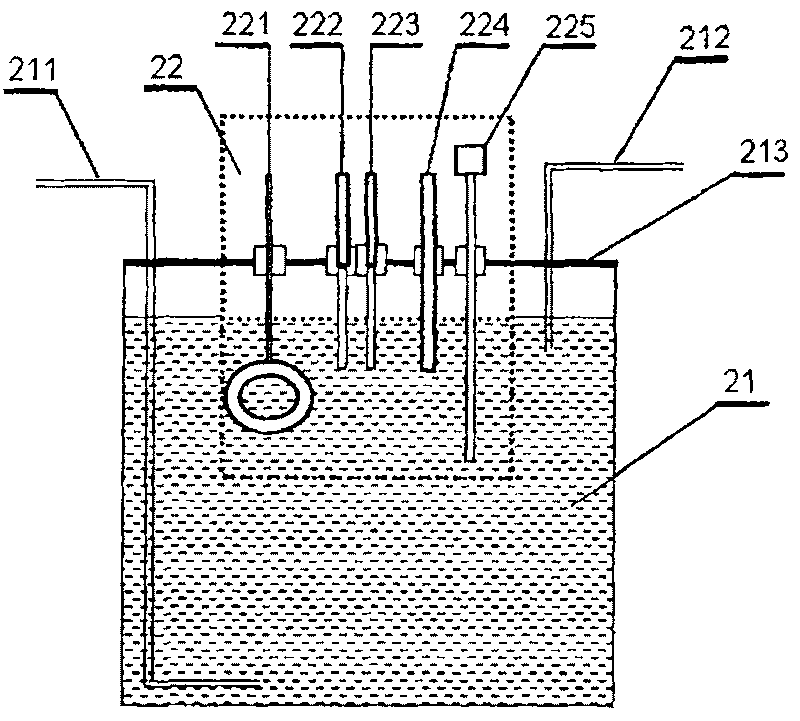
InactiveCN101201404ASolve drying problemsRealize automatic continuous detectionSeismic signal receiversGas phaseCollection system
The invention relates to an automatic synthetic information monitoring system for the underground liquid. The invention is characterized in that the system comprises an underground liquid collection system, a combinational electrode measure system, an automatic degassing and drying system, a sensor system, an automatic gas chromatography analysis system and an intelligent measure control and computer system. The underground liquid collection system is connected with the combinational electrode measure system and the automatic gas escaping and drying system; after being distributed by gas transmission pipes, the automatic gas escaping and drying system is respectively connected with the sensor system and the automatic gas chromatography analysis system; the combinational electrode measure system and the sensor system are connected with the intelligent measure control and computer system in a CAN main line way. The invention has the advantage that the invention is capable of obtaining all kinds of real-time monitoring information from the automatic gas chromatography analysis system, the sensor and the electrode system through one automatic sampling. The information comprises the synthetic information of the underground liquid, such as hydrogen, helium, oxygen, argon, nitrogen, methane, carbon dioxide, sulfur hydrogen, conductance, acidity and alkalinity, chlorine ion, fluorine ion and temperature etc.
Owner:SHANGHAI GASOLINEEUM & CHEM EQUIP +1
Hydrochloric acid cefepime raw material and method for measuring content of N-methyl pyrrolidine in preparation thereof
ActiveCN101226174AImprove balanceShort measurement timeComponent separationCefepime hydrochlorideRetention time
The invention relates to a measurement method of N-methylpyrrolidine content of cefepime dihydrochloride material and relative agent, for evaluating the quality of cefepime dihydrochloride material and relative agent. The invention discloses a liquid chromatograph which comprises using carboxylic cationic column as analysis column, uses a conductive detector to check, preparing flow phase, preparing sample solution and reference substance solution, using test method