Novel vaccine adjuvant and application thereof in novel coronavirus pneumonia vaccine and other vaccines
A vaccine adjuvant and vaccine technology, applied in the field of biomedicine, can solve the problem of ineffective antigen-specific cellular immunity, achieve the effect of enhancing fat solubility and stability, and improving hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
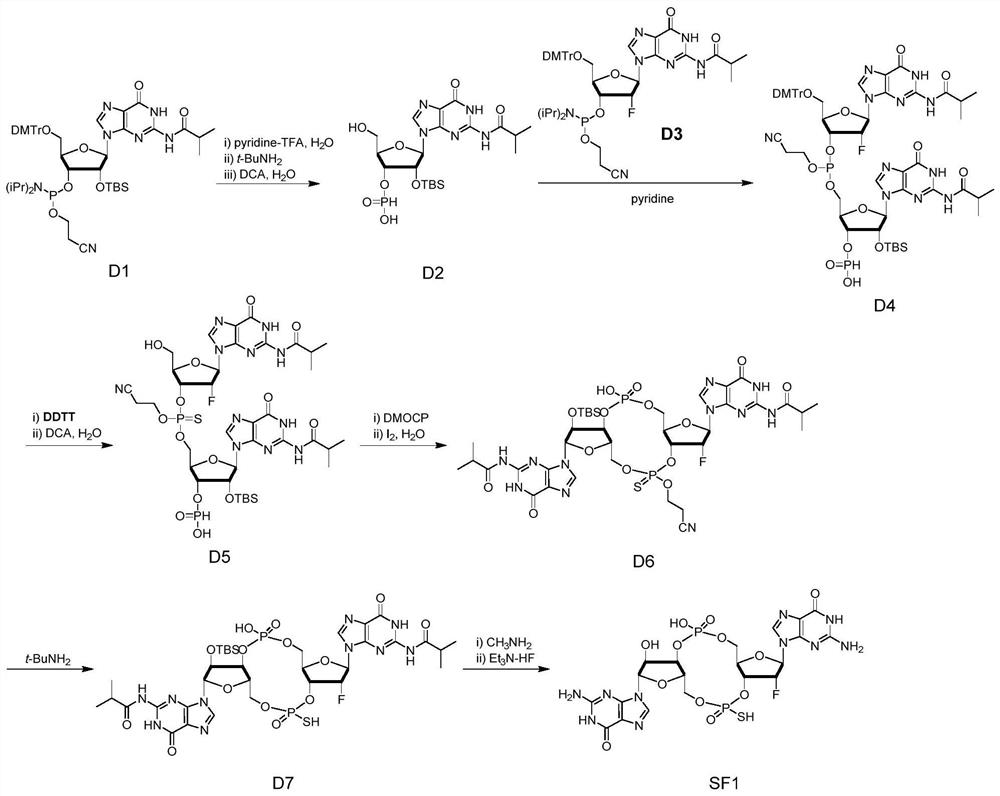
[0112] The synthesis of embodiment 1SF1
[0113] In this example, guanylic acid was taken as an example to synthesize SF1. SF1 is a cyclic dinucleotide modified with monothiomonofluoride on the same side. Its preparation method is as follows. For specific steps, please refer to the attached figure 1 Reaction formula:
[0114] Compound D2
[0115] Weigh 0.5mmol of guanylate phosphoramidite monomer (S1) and 0.116g (0.6mmol) of pyridine trifluoroacetate into a 50mL round bottom flask, add magnetron and 2.5mL of acetonitrile and stir to dissolve, then add 18μL of water The reaction was stirred for 1 min. Then add 2.5mL t-BuNH 2 The reaction was stirred for 10 min. After the reaction is over, remove the solvent under negative pressure until it becomes fluffy, then add 5 mL of acetonitrile and rotate until it becomes fluffy, and repeat the operation twice to remove t-BuNH 2 . Add 6 mL of dichloromethane (DCM) to dissolve the solid, then add 90 μL of water and 6 mL of 6% dichl...
Embodiment 2
[0125] Add 10 mL of 33% methylamine absolute ethanol solution (mass ratio) to the round-bottomed flask containing D6, put on a rubber stopper and stir for 1.5 hours, and take a sample in the middle for ESI-MS detection to determine the removal of the isobutyryl (iBu) protecting group Condition. After the reaction was completed, it was concentrated to an oily state, and 400 μL pyridine and 200 μL triethylamine were added to continue rotary evaporation to an oily state, and the operation was repeated three times to convert the product from t-BuNH 2 The salt form is converted to the triethylamine salt form. Then add 400 μL of pyridine to dissolve the oil, stopper the bottle mouth with a rubber stopper, and place the round bottom flask in a 50°C oil bath for stirring. Use a syringe to draw 1.4mL triethylamine and 0.83mL triethylamine hydrofluoride respectively and inject them into the round bottom flask synchronously and slowly for 1min. After the injection was completed, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com