Method for simultaneously measuring content of multiple active ingredients in eucommia ulmoides
A technology for active ingredients and content, applied in the field of simultaneous determination of the content of various active ingredients in Eucommia ulmoides, can solve the problems of weakened absorption peak intensity, poor separation of chromatographic peaks, and difficulty in simultaneous determination, etc., to achieve accurate determination methods and improve detection. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Method verification experiment
[0025] 1 Investigation of linear relationship
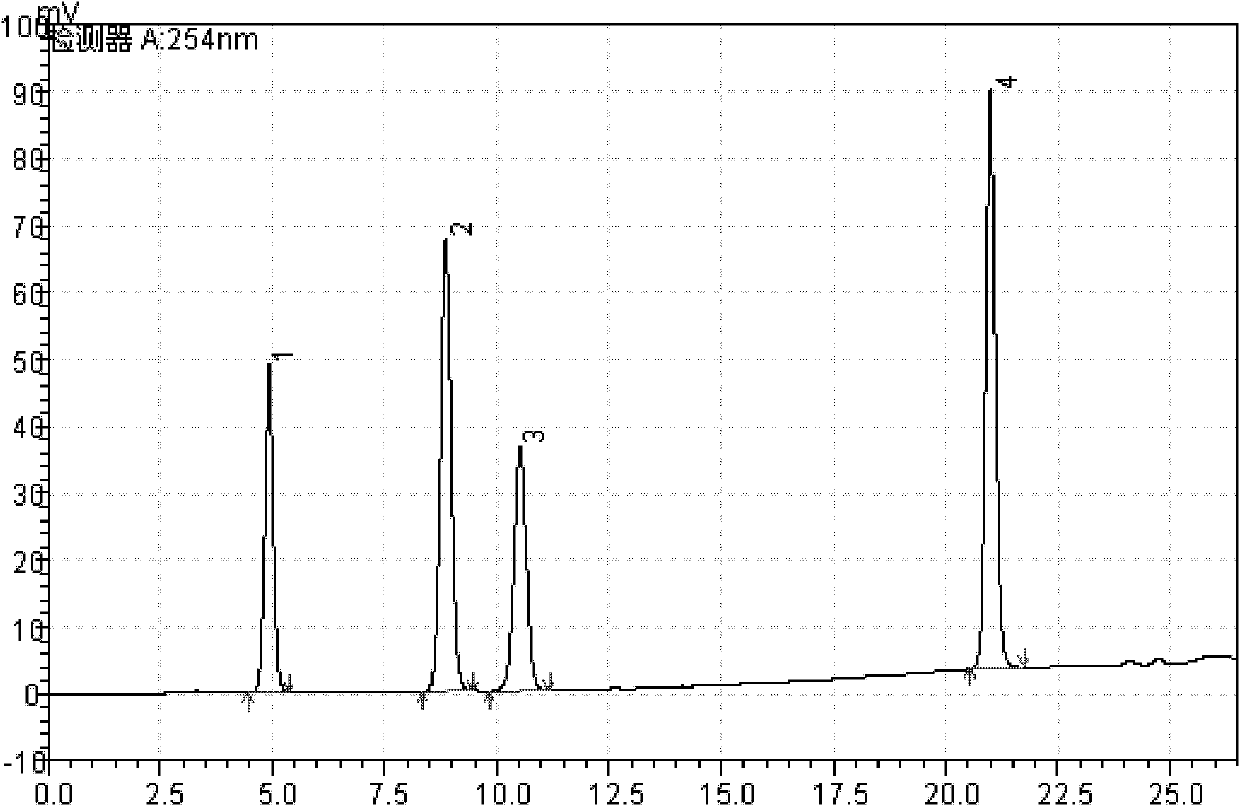
[0026] Accurately weigh the pinoresinol diglucoside, geniposide acid, chlorogenic acid, and rutin reference substance, and dissolve it with methanol to make the concentration 0.11mg·mL -1 , 0.11mg·mL -1 , 0.2mg·mL -1 , 0.1mg·mL -1 , Mix the reference solution, and prepare solutions of different series of concentrations into the high performance liquid chromatograph, and measure the peak area according to the above chromatographic conditions (see the chromatogram figure 1 ), with the injection volume of each reference substance (μg) as the abscissa, and the peak area A as the ordinate, draw a standard curve. The results are shown in Table 3. The results show that the peak area of each reference substance has a good linear relationship with the concentration.