to respectively inject the sample solution and reference substance solution into a liquid chromatograph, recording high pressure liquid chromatographs, and calculating the N-methylpyrrolidine content of the sample. The inventive chromatograph system has easy balance, short sample test time, better repeatability in preserved time, stable baseline, durable column, high quality of chromatograph peak of N-methylpyrrolidine, better repeatability, accurate, simple and reliable process.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
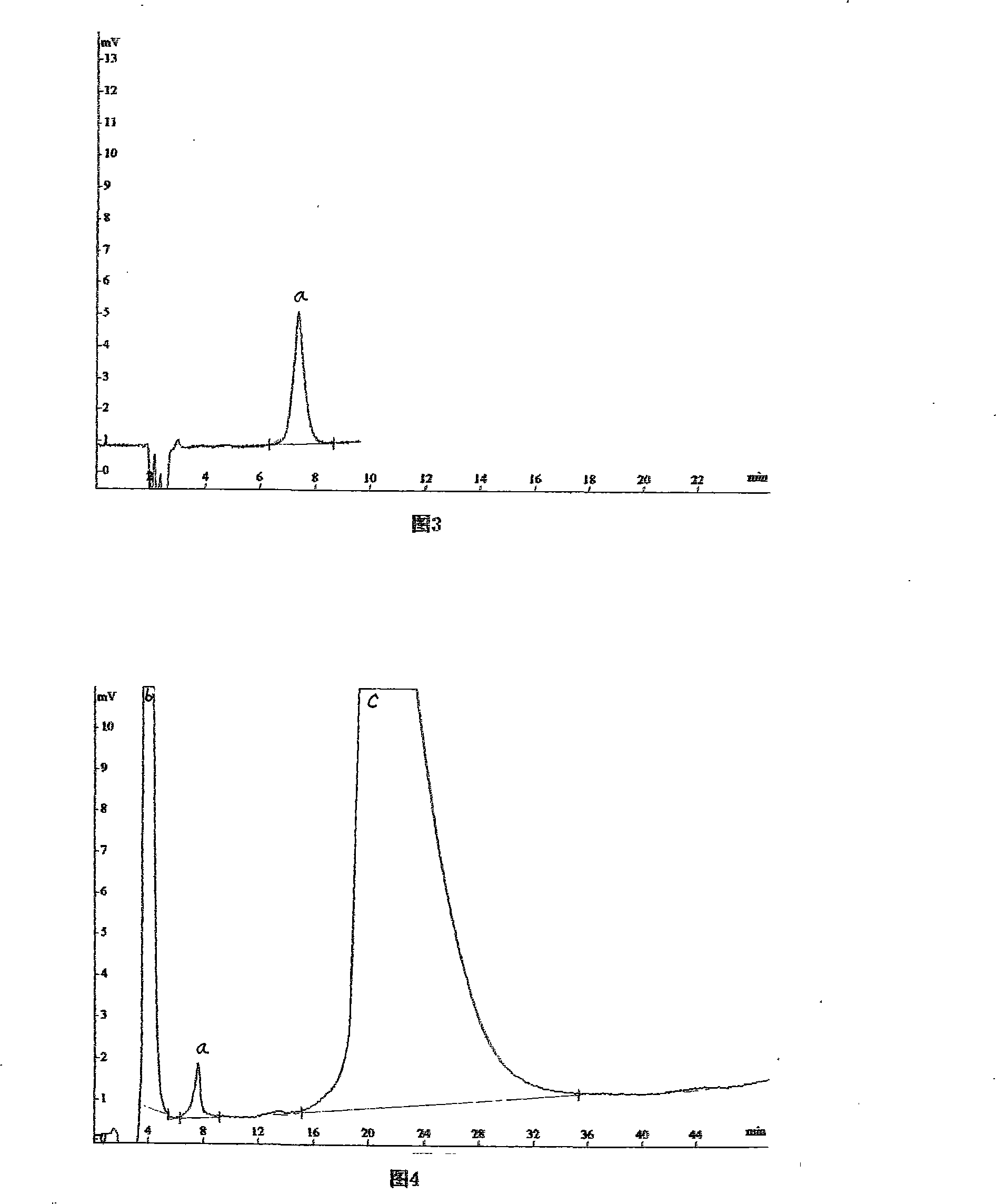
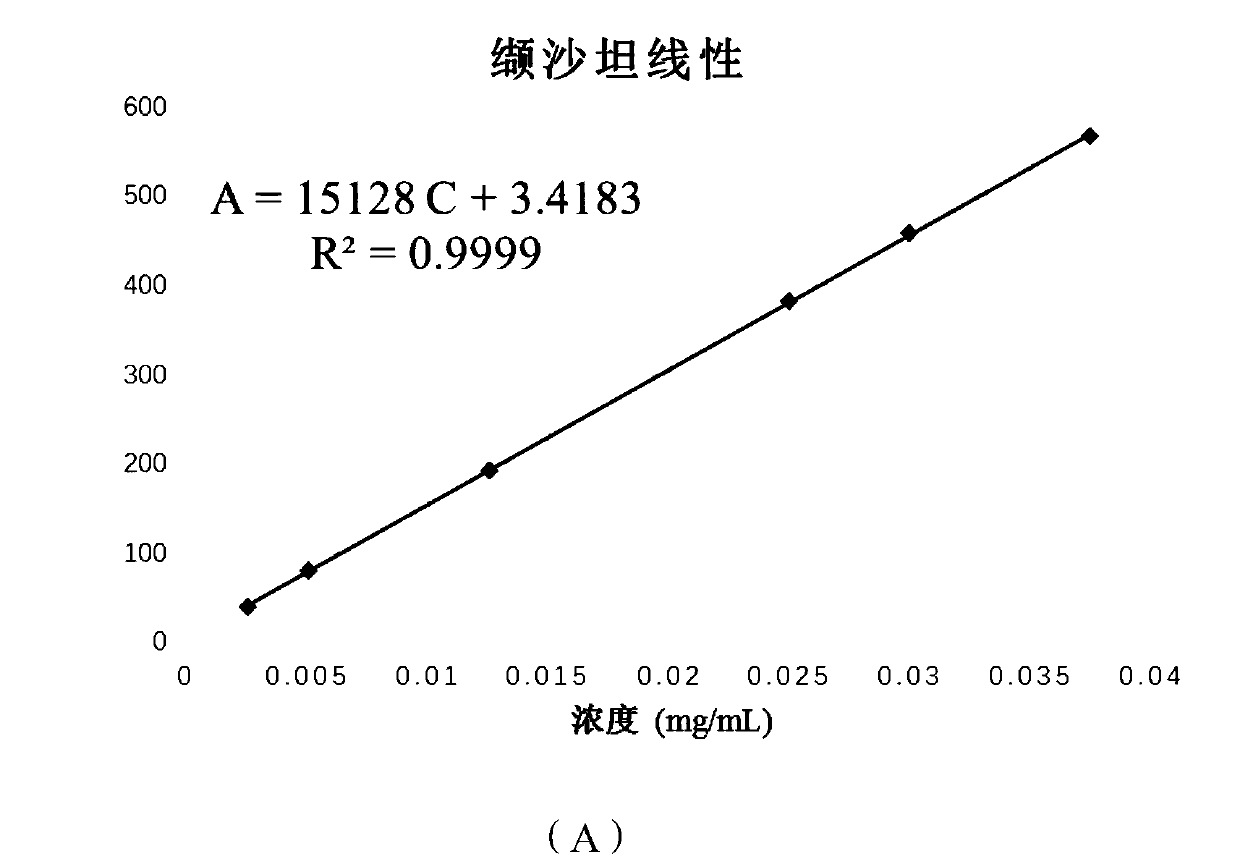
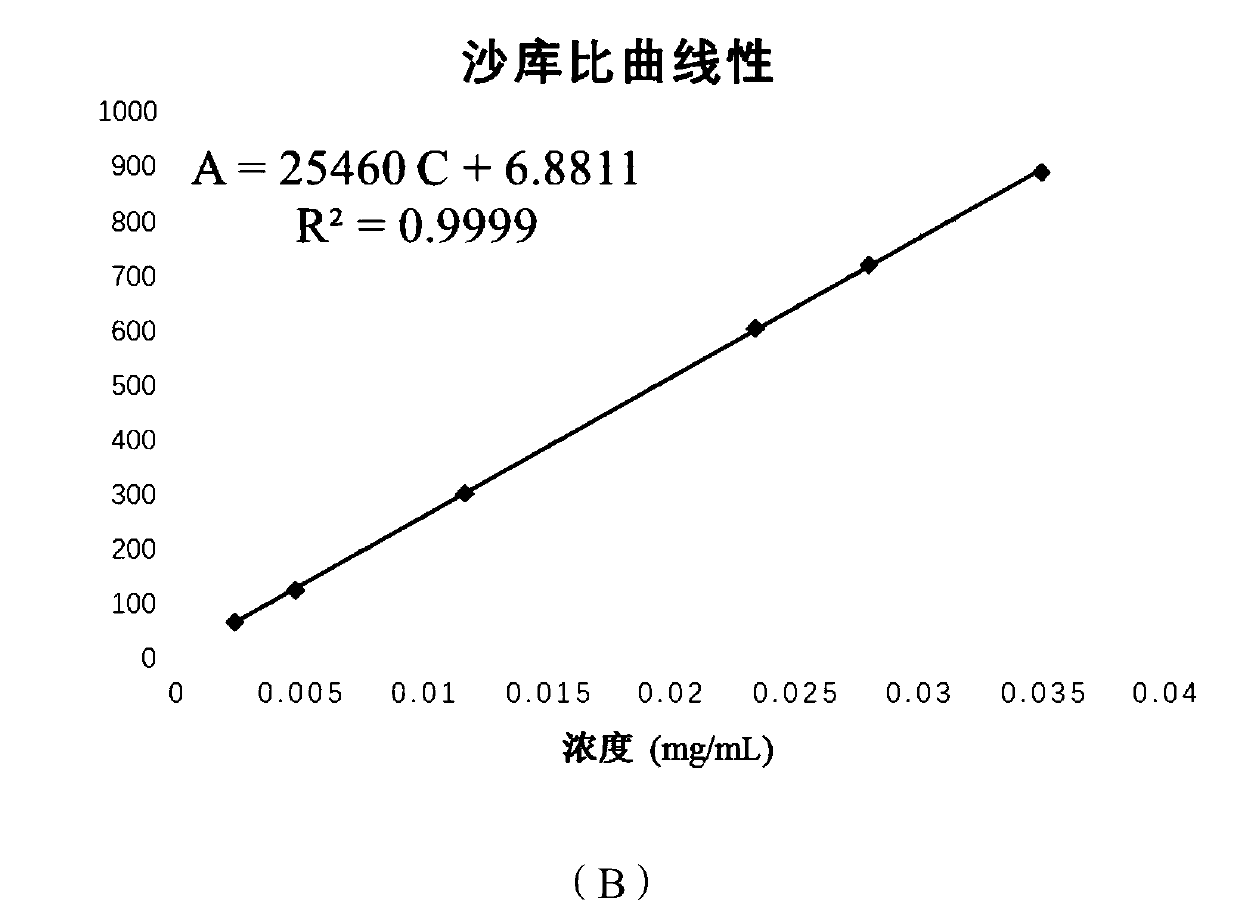
Content determination method for sacubitri valsartan trisodium hemi-pentahydrate capsule effective ingredients
ActiveCN107764910ADetermination method is simpleThe determination method is accurateComponent separationValsartanColumn temperature
The invention discloses a content determination method for sacubitri valsartan trisodium hemi-pentahydrate capsule effective ingredients. The content determination method comprises the following steps: firstly taking an appropriate amount of a sacubitri valsartan trisodium hemi-pentahydrate reference substance to prepare the sacubitri valsartan trisodium hemi-pentahydrate reference substance withthe concentration of 50 micrograms per mL, and taking a sacubitri valsartan trisodium hemi-pentahydrate capsule sample to prepare a sacubitri valsartan trisodium hemi-pentahydrate sample for test withthe concentration of 50 micrograms per mL; then establishing the following chromatographic conditions of a high performance liquid: a C18 chromatographic column (4.6 mm * 250 mm, 5 microns), the moving phase is 0.2% diisopropyl amine water solution-N-methyl pyrrolidone, the detection wavelength is 250 nm, the flow velocity is 0.8 mL / min, the column temperature is 30 DEG C, and the sample size is10 microliters; finally accurately absorbing the reference substance and the sample solution for test into a liquid chromatograph for determination respectively. According to the content determinationmethod for sacubitri valsartan trisodium hemi-pentahydrate capsule effective ingredient disclosed by the invention, the determination method is convenient, accurate and reliable, and the quality of the product can be really reflected.
