Method for determining content of effective components of pirfenidone tablets
A technology for pirfenidone tablets and active ingredients, applied in the field of medicine, can solve the problems of rarely reported content determination methods of pirfenidone tablets and the like, and achieve the effects of effective quality control and convenient determination methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0029] (1) Inspection of linear relationship:
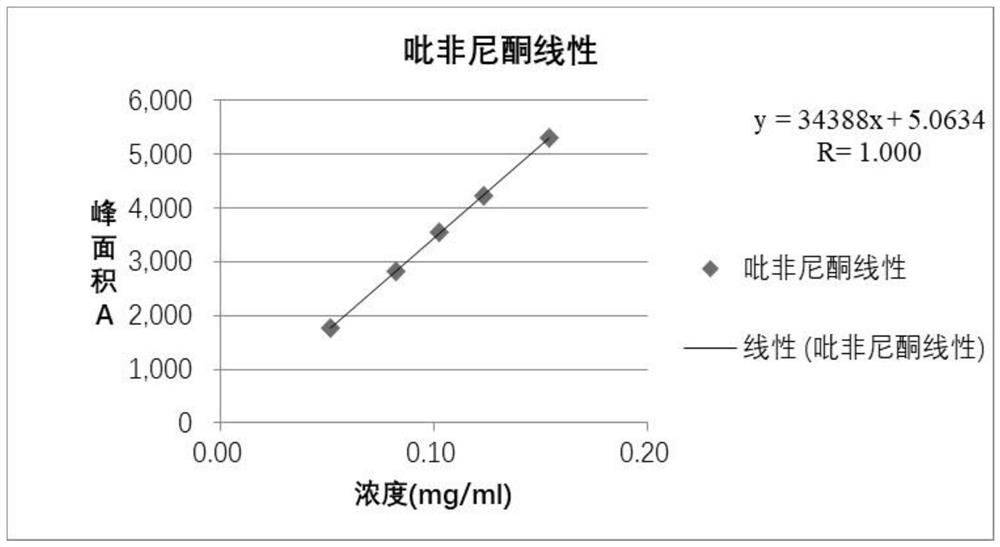
[0030] Accurately weigh an appropriate amount of pirfenidone reference substance, add mobile phase to dissolve and dilute to make a stock solution of 0.2 mg / ml, and use mobile phase to gradually dilute to concentrations of 0.05 mg / ml, 0.08 mg / ml, and 0.10 mg / ml , 0.12mg / ml, and 0.15mg / ml solutions, respectively accurately measure 10 μ L of each solution and inject it into a liquid chromatograph, and record the chromatogram; take the pirfenidone peak area (A) as the ordinate, and the concentration of the pirfenidone solution (C) is the abscissa, and the regression equation y=34388x+5.0634 (R 2 =1.000), such as figure 1 , the results showed that pirfenidone had good linearity in the range of 0.051-0.154mg / mL.
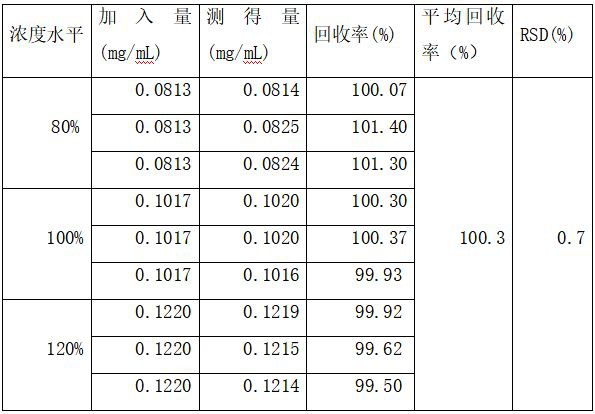
[0031] (2), accuracy test:
[0032] Accurately weigh about 16mg, 20mg, and 24mg of pirfenidone samples in three parts, place them in 20ml volumetric flasks respectively, add mobile phase to dilute to the mark, shake well,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com