Patents
Literature
114 results about "Family Circoviridae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
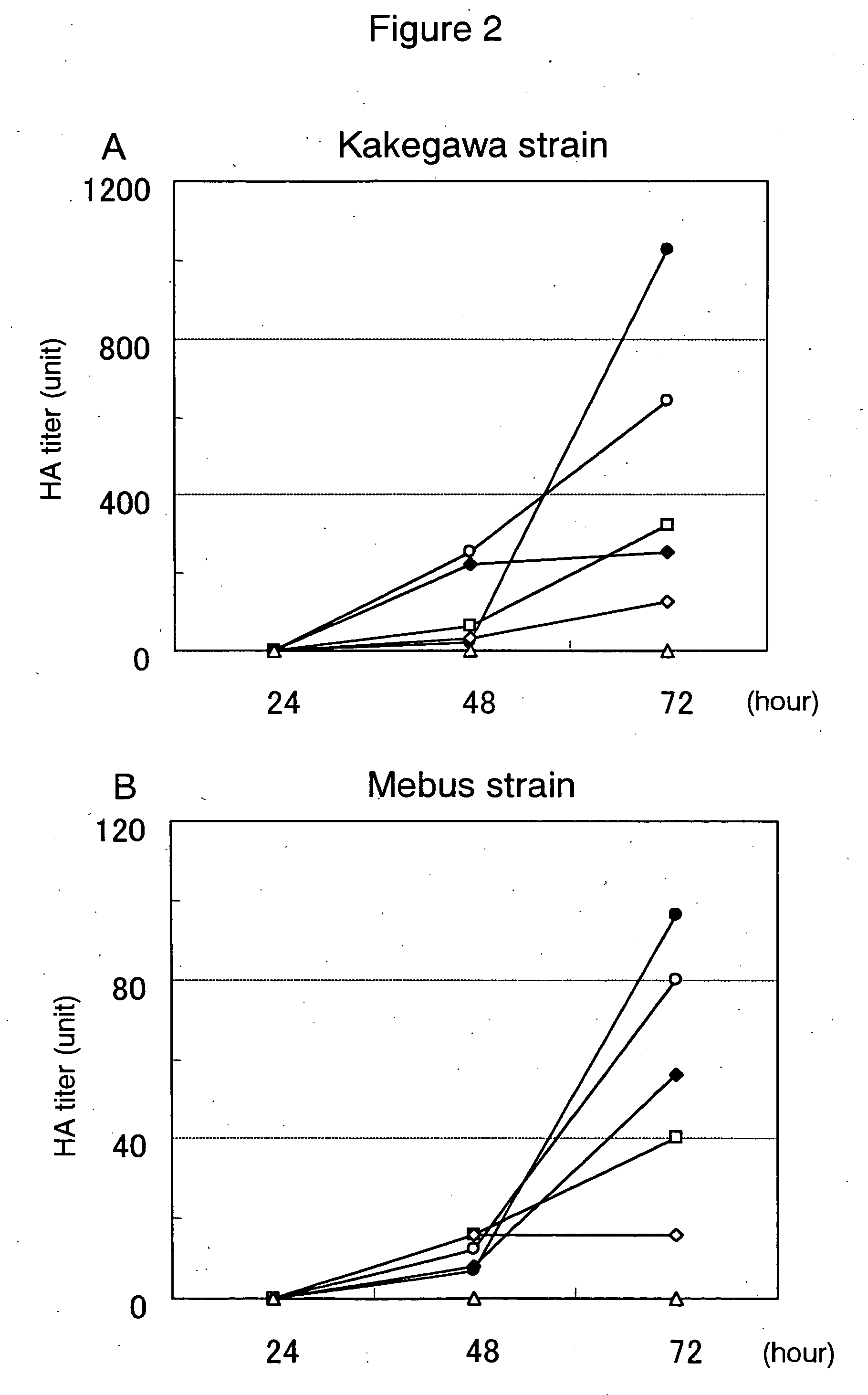
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
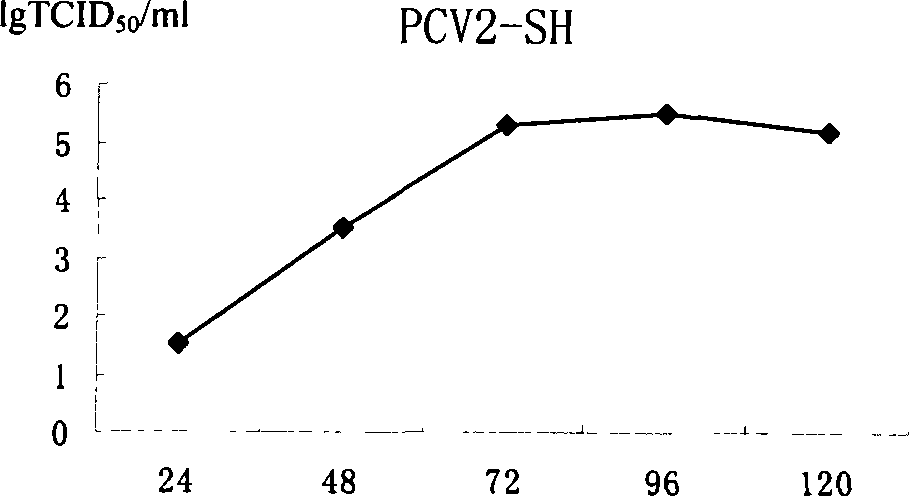
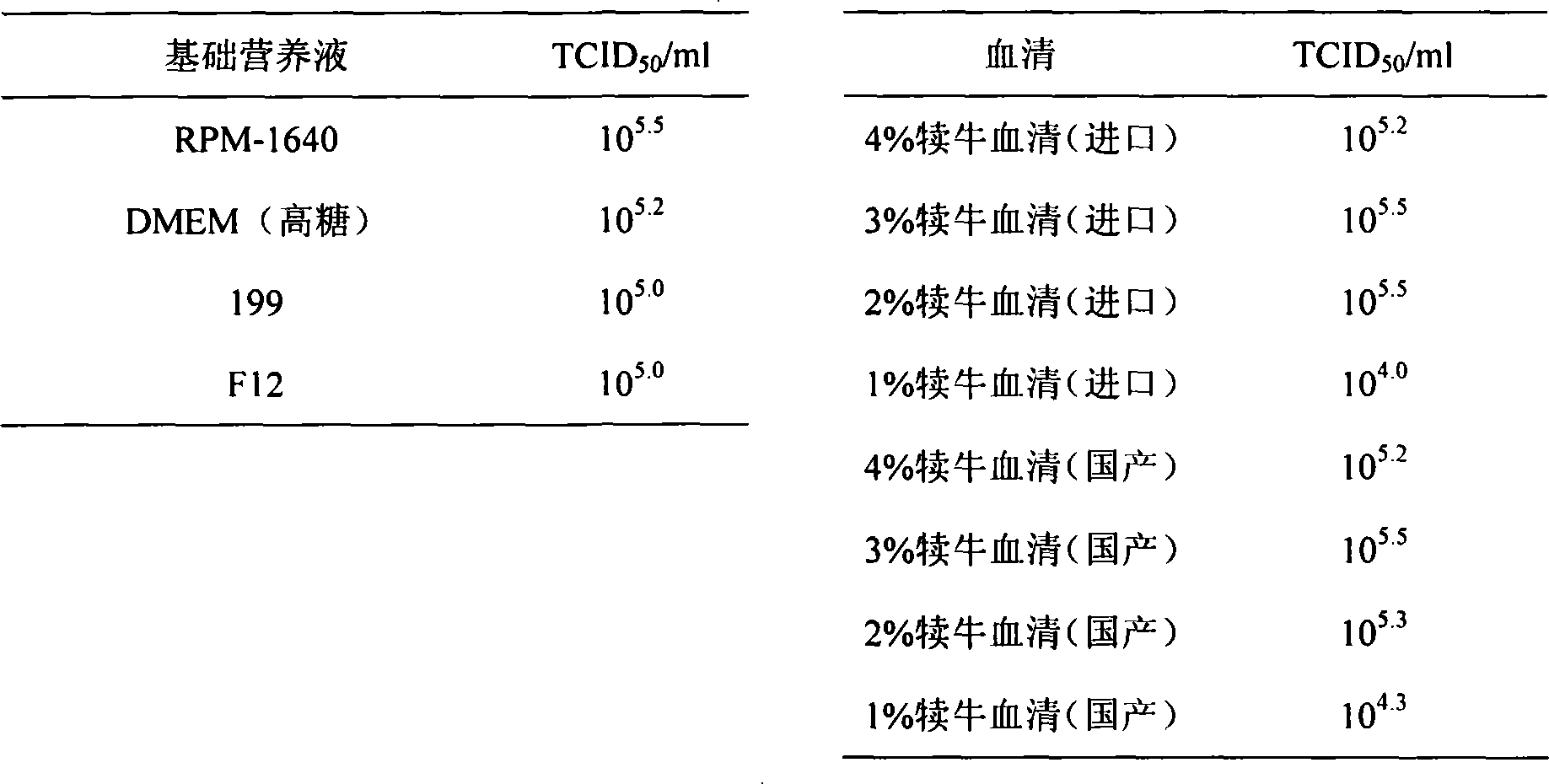
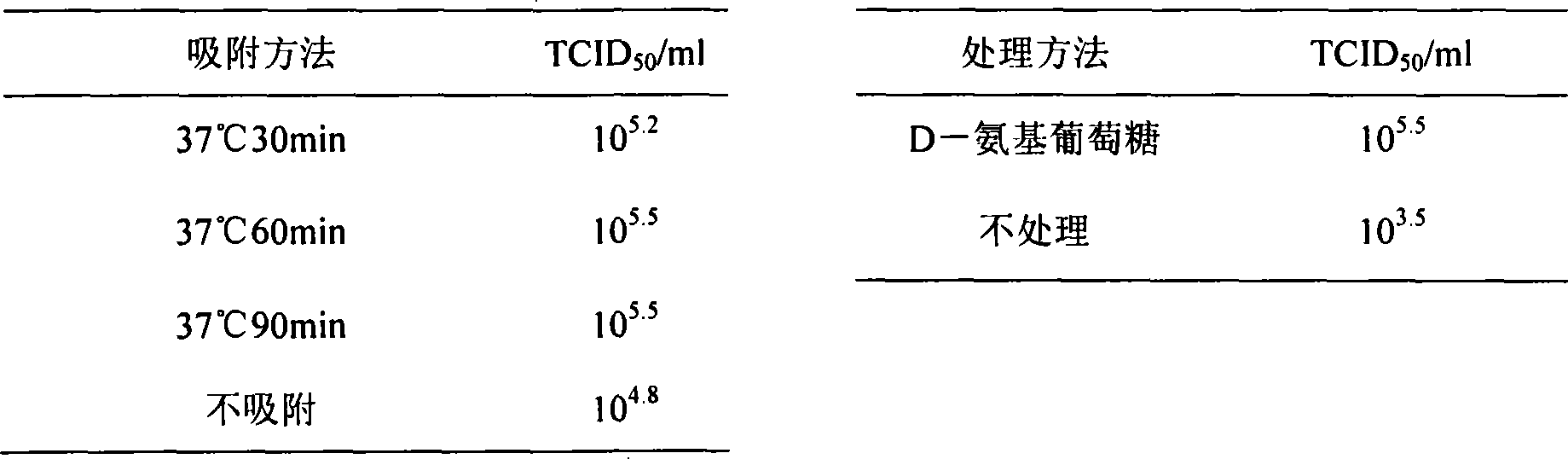
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
2′-branched nucleosides and Flaviviridae mutation
ActiveUS7824851B2High sensitivityReduce Flaviviridae infectionBiocideSsRNA viruses positive-senseAmino acidMutant strain
The present invention discloses a method for the treatment of Flaviviridae infection that includes the administration of a 2′-branched nucleoside, or a pharmaceutically acceptable prodrug and / or salt thereof, to a human in need of therapy in combination or alternation with a drug that directly or indirectly induces a mutation in the viral genome at a location other than a mutation of a nucleotide that results in a change from seine to a different amino acid in the highly conserved consensus sequence, XRXSGXXXT (Sequence ID No. 63), of domain B of the RNA polymerase region, or is associated with such a mutation. The invention also includes a method to detect a mutant strain of Flaviviridae and a method for its treatment.