Owner:HUAQIAO UNIVERSITY
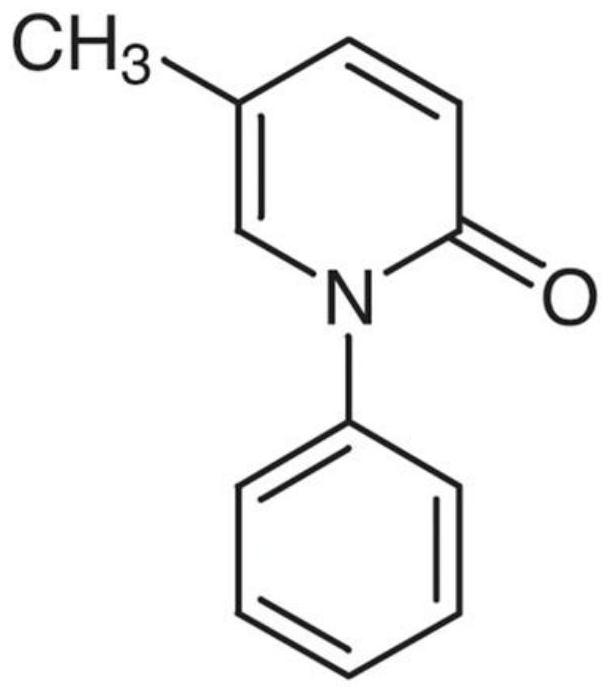
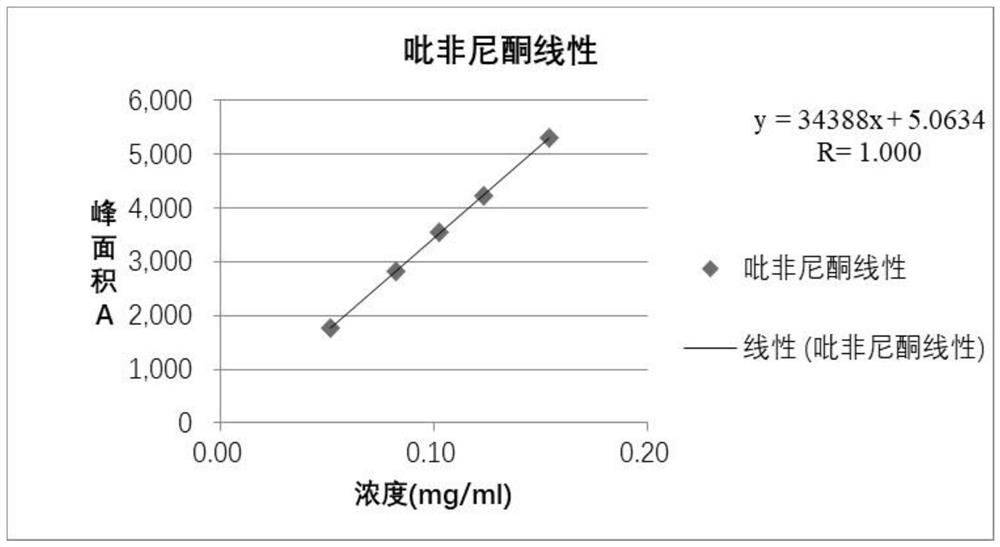
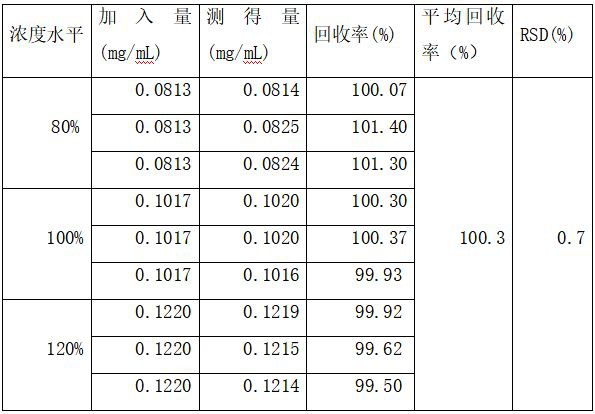
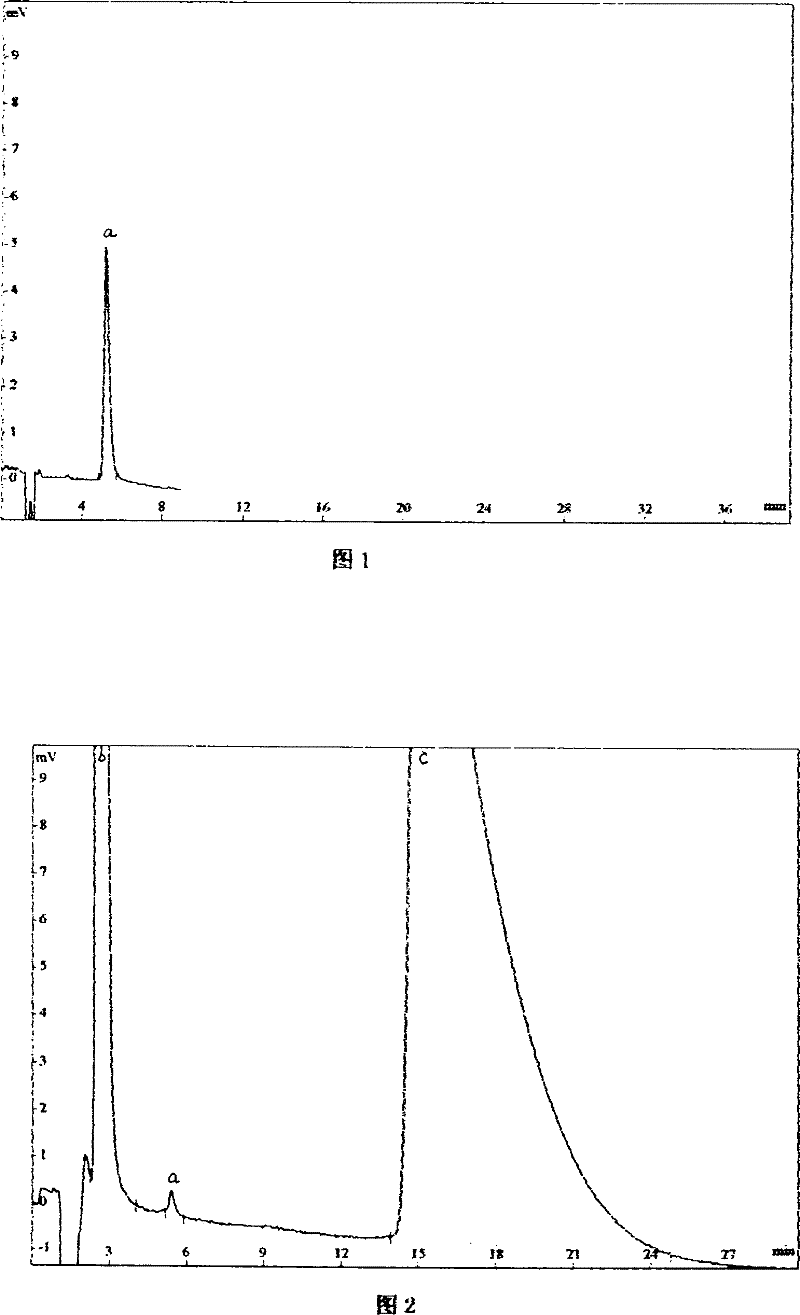
Method for determining content of effective components of pirfenidone tablets
PendingCN114113397AEffective quality controlDetermination method is simpleComponent separationIsocratic elutionCombinatorial chemistry
The invention discloses a method for detecting the content of effective components in pirfenidone tablets, which comprises the following steps of: taking a proper amount of ground pirfenidone tablet sample, putting the ground pirfenidone tablet sample into a volumetric flask, adding a mobile phase, dissolving and diluting to obtain a test solution; taking a proper amount of a pirfenidone reference substance, placing the pirfenidone reference substance in a volumetric flask, and dissolving and diluting the pirfenidone reference substance with a mobile phase to obtain a reference substance solution; precisely measuring 10 microliters of the test solution and 10 microliters of the reference solution, respectively injecting the test solution and the reference solution into a high performance liquid chromatograph, and recording chromatograms, isocratic elution is carried out by using octadecylsilane chemically bonded silica as a filler and water-acetonitrile as a mobile phase, the determination method is convenient, accurate and reliable, and effective control on the quality of the pirfenidone tablet can be realized.
Owner:JIANGSU SEMPOLL PHARMA
Method for testing content of sugar in fermentation liquor and application of method
InactiveCN106770991AMeet the conditions of the determinationEasy to usePreparing sample for investigationMicroorganismDiluent
The invention provides a method for testing the content of sugar in fermentation liquor and application. The method comprises the following steps: performing centrifugal treatment on the fermentation liquor, diluting supernatant, adjusting the pH value, and testing the content of the sugar of the diluent by using a glucometer. The invention further provides the application of the method in testing the glucose content of the fermentation liquor, and preferably, the fermentation liquor includes fermentation liquor and microorganism fermentation liquor.
