Adiponectin chemiluminescence immunoassay kit and its preparation method and use
A technology of chemiluminescence immunity and detection kits, which is applied in the direction of chemiluminescence/bioluminescence, anti-hormonal immunoglobulin, and analysis by making materials undergo chemical reactions, which can solve the problem of inaccurate, complicated, and complex reagent or sample additions. Problems such as poor detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
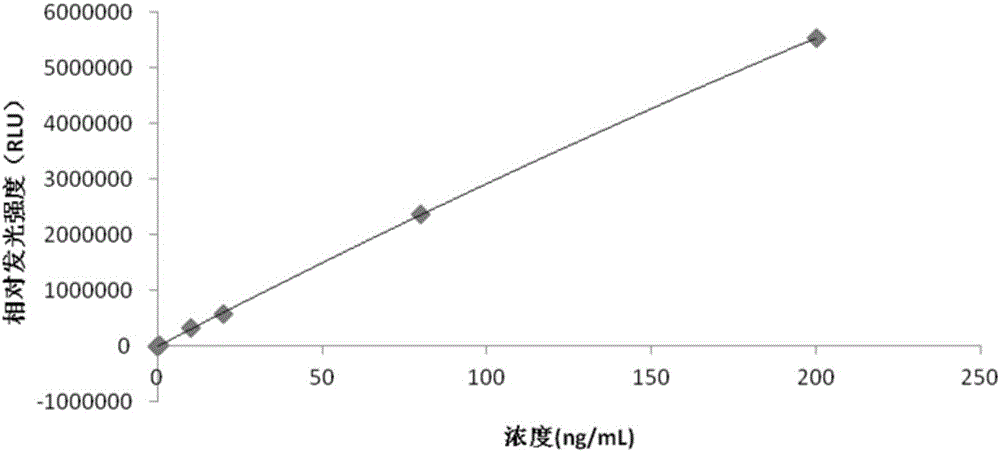
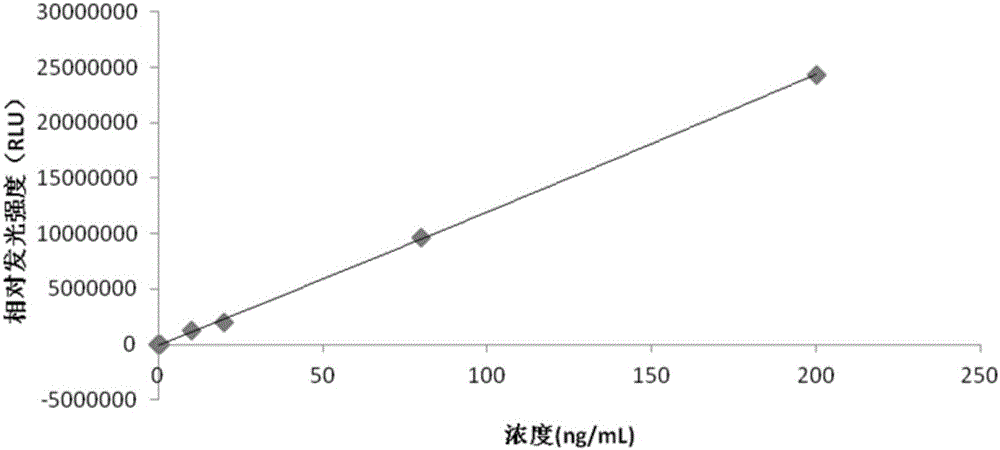
[0062] The preparation process of the adiponectin calibrator is as follows: the adiponectin is prepared into adiponectin solutions with a concentration of 0-200 ng / mL by using the calibrator buffer.
[0063] Specifically, the adiponectin calibration product is a solution of adiponectin with concentrations of 0 ng / mL, 0.5 ng / mL, 10 ng / mL, 20 ng / mL, 80 ng / mL and 200 ng / mL, respectively.
[0064] The adiponectin chemiluminescence immunoassay kit can use a fully automatic chemiluminescence immunoassay analyzer as a detection tool to complete the detection of adiponectin. This adiponectin chemiluminescent immunoassay kit, after experiments, has a detection sensitivity of 0.01ng / mL, which is at least 10 times more sensitive than the traditional adiponectin detection method. The detection accuracy of the kit is high.
[0065] When this adiponectin chemiluminescence immunoassay kit is used, the adiponectin calibration product is detected by a fully automatic chemiluminescence immunoa...
Embodiment 1
[0117] Example 1: Preparation of adiponectin chemiluminescent immunoassay kit
[0118] (1) Preparation of magnetic particles coated with adiponectin monoclonal antibody:
[0119] Take 50 mg of carboxylated magnetic particles (MagnaBind TM , Cat. No. 21353) suspension, magnetically separated to remove the supernatant, resuspended with 0.02M, pH 5.5 MES buffer, added 0.5mL~2mL of newly prepared 10mg / mL EDC aqueous solution to activate the carboxyl groups on the surface of the magnetic beads, and added 3mg ~5 mg of adiponectin monoclonal antibody (Novus, NB100-65810), suspended at room temperature for 2h~10h, magnetically separated, removed the supernatant, and resuspended with 0.1M Tris buffer at pH 8.0 containing 2% BSA to 1mg / mL to obtain adiponectin monoclonal antibody-coated magnetic particles, and store in 5mL bottles at 4°C for future use.
[0120] (2) Preparation of adiponectin monoclonal antibody labeled with acridinium ester:
[0121] Take 500 μL 1.0 mg / mL adiponecti...
Embodiment 2
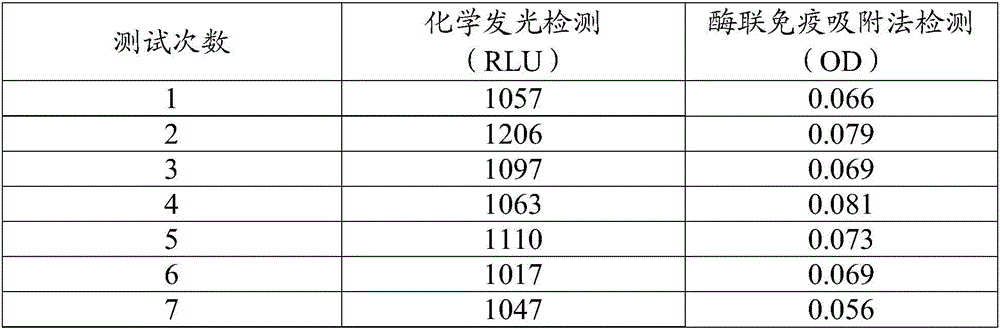
[0124] Example 2: Chemiluminescent immunoassay method for adiponectin acridinium ester
[0125] The present invention uses a full-automatic chemiluminescence immunoassay analyzer and the adiponectin chemiluminescence immunoassay kit prepared in Example 1 as detection tools. Magnetic particles coated with adiponectin monoclonal antibody and 50 μL of acridinium ester-labeled adiponectin monoclonal antibody were reacted for 10 minutes and then magnetically separated. The instrument sent the reaction mixture into a dark room, and sequentially added luminescent substrate A solution ( Contains 0.1M HNO 3 and 0.5%H 2 o 2 ) and B solution (containing 0.25M NaOH) for luminescence reaction, and finally record the luminescence intensity, and calculate the adiponectin content of the tested sample from the standard curve.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com