[0027] Table 3 Results of linear relationship investigation
[0028]
[0029]
[0030] 3. Precision experiment
[0031] Precisely d...
Embodiment 2
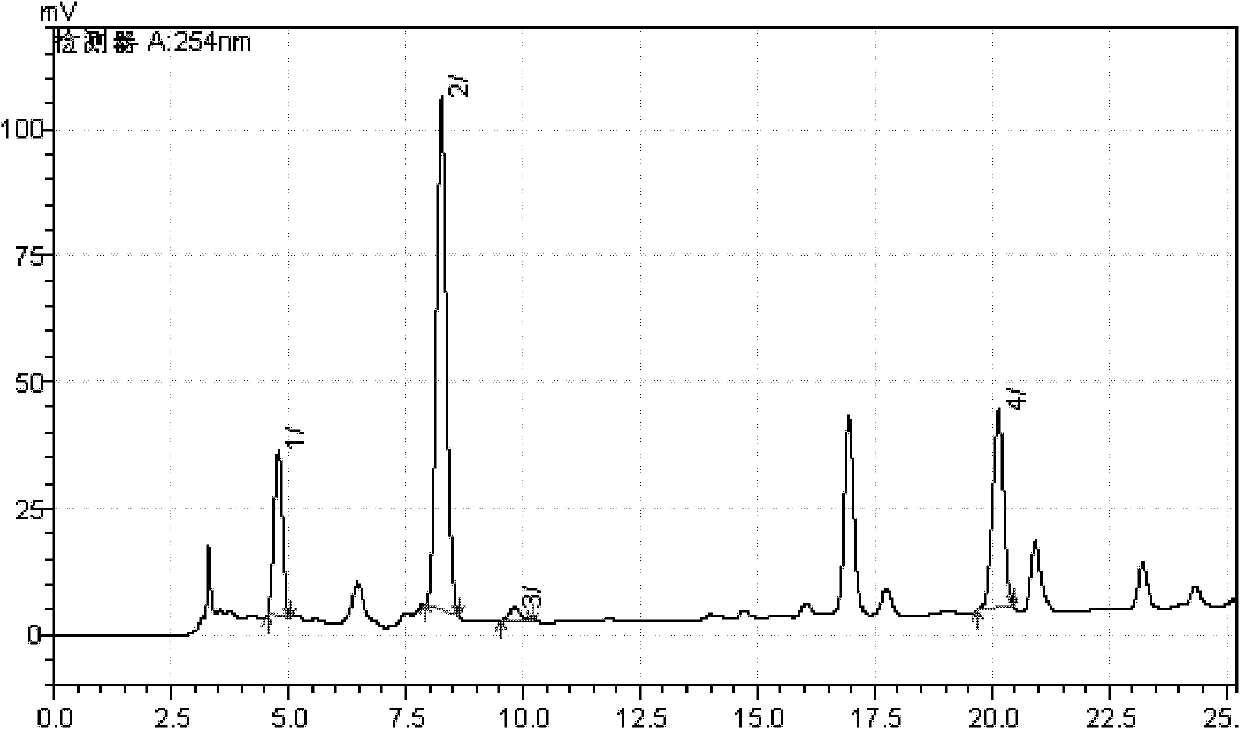
[0039] Example 2 Test of active ingredients in Eucommia bark
[0040] The octadecylsilane bonded silica gel is used as a filler; the mobile phase A is methanol, and the mobile phase B is an aqueous solution of phosphoric acid with a mass concentration of 0.1%. The mobile phase gradient elution program and detection wavelength program are shown in Table 1 and Table 2: Flow rate: 0.8ml.min -1 ; Column temperature: 30℃; Injection volume is 5μl.
[0041] Preparation of reference solution: accurately weigh pinoresinol diglucoside, geniposide acid, chlorogenic acid, and rutin reference substance, and dissolve it with methanol to make the concentration 0.11mg·mL -1 , 0.11mg·mL -1 , 0.2mg·mL -1 , 0.1mg·mL -1 The mixed reference solution is injected with the resolution not less than 1.5 and the theoretical plate is not less than 5000.