Owner:AGRO ENVIRONMENTAL PROTECTION INST OF MIN OF AGRI
Measurement method of inorganic germanium content in carboxyethylated germanium sesquioxide
ActiveCN102841064AThe determination method is accurate and reliablePreparing sample for investigationColor/spectral properties measurementsChemical productsCarboxyethylgermanium sesquioxide
The invention relates to the technical field of chemical product testing, in particular to a measurement method of inorganic germanium content in carboxyethylated germanium sesquioxide (Ge-132). Hydrochloric acid is used for distilling and separating inorganic germanium (IV) ions in a carboxyethylated germanium sesquioxide solution, then phenylfluorone-CTMAB- is adopted on the separated inorganic germanium (IV) ions for developing, and a spectrophotometric method is used for measurement. The measurement method of the inorganic germanium content in carboxyethylated germanium sesquioxide is reliable.
Owner:WUHAN YUNJINGFEI OPTICAL FIBER MATERIAL
Hydrochloric acid cefepime raw material and method for measuring content of N-methyl pyrrolidine in preparation thereof
ActiveCN101226174BImprove balanceShort measurement timeComponent separationCefepime hydrochlorideTested time
The invention relates to a measurement method of N-methylpyrrolidine content of cefepime dihydrochloride material and relative agent, for evaluating the quality of cefepime dihydrochloride material and relative agent. The invention discloses a liquid chromatograph which comprises using carboxylic cationic column as analysis column, uses a conductive detector to check, preparing flow phase, preparing sample solution and reference substance solution, using test method to respectively inject the sample solution and reference substance solution into a liquid chromatograph, recording high pressureliquid chromatographs, and calculating the N-methylpyrrolidine content of the sample. The inventive chromatograph system has easy balance, short sample test time, better repeatability in preserved time, stable baseline, durable column, high quality of chromatograph peak of N-methylpyrrolidine, better repeatability, accurate, simple and reliable process.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
A method for the determination of newly added detection components of Tang Herb Tablets, a traditional Chinese medicine for treating AIDS
The invention provides a method for detecting newly increased detection components in a traditional Chinese medicinal Tang Herb for treating AIDS. The method for detecting newly increased detection components comprises the following steps: 1, discriminating Hedyotis diffusa and Medicine Terminalia Fruit components in the Tang Herb through adopting thin-layer chromatography; and 2, simultaneously detecting gallic acid, chlorogenic acid, liquiritin and picroside II through gradient elution of a mobile phase under same high performance liquid chromatographic conditions. The content of gallic acid in every tablet of Tang Herb is not less than 0.9 mg, the content of chlorogenic acid in every tablet of the Tang Herb is not less than 0.5 mg, the content of liquiritin in every tablet of the Tang Herb is not less than 0.25 mg, and the content of the picroside II in every tablet of the Tang Herb is not less than 0.45 mg. Full-inspection projects confirm that the newly increased detection component detection method can be added to an original detection method in order to further improve the detection kind and the detection method of a medicine and facilitate the batch production determination of the traditional Chinese medicine Tang Herb and the improvement of the product quality. The detection method provided by the invention has the advantages of good stability and reliability, high specificity and large application values.
Owner:SHANGHAI HUNDREDS HERBAL PHARMA
Measurement method of inorganic germanium content in carboxyethylated germanium sesquioxide
ActiveCN102841064BThe determination method is accurate and reliablePreparing sample for investigationColor/spectral properties measurementsChemical productsCarboxyethylgermanium sesquioxide
The invention relates to the technical field of chemical product testing, in particular to a measurement method of inorganic germanium content in carboxyethylated germanium sesquioxide (Ge-132). Hydrochloric acid is used for distilling and separating inorganic germanium (IV) ions in a carboxyethylated germanium sesquioxide solution, then phenylfluorone-CTMAB- is adopted on the separated inorganic germanium (IV) ions for developing, and a spectrophotometric method is used for measurement. The measurement method of the inorganic germanium content in carboxyethylated germanium sesquioxide is reliable.
Owner:WUHAN YUNJINGFEI OPTICAL FIBER MATERIAL
Toad skin total alkaloid and its prepn, analysis and prepn process
InactiveCN101019891BRaise quality standardsThe determination method is accurate and reliableAmphibian material medical ingredientsOrganic active ingredientsMedicineToad skin
The present invention provides preparation process of toad skin total alkaloid. The toad skin total alkaloid contains indole total alkaloid 50-95 wt%, cinobufagin and lipo bufogenin 0.02-0.08 wt%, and toad thetine 20-50 wt%. The present invention provides also the method of measuring toad thetine content, and the injection, powder for injection, dripping pill, capsule, oral liquid, etc with the oad skin total alkaloid as effective component and their preparation process.
Owner:周亚球 +1
System for automatically monitoring summarized information of underground fluid
InactiveCN101201404BSolve drying problemsRealize automatic continuous detectionSeismic signal receiversGas phaseCollection system
The invention relates to an automatic synthetic information monitoring system for the underground liquid. The invention is characterized in that the system comprises an underground liquid collection system, a combinational electrode measure system, an automatic degassing and drying system, a sensor system, an automatic gas chromatography analysis system and an intelligent measure control and computer system. The underground liquid collection system is connected with the combinational electrode measure system and the automatic gas escaping and drying system; after being distributed by gas transmission pipes, the automatic gas escaping and drying system is respectively connected with the sensor system and the automatic gas chromatography analysis system; the combinational electrode measure system and the sensor system are connected with the intelligent measure control and computer system in a CAN main line way. The invention has the advantage that the invention is capable of obtaining all kinds of real-time monitoring information from the automatic gas chromatography analysis system, the sensor and the electrode system through one automatic sampling. The information comprises the synthetic information of the underground liquid, such as hydrogen, helium, oxygen, argon, nitrogen, methane, carbon dioxide, sulfur hydrogen, conductance, acidity and alkalinity, chlorine ion, fluorine ion and temperature etc.
Owner:SHANGHAI GASOLINEEUM & CHEM EQUIP +1
Black ginseng extract and black ginseng quick release mini-pill for treating renal interstitial fibrosis, and application of black ginseng extract and black ginseng quick release mini-pill
ActiveCN111956677AImprove extraction efficiencyProcess stabilityPill deliveryUrinary disorderImmediate releaseDrug efficiency
The invention provides a black ginseng extract and a black ginseng quick release mini-pill for treating renal interstitial fibrosis, and application of the black ginseng extract and the black ginsengquick release mini-pill, and belongs to the technical field of medicines. The black ginseng extract efficiently extracts ginseng saponin Rg3, Rg5 and Rk1 from the black ginseng by an ultrasonic extraction method. The invention also provides a black ginseng quick release mini-pill, which comprises the following ingredients in parts by weight: 15-35% of black ginseng extract, 25-47% of microcrystalline cellulose, 23-26% of lactose, 5-8% of crosslinked carboxymethyl starch sodium, 5-8% of low-substituted hydroxypropyl cellulose and the balance of ethyl alcohol aqueous solution of which the volumefraction is 50-65%. In vivo and vitro experiment investigates a medicine efficacy function and a pharmacology mechanism of the black ginseng extract and the black ginseng quick release mini-pill forintervening in the renal interstitial fibrosis so as to indicate that the black ginseng extract or the black ginseng quick release mini-pill has potential for preparing a medicine for treating the renal interstitial fibrosis.
