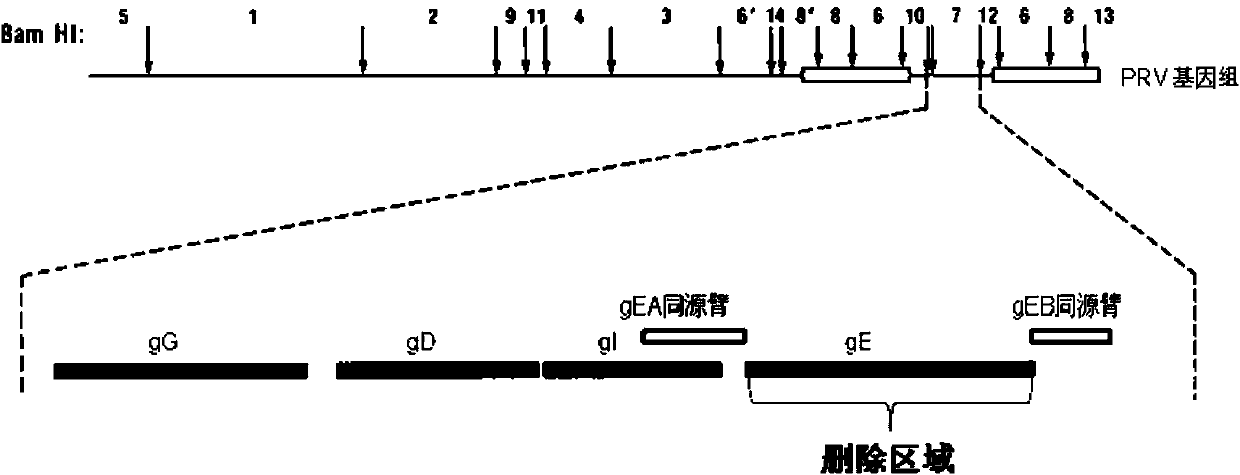
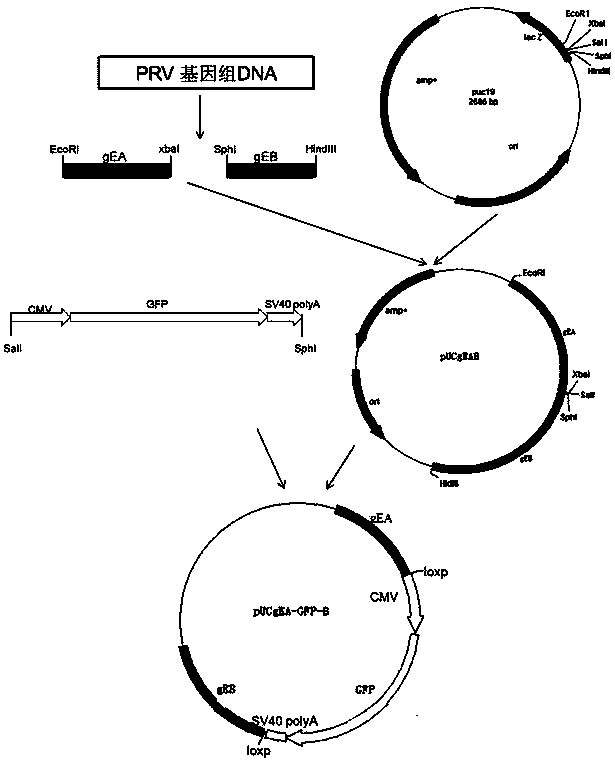
Porcine pseudorabies virus gene deletion strain, vaccine composition, and preparation method and application of vaccine composition
A technology for porcine pseudorabies and vaccine composition, which is applied in the field of animal virology and can solve the problems of inability to identify wild virus strains and vaccine strain infections and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, collection and isolation of virus
[0057] Isolate the sample from the suspected porcine pseudorabies infection sample from Henan, aseptically collect pig brain tissue, add MEM culture solution at 1:10 (volume ratio), grind, prepare tissue suspension, after repeated freezing and thawing for 3 times, 2000r Centrifuge at 1 / min for 15 min, collect the supernatant, and then filter through a 0.2 μm membrane filter, subculture on PK-15 cells at 37°C for 1 hour, replace with MEM medium containing 2% calf serum, and culture at 37°C for 5 days. Harvest the poisonous culture medium, freeze and thaw twice, collect the poison, and add MEM culture medium containing 2% calf serum. Porcine pseudorabies virus PCR detection kit (Beijing Century Yuanheng Animal Epidemic Prevention Technology Co., Ltd.) was used to detect porcine pseudorabies virus, and the result was positive; the isolated virus was detected by using PCR kit to detect exogenous virus (porcine blue ear disea...
Embodiment 2
[0059] Embodiment 2, the genetic characteristic of isolated virus
[0060] The genetic characteristics of the virus isolated in Example 1 were determined by genetic analysis. The genomic DNA of porcine pseudorabies virus isolated on PK15 cells was used as a template, and the primers shown in Table 1 were used for PCR. Primer Premier5.0 was used to design primer sequences for amplifying gB, gC, and gD genes, respectively.
[0061] Using the extracted genomic DNA as a template, prepare the PCR amplification system as follows: template DNA 100 μg, PrimerSTAR HS DNA Polymerase (2.5U / μl) 0.5 μl, 2×PrimerSTAR GC Buffer 25 μl, upstream and downstream primers 1 μl (10 pmol / μl), dNTP Mix (2.5mM each) 4 μl and make up the volume to 50 μl with distilled water. Carry out two-step PCR reactions: denaturation at 98°C for 10 sec, then annealing and extension at 68°C (calculate the required time according to 1 kb / min), a total of 30 cycles. The PCR reaction was terminated at 4°C. The resu...
Embodiment 3
[0064] Embodiment 3, the pathogenicity test of virus
[0065] 3.1 Pathogenicity of piglets of different ages
[0066] Six 34- to 35-day-old swine pseudorabies antibody-negative piglets were randomly divided into 2 groups, 4 pigs / group (test group) and 2 pigs / group (control group), and were inoculated with porcine pseudorabies virus HN1201 strain (challenge dose 2×10 8.0 TCID 50 / head), the control group was inoculated with DMEM medium; at the same time, four 49-day-old piglets were inoculated with the highly virulent HN1201 strain (the challenge dose was 2×10 8.0 TCID 50per head), and 35-day-old piglets were still used as the control. After the virus inoculation, the body temperature of the piglets was measured every day, and the clinical symptoms and death were observed. The specific results are shown in Table 2.
[0067] Table 2 Pathogenicity of porcine pseudorabies virulent HN1201 strain to piglets of different ages
[0068]
[0069]
[0070] The results showed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com