Content determination method for sacubitri valsartan trisodium hemi-pentahydrate capsule effective ingredients
A technology for pentahydrate and active ingredients, which is applied in the field of content determination of active ingredients in sacubitril valsartan trisodium hemipentahydrate capsules, and can solve problems such as detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
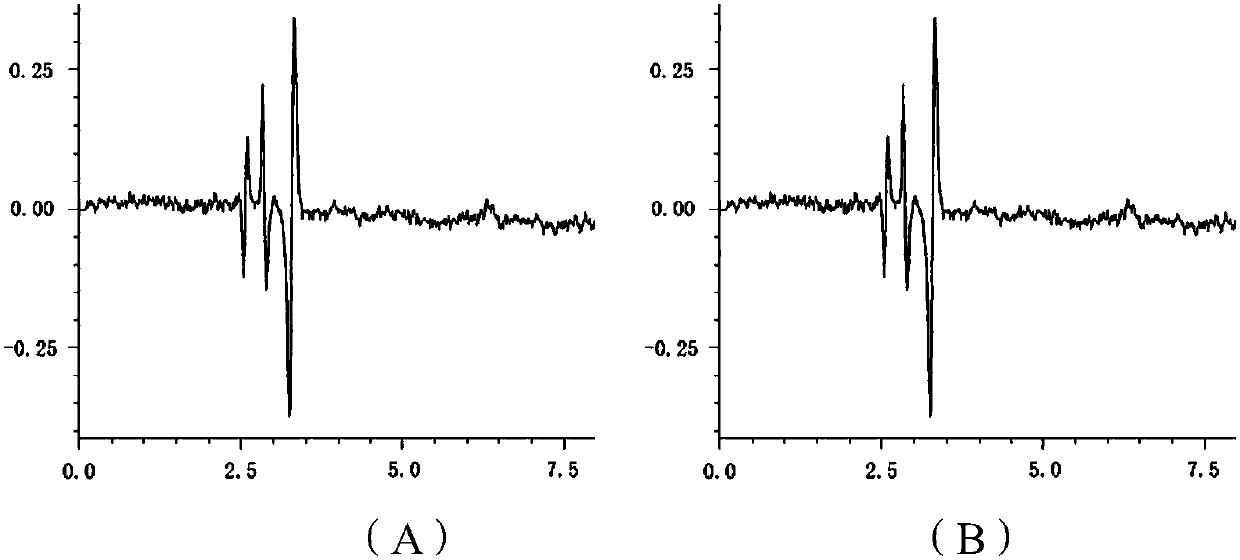
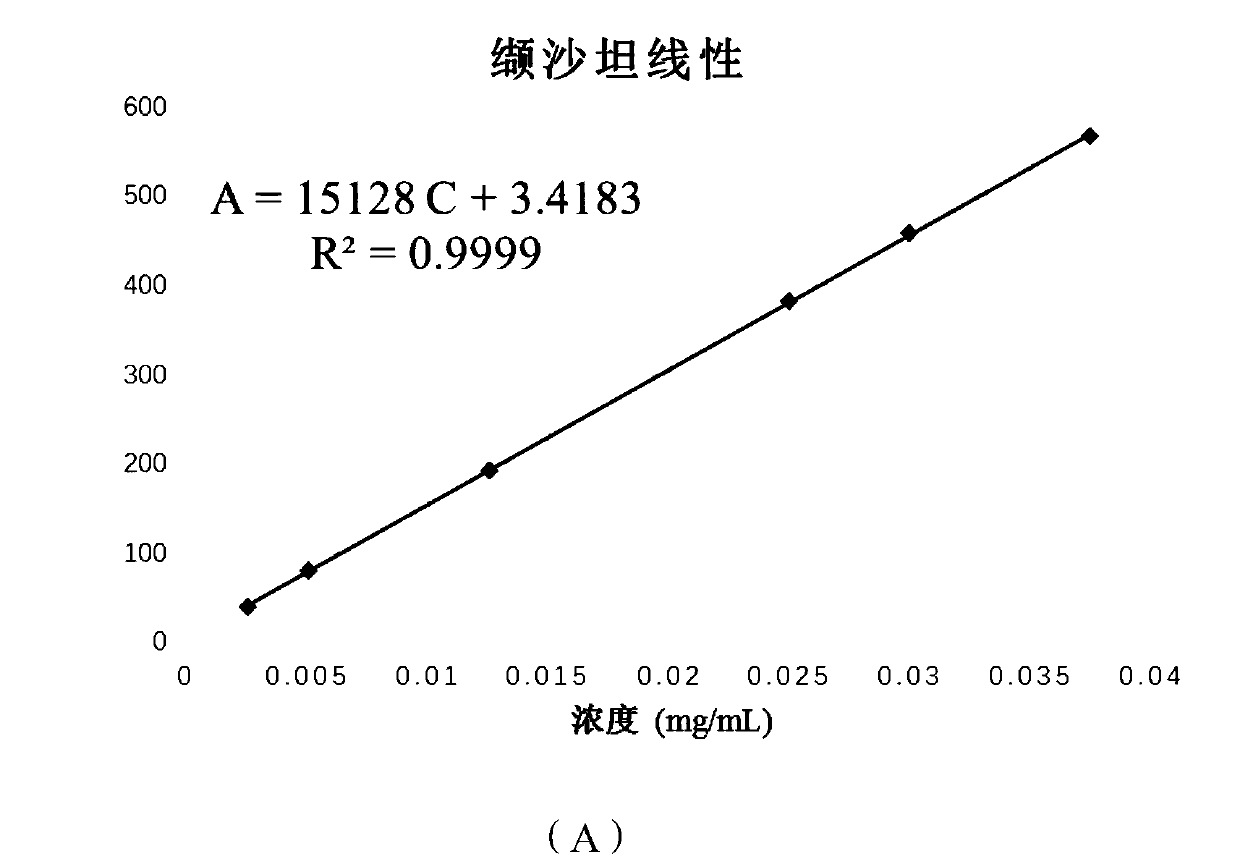
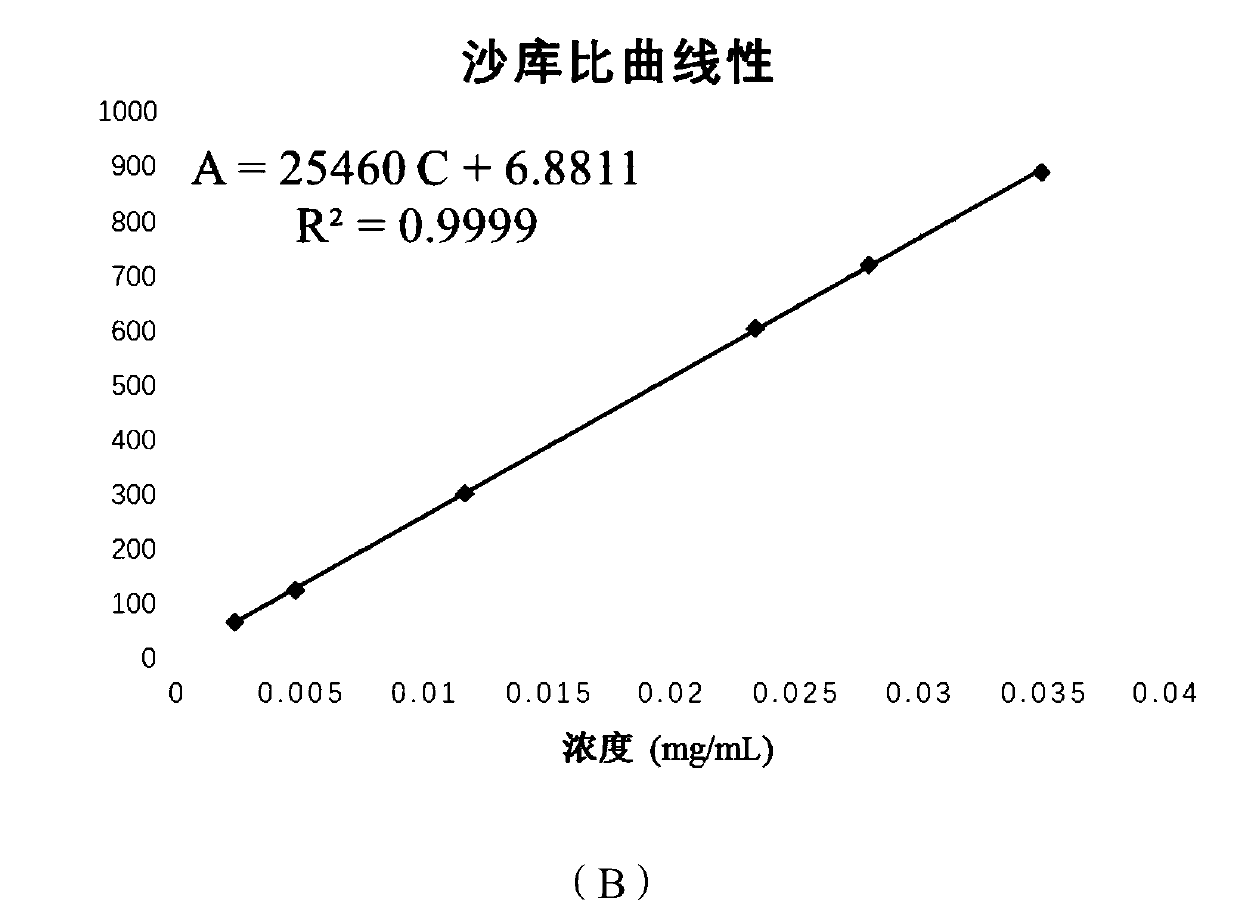
[0069] A method for determining the content of active ingredients of sacubitril valsartan trisodium hemipentahydrate capsules, comprising the steps of:
[0070] (1) Preparation of reference substance: Accurately weigh an appropriate amount of sacubitril-valsartan trisodium hemipentahydrate reference substance, add methanol to make a drug containing 50 μg of sacubitril-valsartan trisodium hemipentahydrate per 1 ml solution;
[0071] (2) Preparation of the test product: Take 20 capsules of sacubitril-valsartan trisodium hemipentahydrate from different batches, put them in a mortar and grind them finely through a No. 4 sieve, and take sacubitril-valsartan trisodium An appropriate amount of the content of the semi-pentahydrate capsule sample (approximately equivalent to 10 mg of sacubitril-valsartan trisodium hemi-pentahydrate), accurately weighed, placed in a 20mL volumetric flask, added methanol and shaken to dissolve, filtered, and the filtrate was placed Cool to room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com