Owner:YANBIAN UNIV
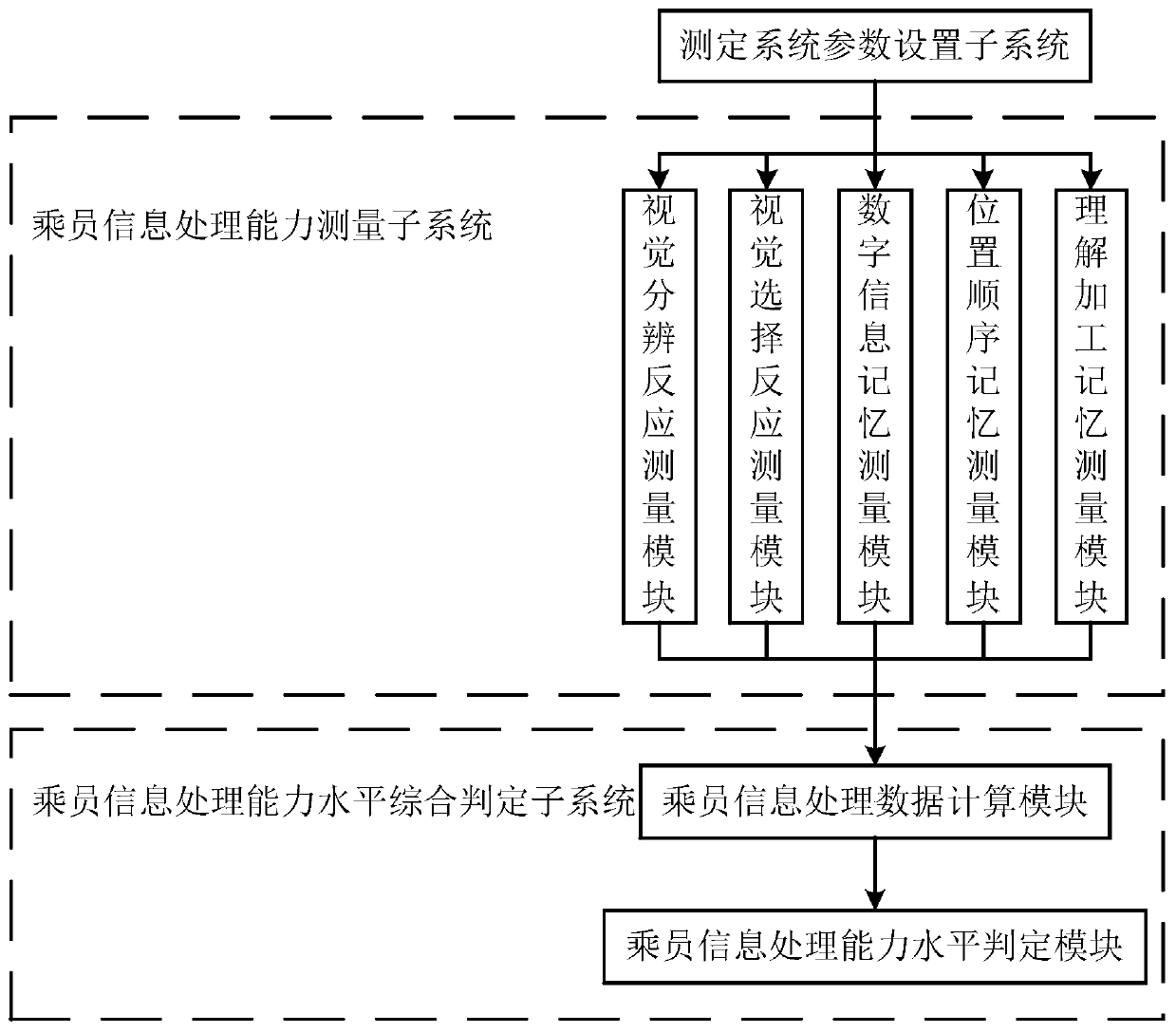
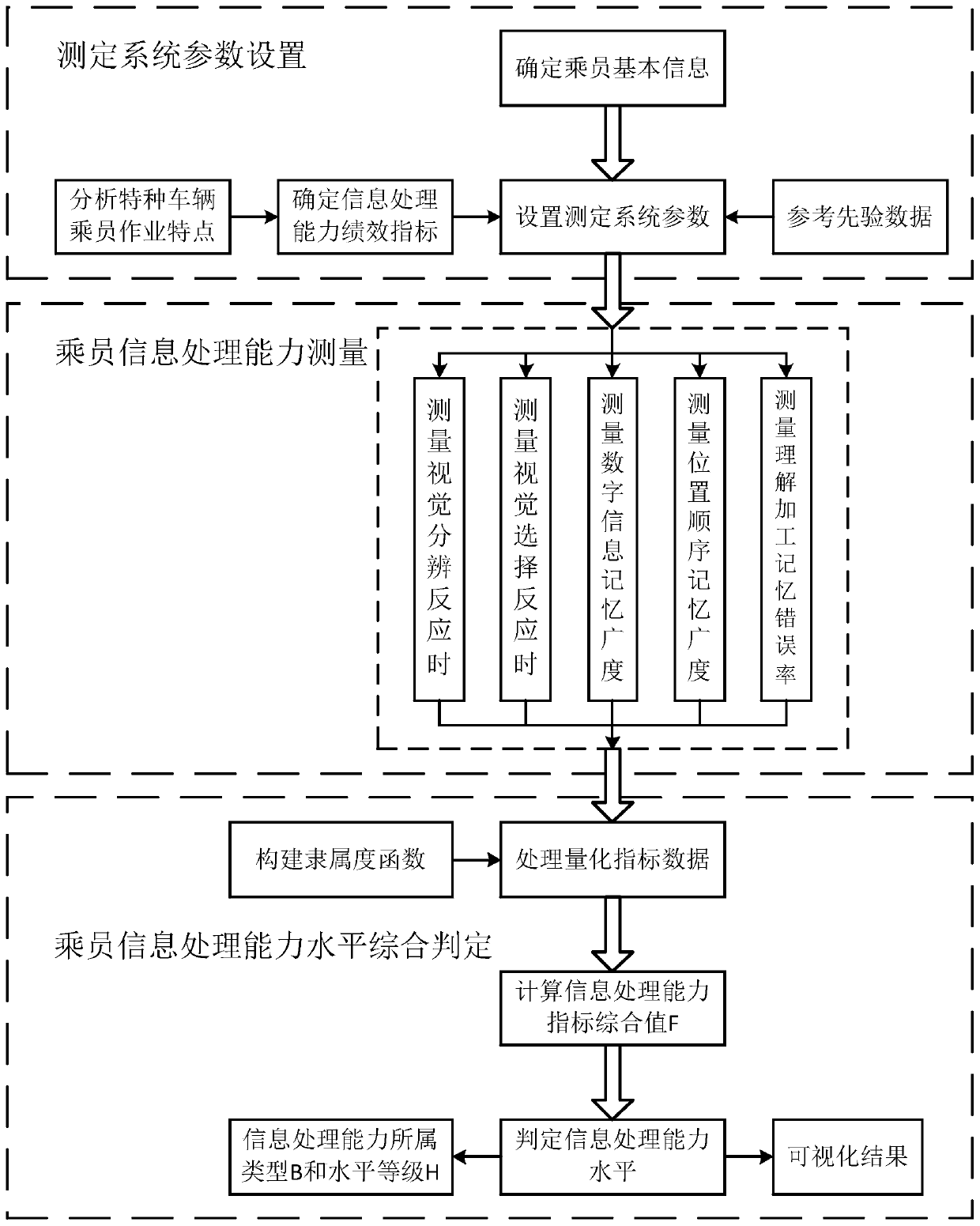
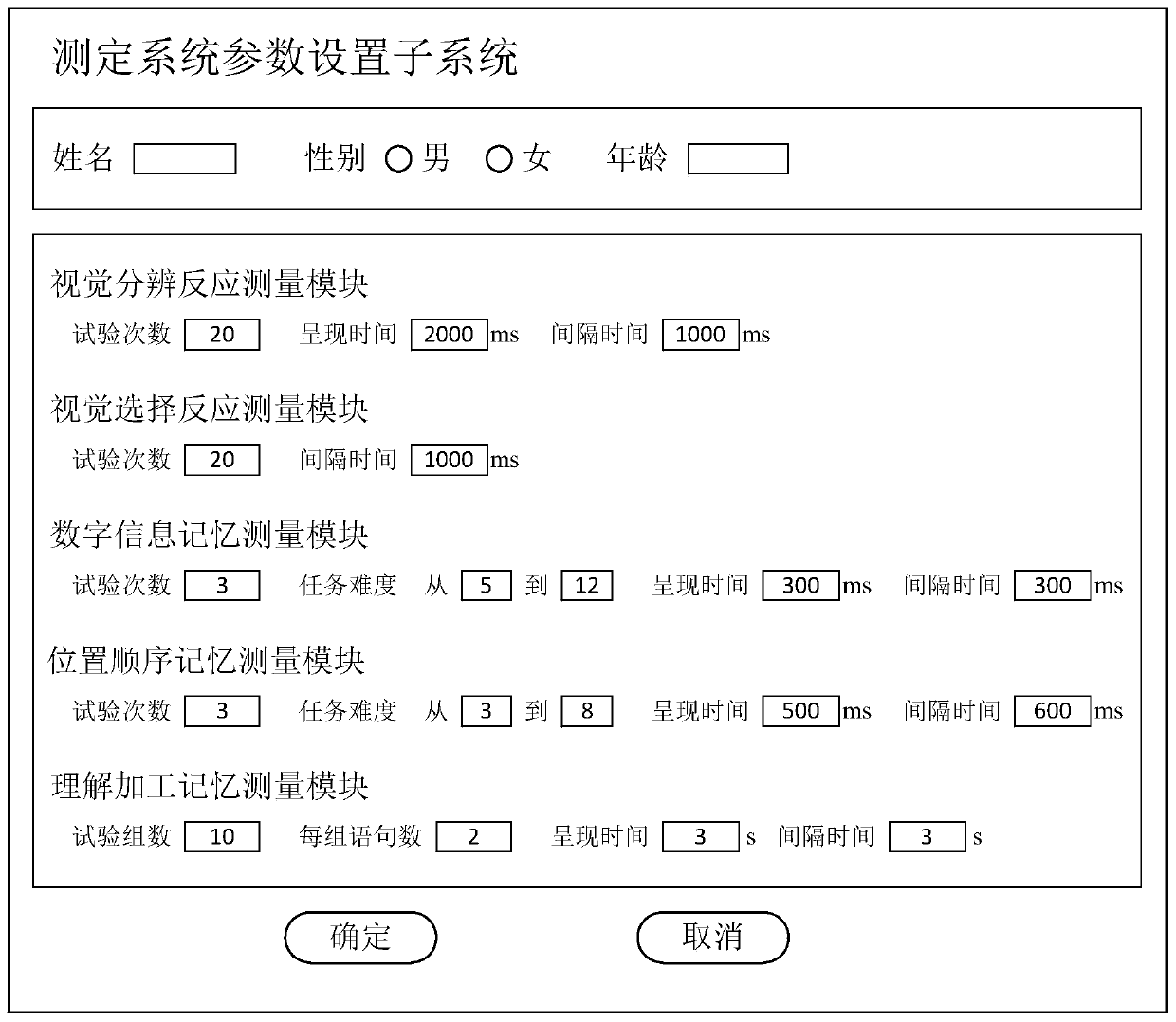
System and method for measuring occupant information processing capability of special vehicles
ActiveCN107025372BTimely processingEasy to handleComputer-assisted medical data acquisitionSensorsInformation processingComputer module
The invention discloses a special vehicle passenger information processing capacity measurement system and method. The system comprises a measurement system parameter setting subsystem, a passenger information processing capacity measurement subsystem and a comprehensive passenger information processing capacity level judgment system, wherein the passenger information processing capacity measurement subsystem comprises a visual discrimination reaction measurement module, a visual selection reaction measurement module, a digital information memory measurement module, a position order memory measurement module and an understanding processing memory measurement module; the comprehensive passenger information processing capacity level judgment system comprises a passenger information processing data calculation module and a passenger information processing capacity level judgment module. With the adoption of the special vehicle passenger information processing capacity measurement system and method, visual discrimination reaction time, visual selection reaction time, digital information memory breadth, position order memory breadth and understanding processing memory error rate can be processed quickly and accurately, measured person information processing capacity index comprehensive value is obtained, and the measured person information processing capacity level is obtained.
