Method for determining content of (2E, 4E)-ethyl-4-(pyridine-2-yl imino)-2-ethyl crotonate
A technology of ethyl crotonate and imino group, applied in the field of medicine, can solve problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
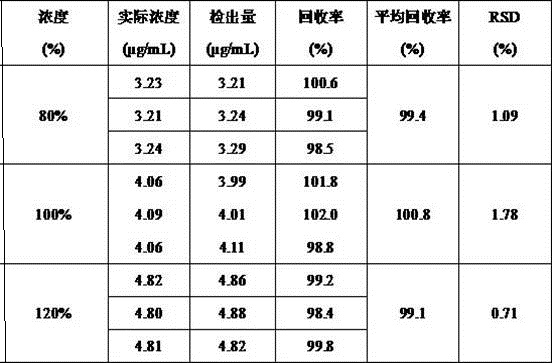
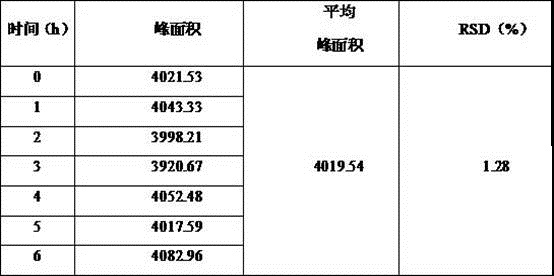
[0047] A method for determining the content of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester", comprising the steps of:
[0048] (1) Preparation of reference substance: Accurately weigh 5 mg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" and dissolve it in mobile phase Finally, put it in a 100 mL volumetric flask, add mobile phase to dilute to the mark, and prepare a reference substance mother solution with a concentration of 50 μg / mL, take 1 mL of the reference substance mother solution in a 10 mL volumetric flask, and dilute to the mark with mobile phase , configured as a reference substance solution containing 5 µg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" per 1 ml;
[0049] (2) Preparation of the test sample: Accurately weigh 25 mg of minodronic acid hydrate, add 4 g / L sodium hydroxide solution to dissolve, sonicate for 5 min, let cool to room temperature, place in a 50 mL measuring bottle, Add mobile ...
Embodiment 2
[0056] A method for determining the content of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester", comprising the steps of:
[0057] (1) Preparation of reference substance: Accurately weigh 5 mg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" and dissolve it in mobile phase Finally, put it in a 100 mL volumetric flask, add mobile phase to dilute to the mark, and prepare a reference substance mother solution with a concentration of 50 μg / mL, take 1 mL of the reference substance mother solution in a 10 mL volumetric flask, and dilute to the mark with mobile phase , configured as a reference substance solution containing 5 µg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" per 1 ml;
[0058] (2) Preparation of the test sample: Accurately weigh 25 mg of minodronic acid hydrate, add 4 g / L sodium hydroxide solution to dissolve, sonicate for 5 min, let cool to room temperature, place in a 50 mL measuring bottle, Add mobile ...
Embodiment 3
[0065] A method for determining the content of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester", comprising the steps of:
[0066] (1) Preparation of reference substance: Accurately weigh 5 mg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" and dissolve it in mobile phase Finally, put it in a 100 mL volumetric flask, add mobile phase to dilute to the mark, and prepare a reference substance mother solution with a concentration of 50 μg / mL, take 1 mL of the reference substance mother solution in a 10 mL volumetric flask, and dilute to the mark with mobile phase , configured as a reference substance solution containing 5 µg of "(2E, 4E)-ethyl-4-(pyridin-2-ylimino)-2-butenoic acid ethyl ester" per 1 ml;
[0067] (2) Preparation of the test sample: Accurately weigh 25 mg of minodronic acid hydrate, add 6 g / L sodium hydroxide solution to dissolve, sonicate for 5 min, let it cool to room temperature, put it in a 50 mL measuring bottle, Add mob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com