Owner:INDENIX PHARM LLC
Antisense antiviral compound and method for treating ssRNA viral infection
ActiveUS20060269911A1Promote absorptionSsRNA viruses positive-senseMicrobiological testing/measurementOligonucleotideAstroviridae
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Flaviviridae, Picomoviridae, Caliciviridae, Togaviridae, Arteriviridae, Coronaviridae, Astroviridae and Hepeviridae families in the treatment of a viral infection. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of 1240 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 5′-terminal end 40 bases of the positive-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
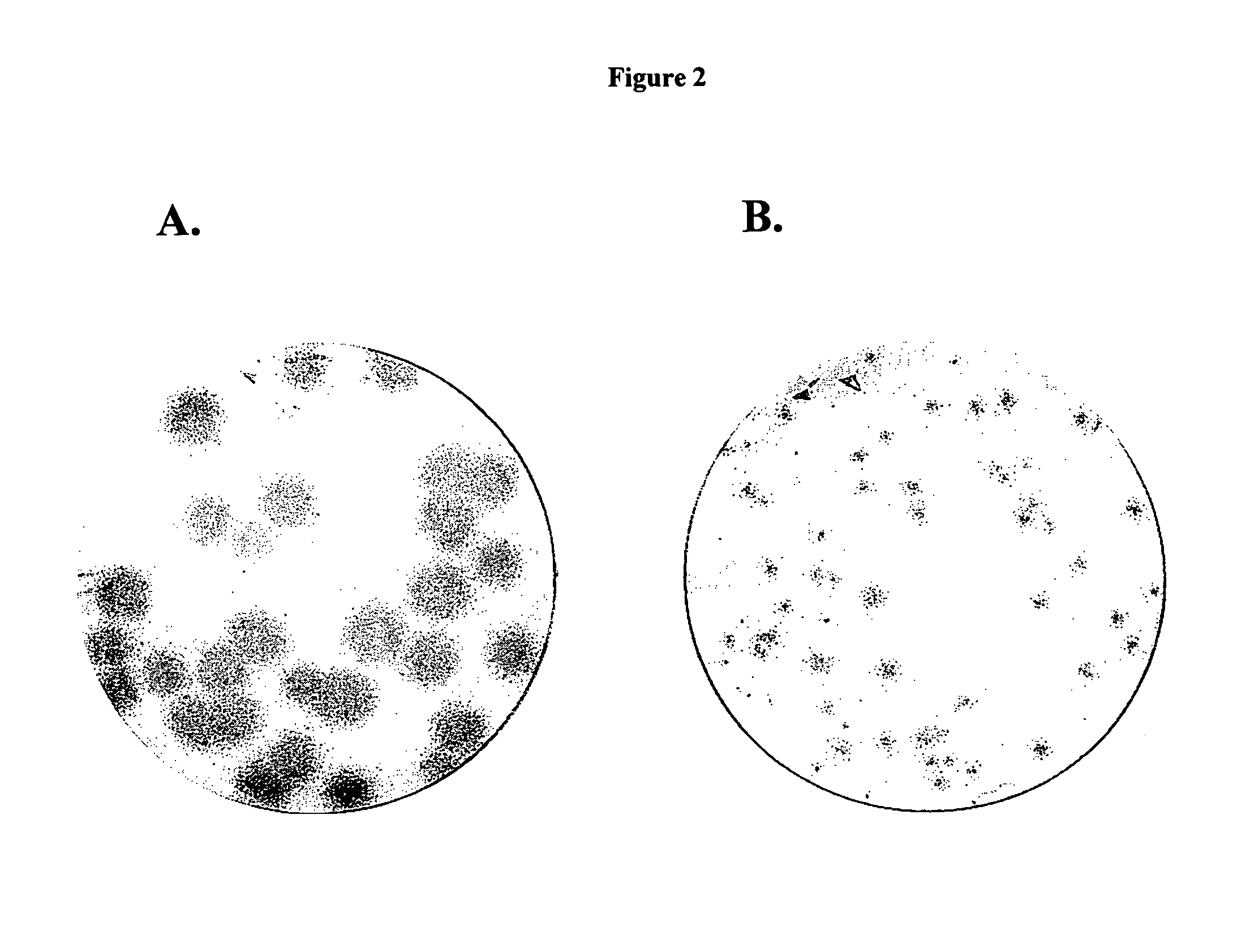
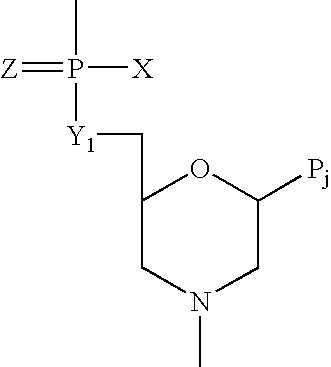
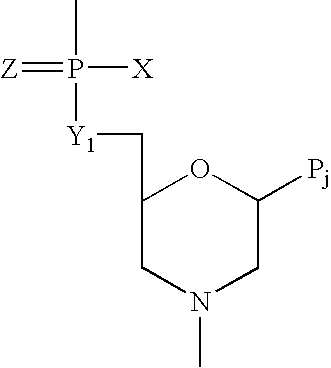
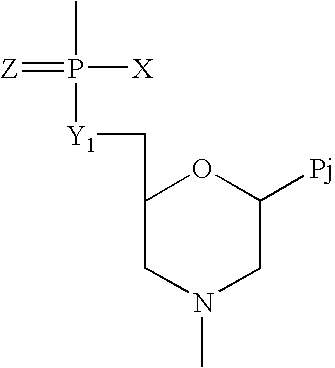
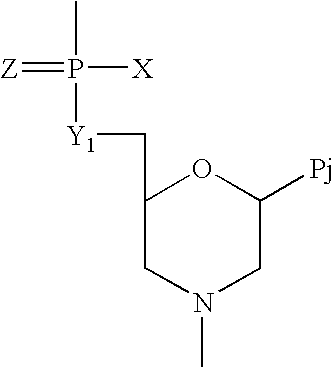
Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections
InactiveUS20040082574A1Potent and selective activityPotent activityBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
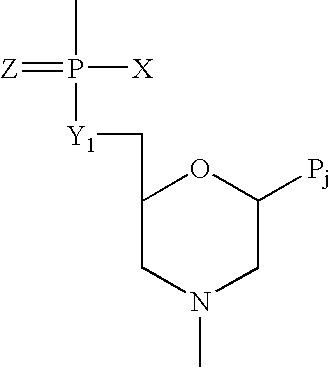
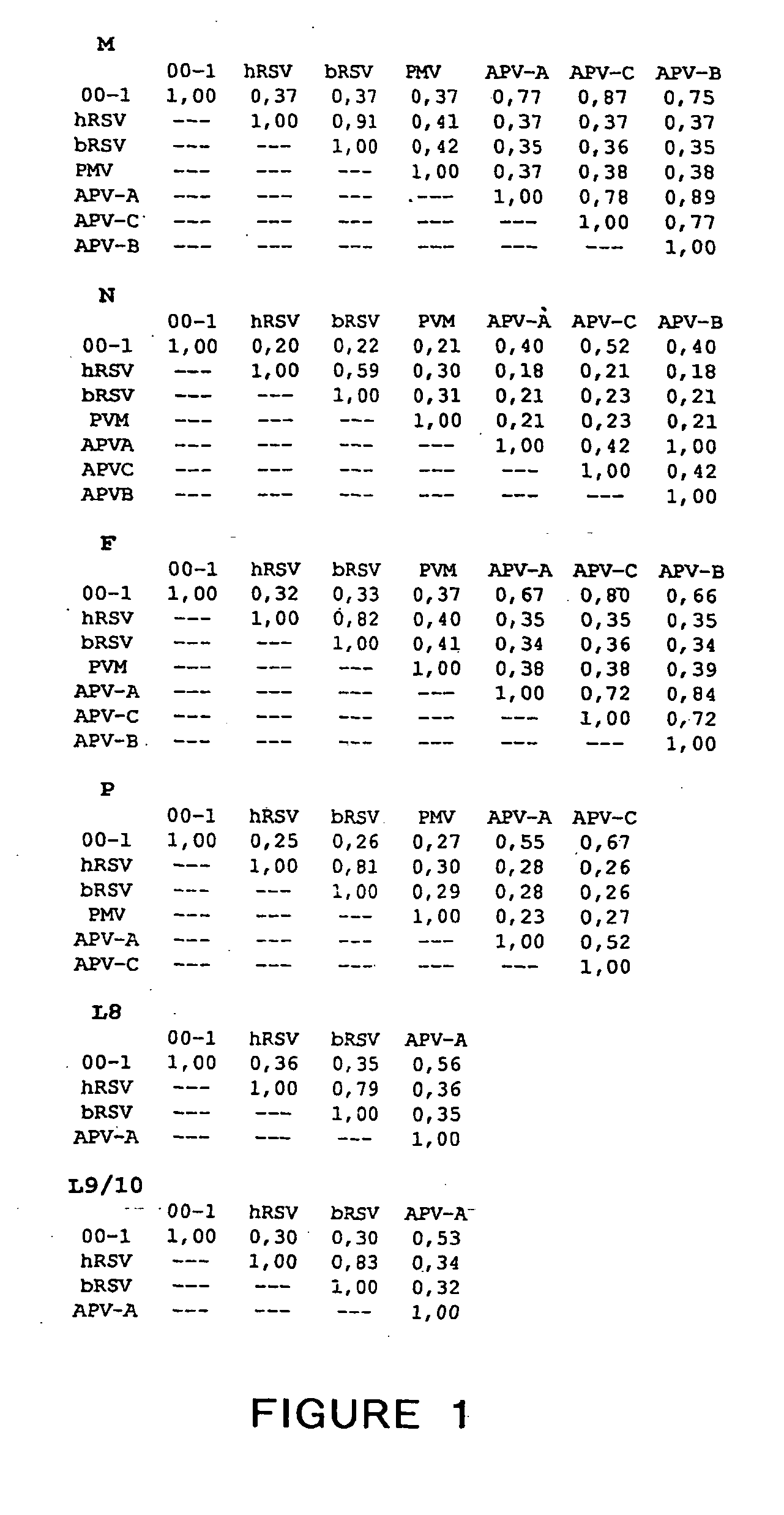
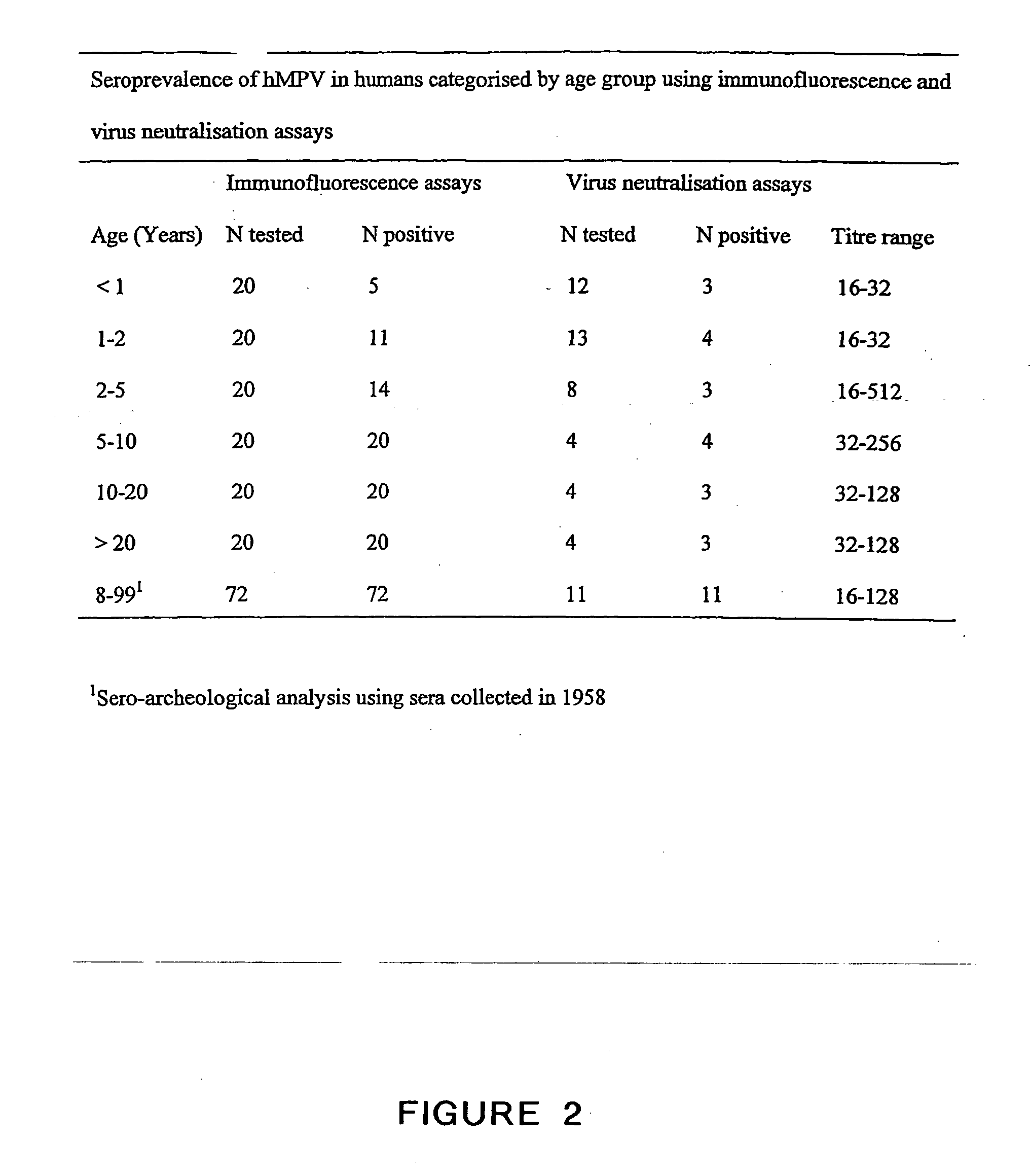
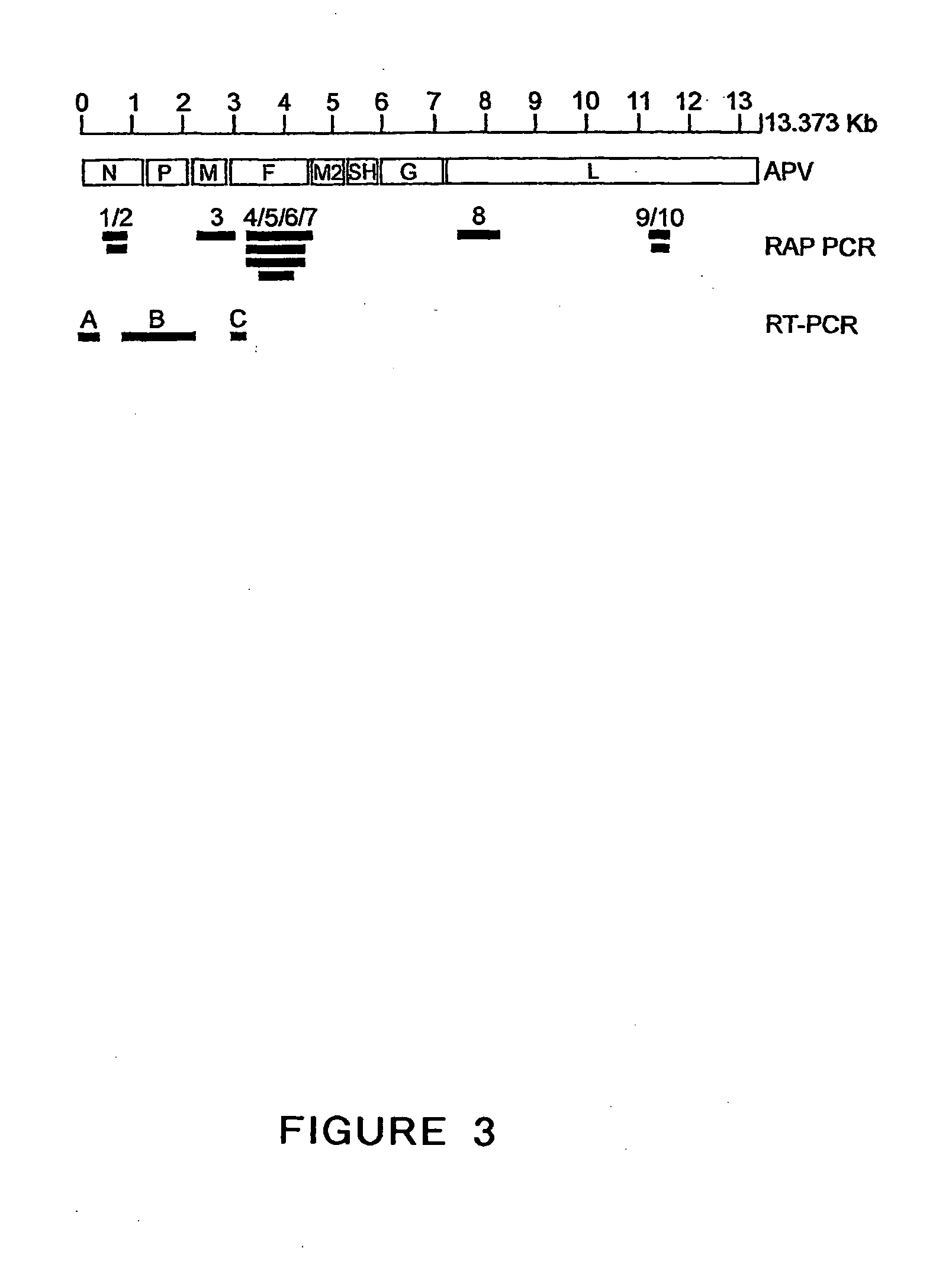
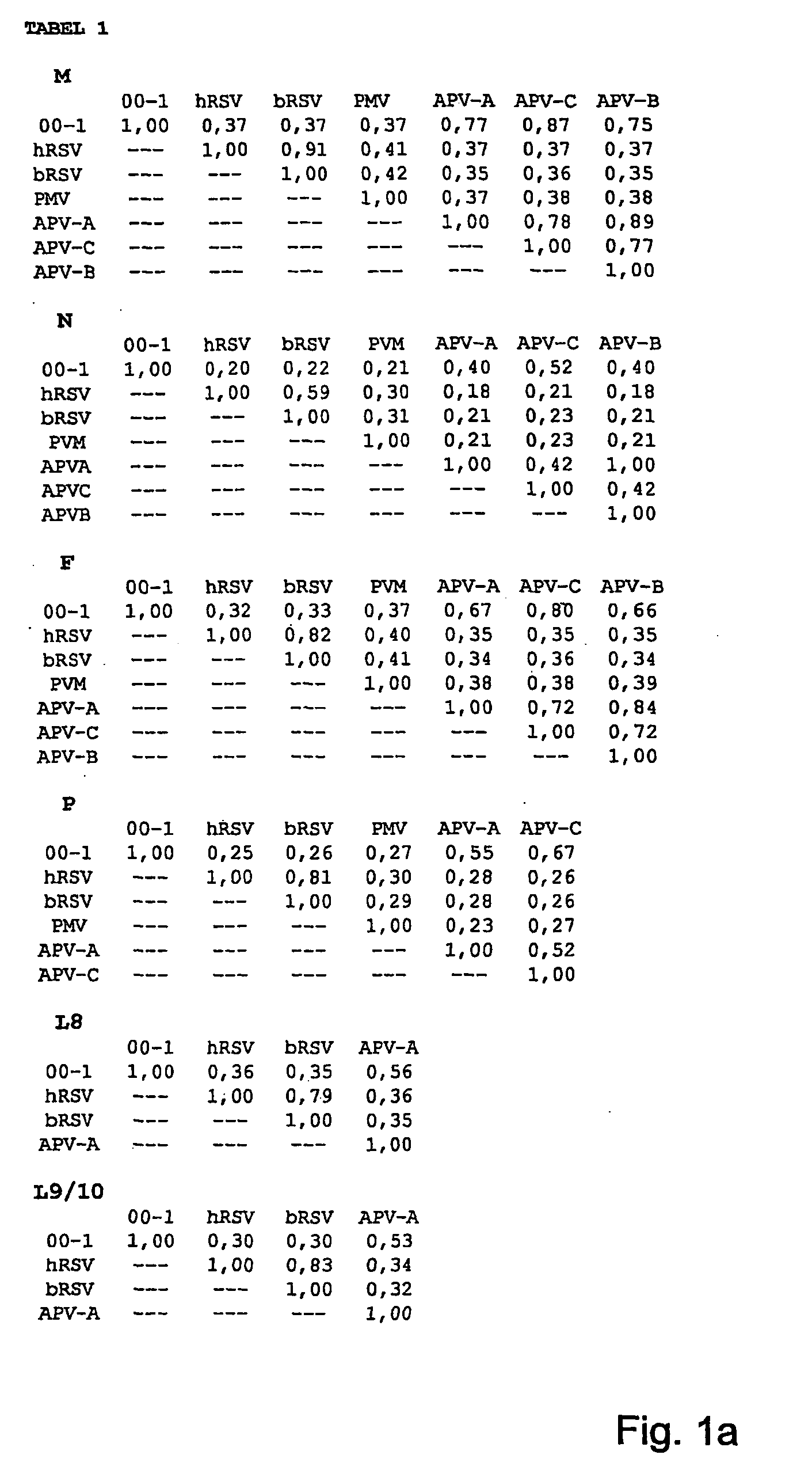
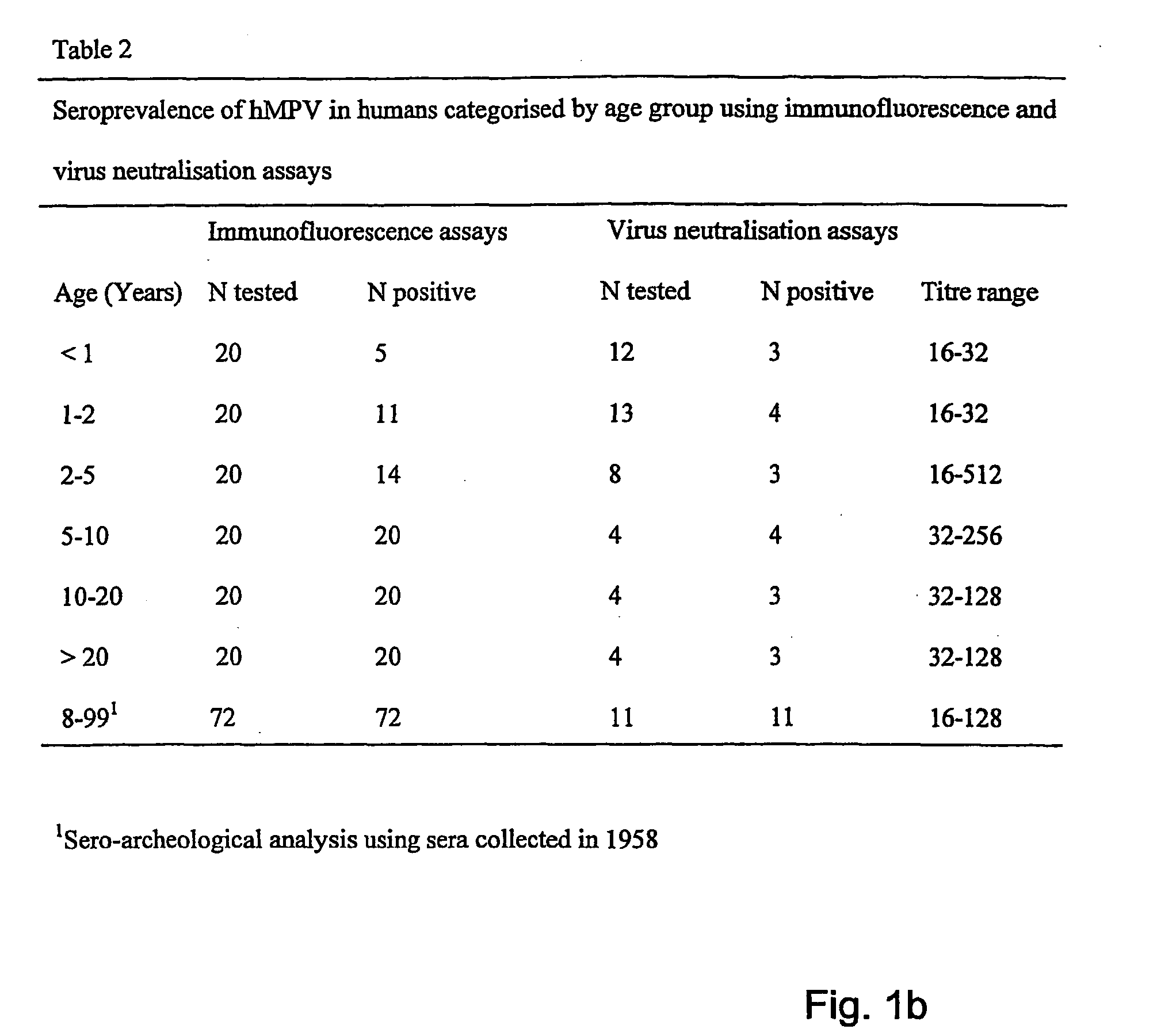
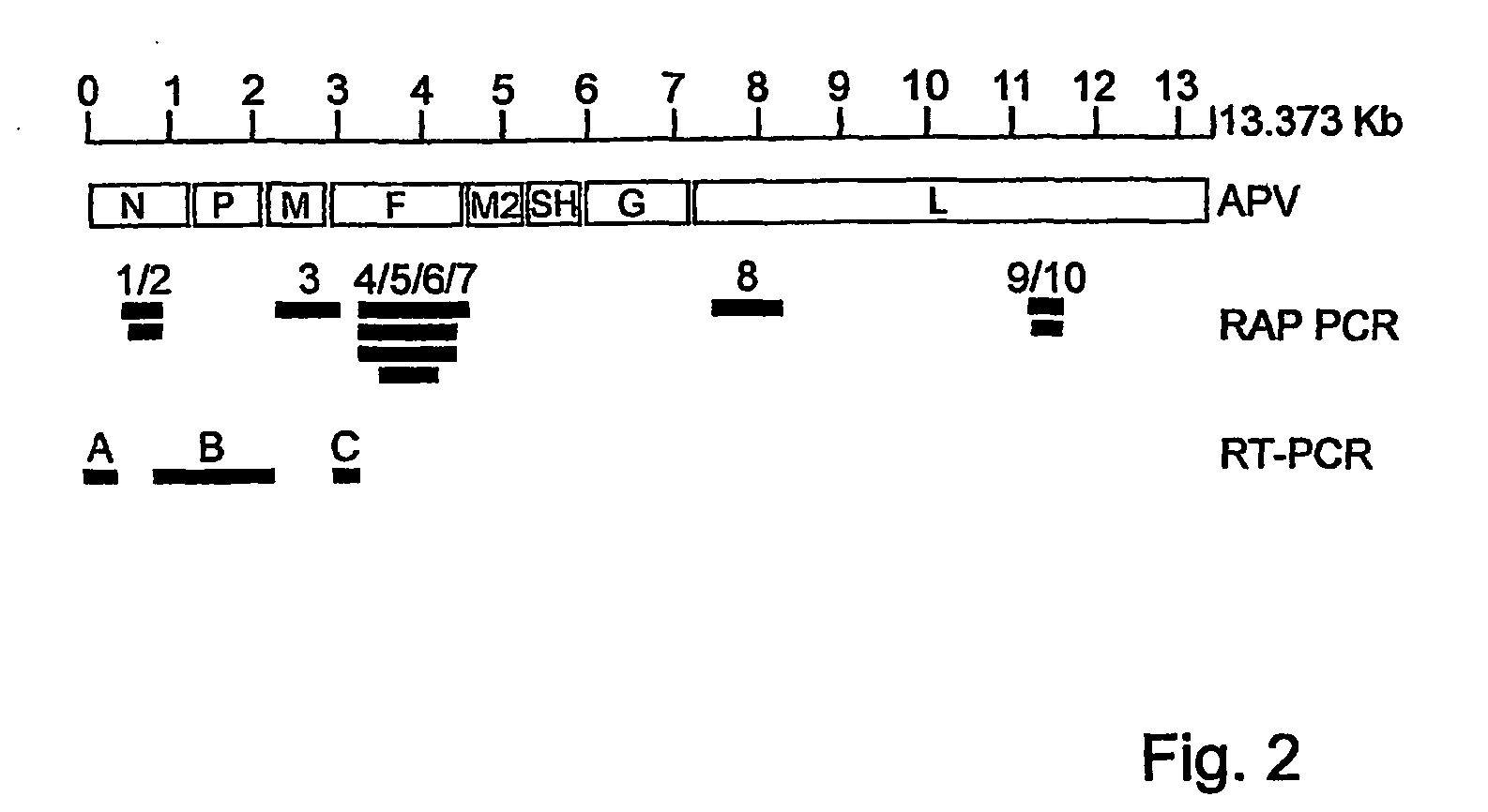
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