Owner:ACADEMY OF ARMORED FORCES ENG PLA
Method for simultaneously measuring content of multiple active ingredients in eucommia ulmoides
InactiveCN102890126BImprove detection efficiencyThe determination method is accurateComponent separationO-Phosphoric AcidChlorogenic acid
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
Method for determining vanadium in silicon-vanadium alloy
ActiveCN102928425BSolve technical problemsSolve the defect of low measurement resultsMaterial analysis by observing effect on chemical indicatorHydrofluoric acidAmmonium ferrous sulfate
The invention belongs to the field of analytical chemistry, and particularly relates to a method for determining vanadium in a silicon-vanadium alloy. According to the method for determining vanadium in a silicon-vanadium alloy, the vanadium is determined by a steel and alloy chemical analysis method-ammonium ferrous sulfate titration method, and hydrofluoric acid is added after concentrated hydrochloric acid is added. Through the invention, the defect of relatively low determination result is overcome, and an accurate method for determining vanadium in a silicon-vanadium alloy is obtained.
Owner:RUI STEEL INDAL OF PANZHIHUA GANGCHENG GROUP
Simple plant callus subculture weight increase determination method
InactiveCN102914350APromote growthThe determination method is simpleWeighing apparatus for materials with special property/formFilter paperCallus
The invention discloses a simple plant callus subculture weight increase determination method during plant tissue culture. The method includes: firstly, placing a piece of disinfected filter paper on a solidified culture medium, weighing, and dicing a to-be-subcultured callus and inoculating the diced callus on the filter paper prior to weighing, wherein the difference between two-time weighing results serves as initial weight; and after culture, sampling and weighing the grown callus, wherein a numerical value obtained by subtracting the initial weight from grown weight serves as increased weight during subculture. The culture medium supplies water and nutrients to the callus by the aid of a filter paper bridge, the callus is in non-sticky connection with the filter paper, is unattached to the culture medium, can be tweezed directly by tweezers and is less prone to crushing, and the method is simple, convenient, feasible, free of interference during weighing, accurate and reliable.
Owner:胡能兵
Spraying degasser
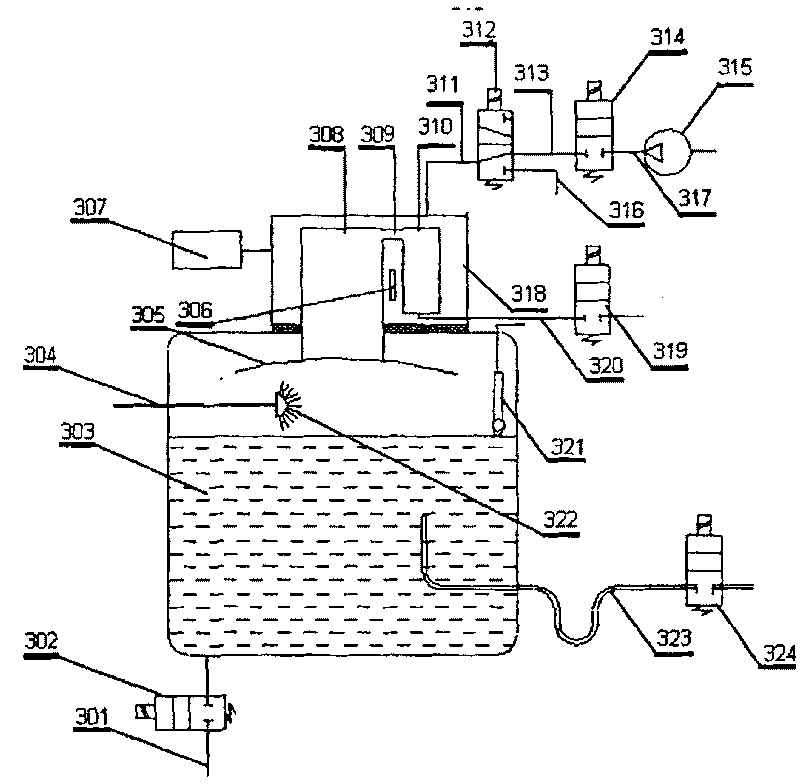
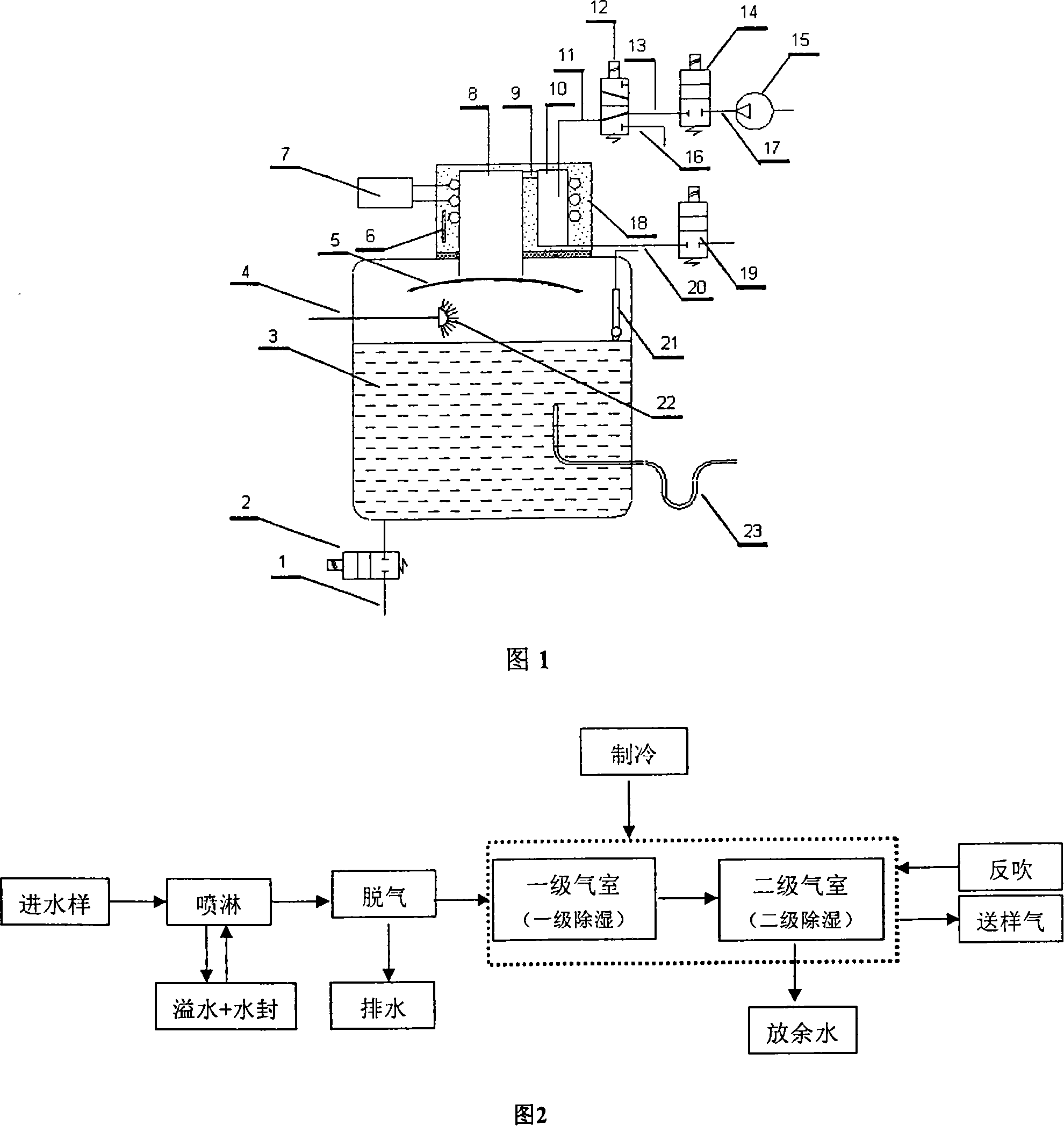
InactiveCN101109734ARealize automatic continuous detectionSolve the moisture problemComponent separationPreparing sample for investigationTemperature controlDegasser
The invention relates to a spraying degassing arrangement, which is characterized in that, a water drain pipe is located at the bottom of a degassing container through a solenoid valve A; a water overflowing pipe is located at one side at the lower end of the degassing container; a level sensor and a nozzle are located at the upper part in the degassing container; the nozzle is connected with a water-in pipe; a baffle is located at the top in the degassing container; an indirectly cooled low-temperature test bath is located above the degassing container; a stage-I air chamber and a stage-II air chamber are arranged in the indirectly cooled low-temperature test bath; the stage-I air chamber is communicated with the degassing container, and is located above the baffle; a temperature controlling system in the indirectly cooled low-temperature test bath is connected with a refrigerating system outside the indirectly cooled low-temperature test bath; the upper end of the stage-I air chamber and that of the stage-II air chamber are connected through a connection pipe A; the upper end of the degassing container is provided with a pipe for draining residual water, and is connected with the 2-position 3-way solenoid valve A through a connection pipe B; one way of the solenoid valve A is connected with a back blowing pump through a connection pipe C, another way of the solenoid valve A is connected with a gas delivery pipe. The invention can be interconnected with an automatic gas-phase chromatograph so as to carry out on line inspection.
Owner:SHANGHAI GASOLINEEUM & CHEM EQUIP +1
A method for the determination of nitrofuran antibiotics in cosmetics
ActiveCN103926340BThe determination method is accurateThe determination method is accurate and reliableComponent separationNitrofurazoneFuraltadone
