Canine parvovirus attenuated strain, vaccine composition prepared from canine parvovirus attenuated strain and application
A technology of canine parvovirus and vaccine composition, which is applied in the direction of viruses, vaccines, antiviral agents, etc., and can solve problems such as harm, loss, and inability to protect dogs in the breeding industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 The acquisition of canine parvovirus JM35 strain
[0043] 1. Take well-grown cat kidney cells (F81 cells), digest them with trypsin, and inoculate them in cell flasks with 90% to 98% volume ratio of RPMI 1640 culture medium and 2% to 10% volume ratio of newborn bovine Serum cell growth solution (pH adjusted to 6.8-7.2) was cultured at 33°C-37°C for virus inoculation.
[0044] 2. The canine parvovirus JM strain was inoculated synchronously with the above passaged cells, and the cell growth liquid (pH adjusted to 6.8-7.2 ) at 33° C. to 37° C. to continue culturing, and after 72 h to 120 h, when more than 80% of the cells have pathological changes, the cell culture virus liquid is harvested.
[0045] 3. Repeat the above steps for continuous subculture to obtain the attenuated canine parvovirus strain, and sequence the obtained attenuated canine parvovirus strain. The results show that the 63rd nucleotide of the VP2 gene undergoes a stable synonymous mutation fro...
Embodiment 2
[0046] Example 2 Study on Biological Characteristics of Canine Parvovirus JM35 Strain
[0047] 1. Pathogenicity test
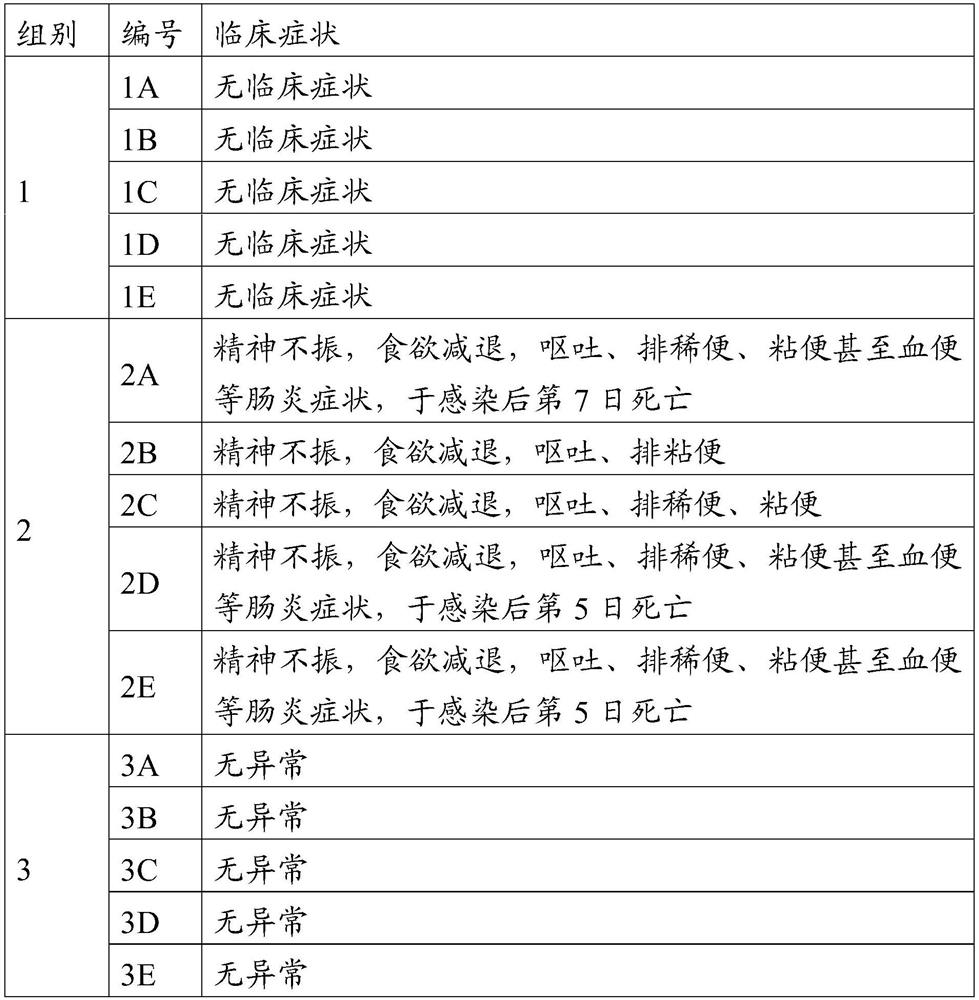
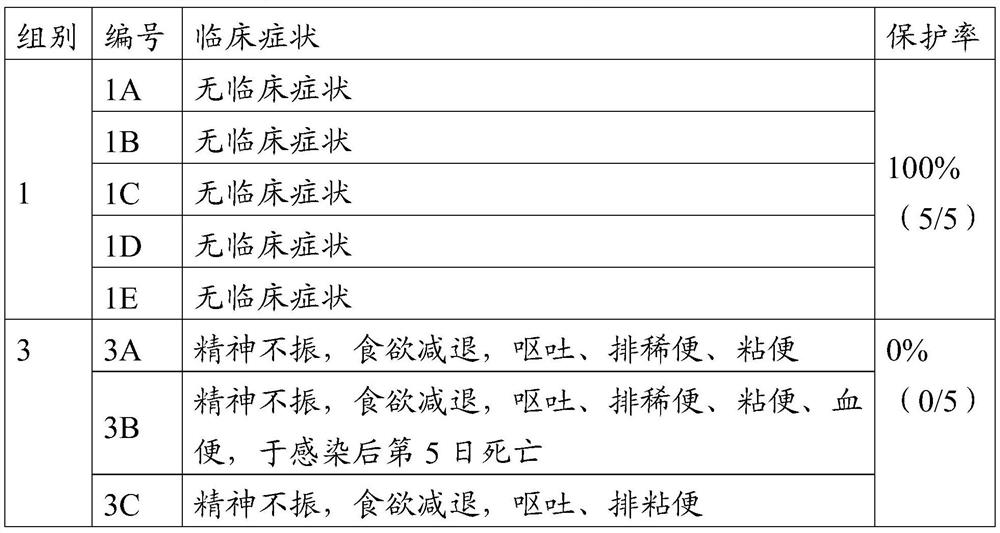
[0048] Fifteen healthy susceptible antigen-negative dogs aged 2 to 3 months were randomly divided into 3 groups with 5 dogs in each group. See Table 1 for the grouping and challenge.