ActiveUS20050019891A1Narrow downSymptoms improvedSsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
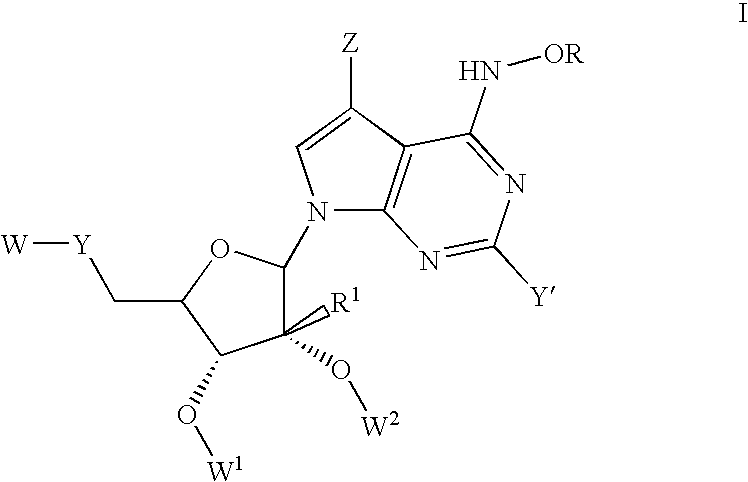
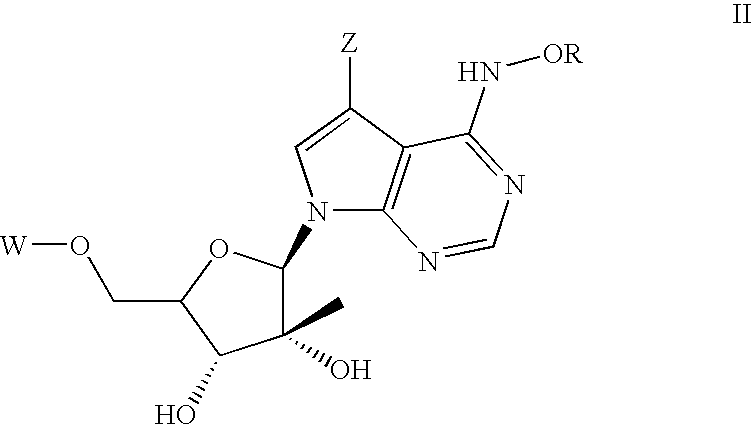
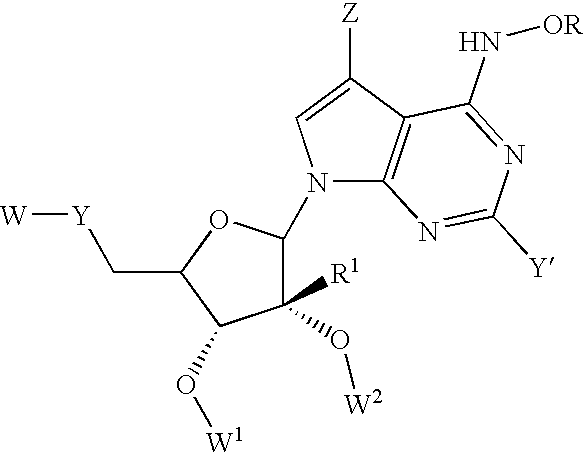
Nucleoside derivatives for treating hepatitis C virus infection
Disclosed are 6-hydroxyamino- or a 6-alkoxyamino-7-deazapurine-ribofuranose derivatives, salts, pharmaceutical compositions, and methods of use thereof for treating viral infections caused by a flaviviridae family virus, such as hepatitis C virus.