The invention discloses a method for measuring nitrofuran antibiotics in cosmetics. The method comprises the following steps: (1) pretreating a sample, namely adding the sample into a mixed solution of acetonitrile and methanol in a volume ratio of 70:30, uniformly mixing, performing ultrasonic extraction, centrifuging, airing supernatant till the supernatant is nearly dry, dissolving residues in a mixed solution of a 15mmol / L ammonium acetate solution and acetonitrile according to a volume ratio of 85:15, and sieving the solution through a micro-porous filter membrane with the size of 0.22 micron, thereby obtaining filtrate for later use; (2) performing high performance liquid chromatography separation on the filtrate, eluting a mobile phase by a gradient elution program, and quantifying according to an external standard method; and (3) confirming a detected positive sample by high performance liquid chromatography-mass spectrometry / mass spectrometry. According to the method, four nitrofuran antibiotics such as macrodantin, furazolidone, furaltadone and nitrofurazone are simultaneously detected. The method is accurate, reliable and high in sensitivity.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Method for determining content of (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-ethyl crotonate
PendingCN114384186ADetermination method is simpleThe determination method is accurateComponent separationMinodronic acid hydratePhysical chemistry
The invention discloses a method for determining the content of (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-ethyl crotonate, which comprises the following steps of: preparing a (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-ethyl crotonate reference substance into a reference substance solution with the concentration of 5mu g / mL, and measuring the content of the (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-ethyl crotonate. Preparing a minodronic acid hydrate test solution with the concentration of 500mu g / mL from a raw material medicine sample of the minodronic acid hydrate, and finally, precisely sucking the reference substance solution and the test solution respectively, injecting the reference substance solution and the test solution into a high performance liquid chromatograph, and measuring; the determination method disclosed by the invention is convenient, accurate and reliable, and the content of the ethyl (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-butenoate in the product can be really reflected.
Owner:王立强
Device for measuring injection volume of ion chromatograph and method for measuring injection volume
ActiveCN109030704BRinsingRaise the level of decontaminationVolume measurement apparatus/methodsComponent separationInjection volumePhysical chemistry
The invention belongs to an ion chromatograph sample injection volume measurement device and a sample injection volume measurement method, and aims at accurately measuring sampling volumes in full quantitative loop sample injection and partial quantitative loop sample injection of ion chromatographs. According to the ion chromatograph sample injection volume measurement device and the sample injection volume measurement method, a sample injection valve is utilized to ensure that inorganic salt solution and a quantitative loop, and flushing fluid and the quantitative loop are communicated withan ion chromatograph of a measurement solution passage or a flushing channel technical scheme in turn to carry out sample injection volume measurement; and by using the sample injection volume measurement method of the device, the technical problems that in existing ion chromatograph sample injection volume measurement devices and sample injection volume measurement methods, the gas flow rate is low to cause the fact that residual liquid exists in quantitative loops, the gas flow rate is high to cause the fact that certain liquid is brought away, and the measured liquid is easy to evaporate tocause loss and then influence the measurement correctness.
Owner:QINGDAO SHENGHAN CHROMATOGRAPH TECH CO LTD
Black ginseng extract, black ginseng quick-release pellets and application thereof for treating renal interstitial fibrosis
ActiveCN111956677BImprove extraction efficiencyProcess stabilityPill deliveryUrinary disorderImmediate releaseEfficacy
The invention provides a black ginseng extract for treating renal interstitial fibrosis, black ginseng quick-release pellets and applications thereof, which belong to the technical field of medicines. Black ginseng extract Ginsenosides Rg3, Rg5 and Rk1 are efficiently extracted from black ginseng by ultrasonic extraction. The present invention also provides black ginseng quick-release pellets, which include the following components in mass percent: 15%-35% of the black ginseng extract, 25%-47% of microcrystalline cellulose, and 23%-26% of lactose , 5% to 8% of croscarmellose sodium starch, 5% to 8% of low-substituted hydroxypropyl cellulose and 50% to 65% ethanol aqueous solution by volume fraction of the balance. The pharmacological effect and pharmacological mechanism of intervening renal interstitial fibrosis were investigated through in vivo and in vitro experiments, indicating that black ginseng extract or black ginseng quick-release pellets have the potential to prepare drugs for treating renal interstitial fibrosis.