[0049] Table 1 Grouping of test animals for pathogenicity of canine parvovirus JM35 strain
[0050] group Inoculation strain Inoculation dose 1 JM35 strain Subcutaneous injection of 4ml (10 6.5 FAID 50 / ml)
2 JM strain Subcutaneous injection of 4ml (10 6.5 FAID 50 / ml)
3 RPMI 1640 Medium Subcutaneous injection 4ml
[0051] Observed for 10 days after virus inoculation, and observed and recorded the clinical manifestations of dogs such as spirit, appetite, and feces every day. The specific results are shown in Table 2.
[0052] Table 2 Pathogenicity of canine parvovirus JM35 strain to dogs
[0053]
[0054] The results sho...
Embodiment 3
[0080] Embodiment 3 Preparation of Canine Parvovirus JM35 Strain Attenuated Live Vaccine
[0081] 1. Proliferation of virus
[0082]The canine parvovirus JM35 strain virus seed prepared in Example 1 was synchronously inserted into the F81 cell suspension, and the RPMI 1640 culture solution containing 90% to 98% by volume and 2% to 10% by volume of newborn bovine serum were added for cell growth solution (pH adjusted to 6.8-7.2) was cultivated at 33°C-37°C. When the cytopathic rate reaches about 80%, the virus is collected by freezing and thawing, and the virus content is measured, and stored at low temperature.
[0083] 2. Preparation of protective agent
[0084] Add 40 g of sucrose and 8 g of gelatin per 100 ml of deionized water, and after fully melting, sterilize with high pressure steam (121° C. for 30 min).
[0085] 3. Vaccine Preparation
[0086] The virus liquid prepared and preserved above is mixed with the protective agent at a ratio of 1:1 (volume ratio), and fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com