[0042] Preparation of test solution: accurately weigh 5 g of Eucommia ulmoides skin after 40 mesh sieve, add 50 mL of methanol in a round bottom flask, and ex...
Embodiment 3
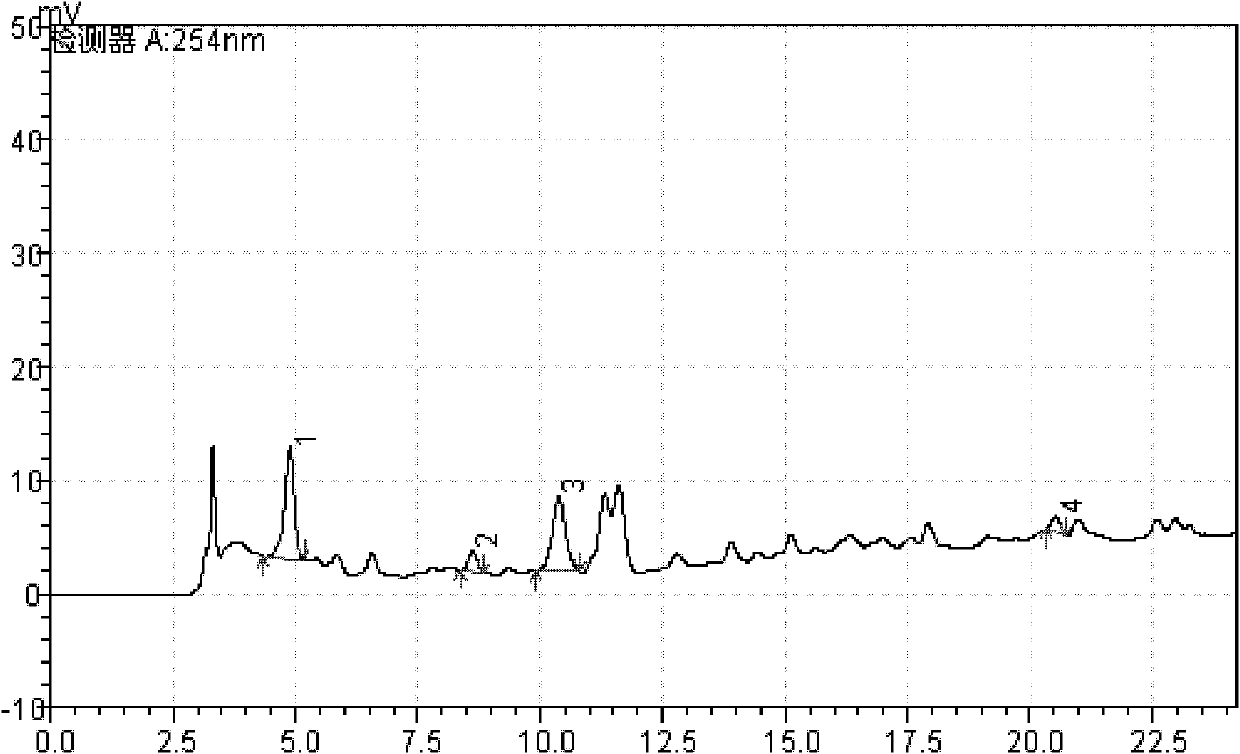
[0043] Example 3 Test of active ingredients in Eucommia ulmoides leaves
[0044] Use octadecylsilane bonded silica gel as a filler; mobile phase A is methanol, mobile phase B is 0.1% phosphoric acid aqueous solution. The mobile phase gradient elution program detection wavelength program is shown in Table 1 and Table 2: Flow rate: 0.8ml.min -1 ; Column temperature: 30℃; Injection volume is 5μl. The column temperature is 30°C.
[0045] Preparation of reference solution: accurately weigh pinoresinol diglucoside, geniposide acid, chlorogenic acid, and rutin reference substance, and dissolve it with methanol to make the concentration 0.11mg·mL -1 , 0.11mg·mL -1 , 0.2mg·mL -1 , 0.1mg·mL -1 The mixed reference solution is injected with the resolution not less than 1.5 and the theoretical plate is not less than 5000.
[0046] Preparation of test solution: accurately weigh 2g of Eucommia ulmoides leaves after 40-mesh sieve, add 30mL methanol in a round-bottomed flask, and extract for 10min i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com