Owner:YANBIAN UNIV
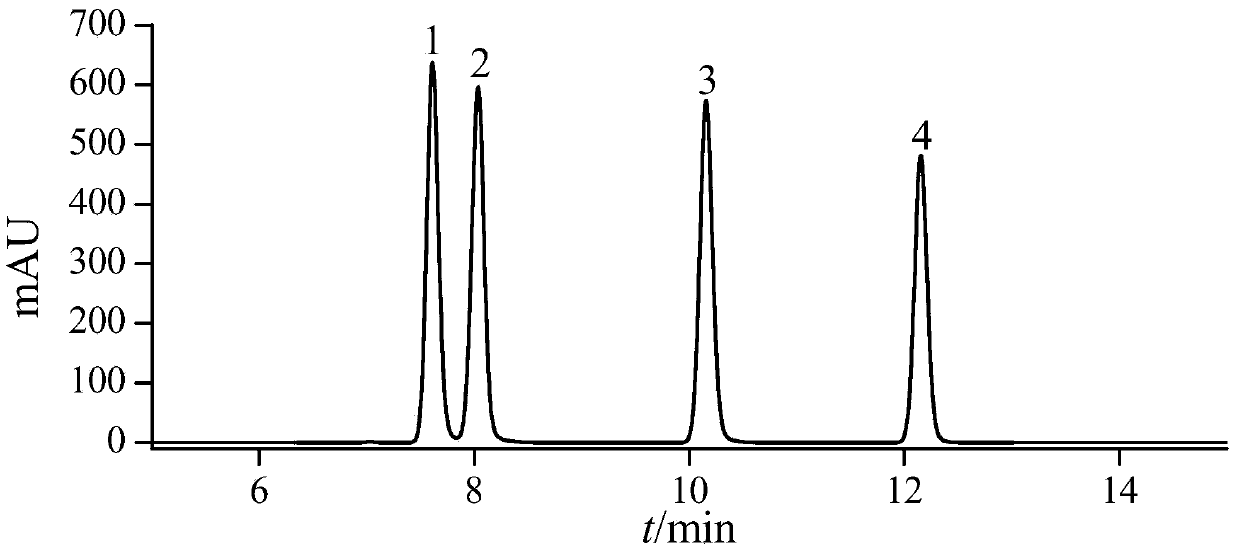
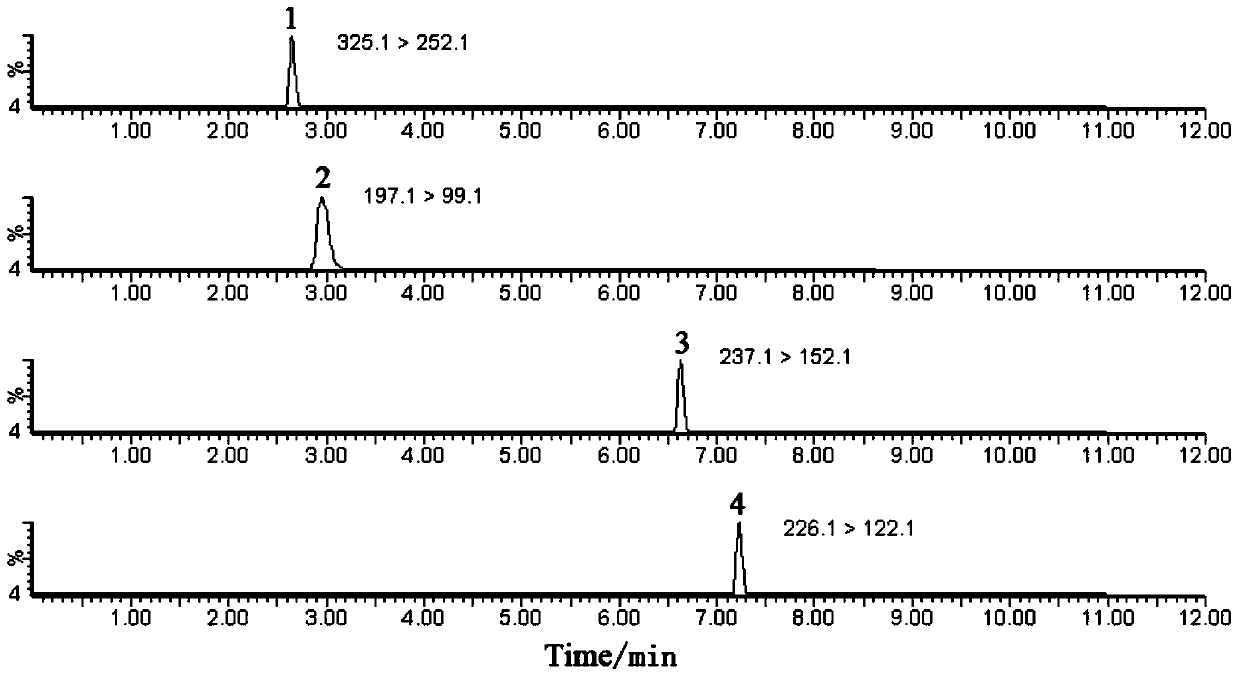
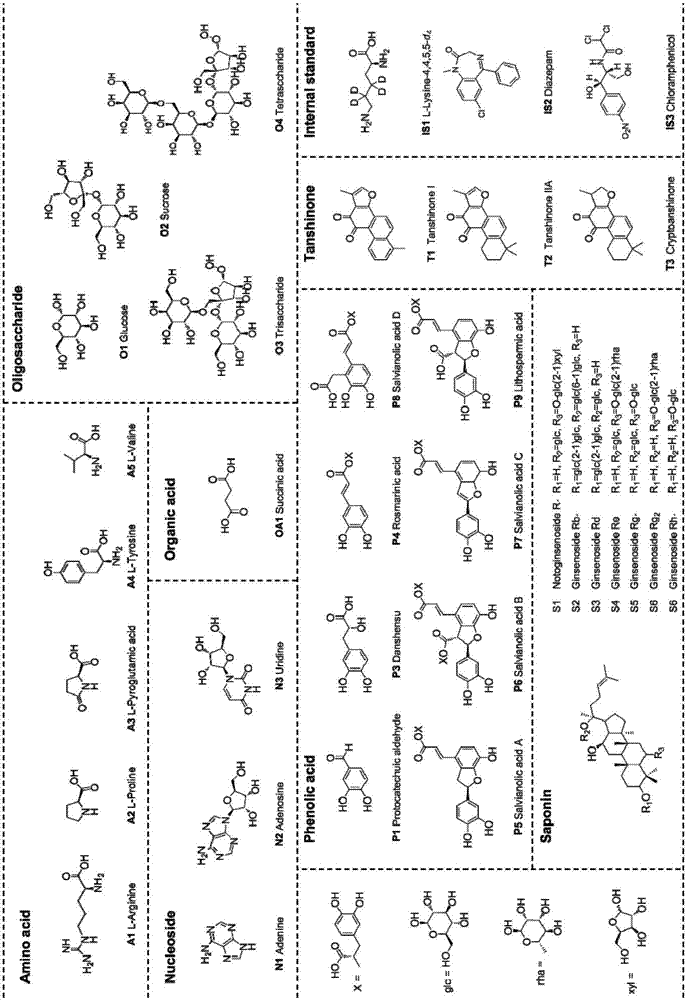
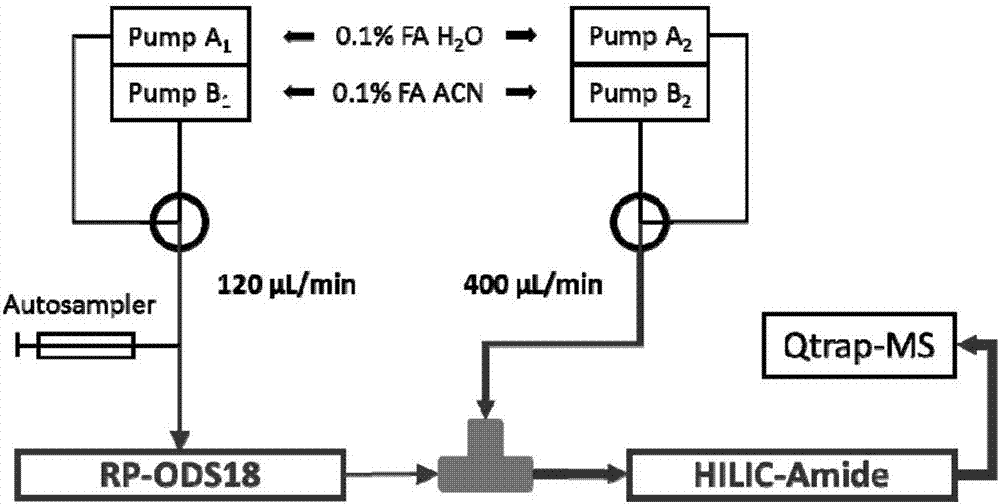
A method for simultaneous determination of 31 components in compound salvia miltiorrhiza extract or related medicinal materials
ActiveCN107991399BReliable methodThe determination method is accurate and reliableComponent separationMedicinal herbsAnalyte
The present invention relates to a method for simultaneously measuring 31 components in a compound salvia miltiorrhiza extract or related medicinal materials. The method adopts a direct series-reversed-phase-hydrophilic interaction chromatography-mass spectrometry method, and the method comprises the following steps: 1 Preparation of sample solution; Step 2 Preparation of standard series solution; Step 3 Preparation of internal standard solution; Step 4 Preparation of test solution; Step 5 Take the solution obtained in Step 4 and inject it into direct series reversed-phase-hydrophilic interaction liquid chromatography The mass spectrometry system obtains the extracted ion chromatograms of 31 analytes, and calculates the content of 31 components in the compound salvia miltiorrhiza extract or related medicinal materials according to the extracted ion chromatograms.
Owner:TIANJIN TASLY PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com