Owner:SMITHKLINE BECKMAN CORP
Heteroaryl derivatives for treating viruses
Disclosed are compounds, compositions, and methods for treating Flaviviridae family virus infections.
Owner:SMITHKLINE BECKMAN CORP
Virus causing respiratory tract illness in susceptible mammals
The invention relates to the field of virology. The invention provides an isolated essentially mammalian negative-sense single stranded RNA virus (MPV) within the sub-family Pneumovirinae of the family Paramyxoviridae and identifiable as phylogenetically corresponding to the genus Metapneumovirus and components thereof.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
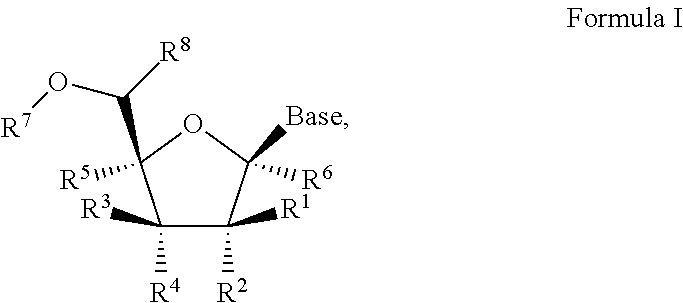
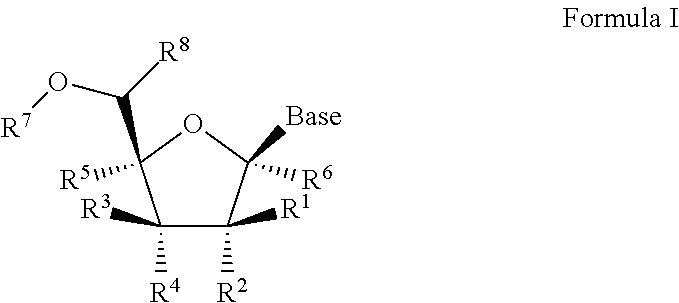
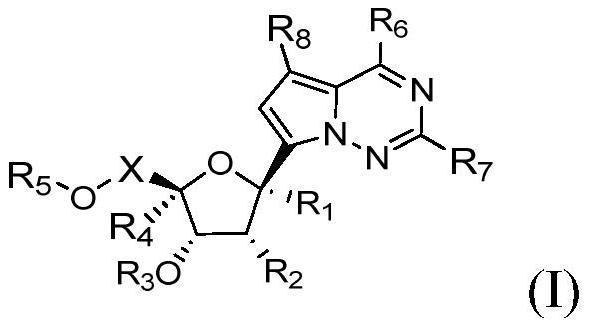
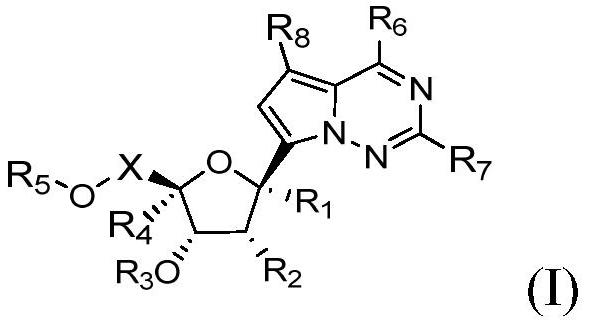
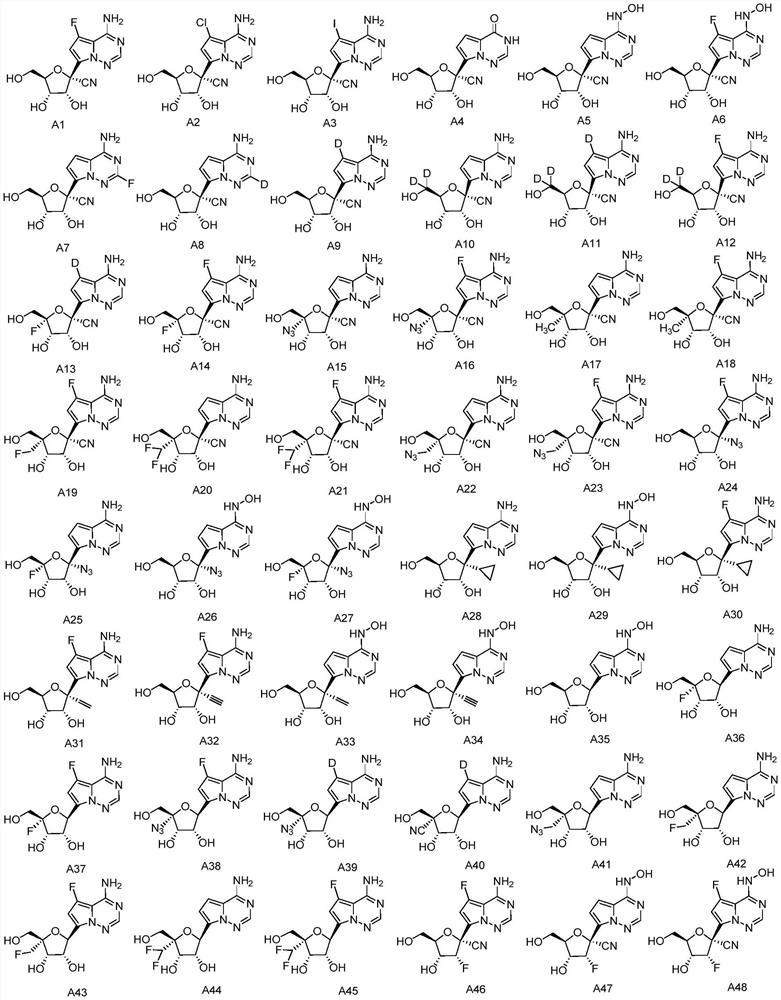
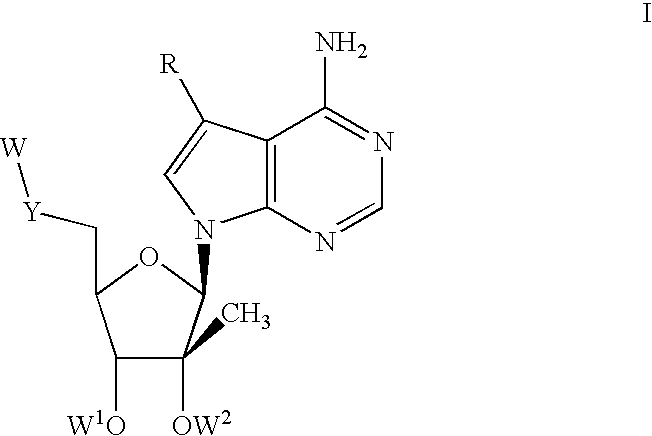
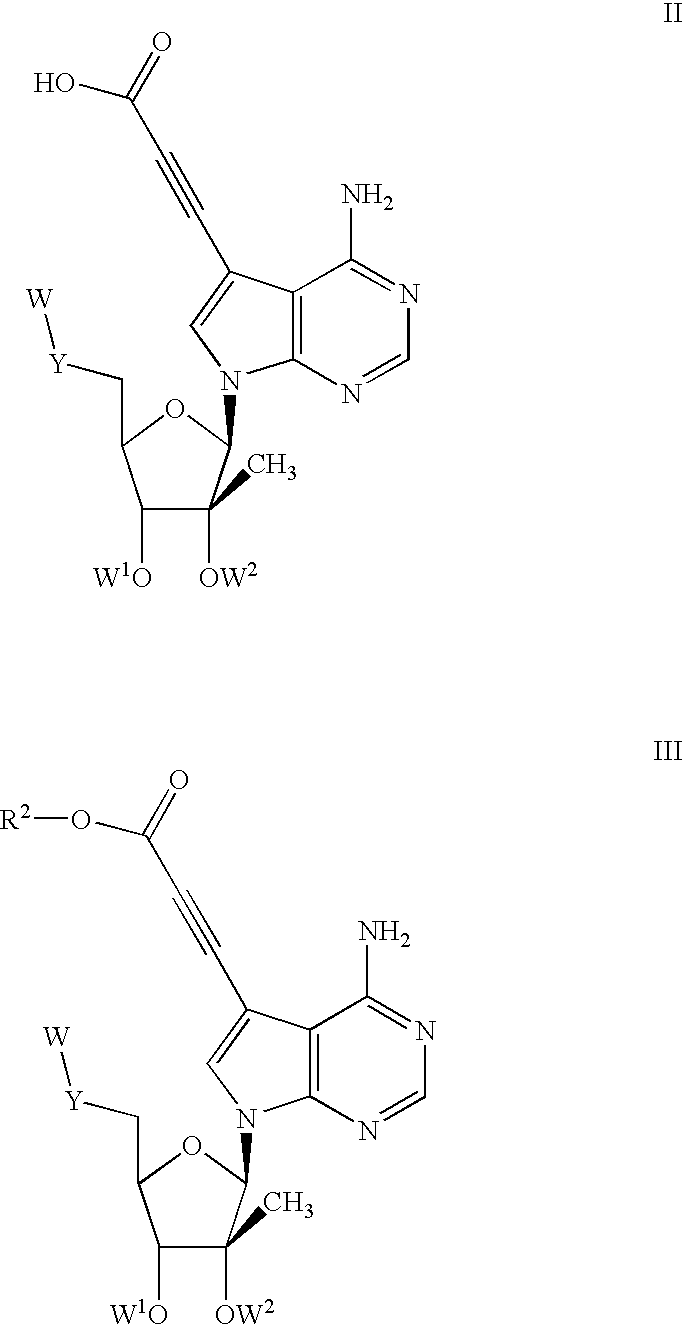
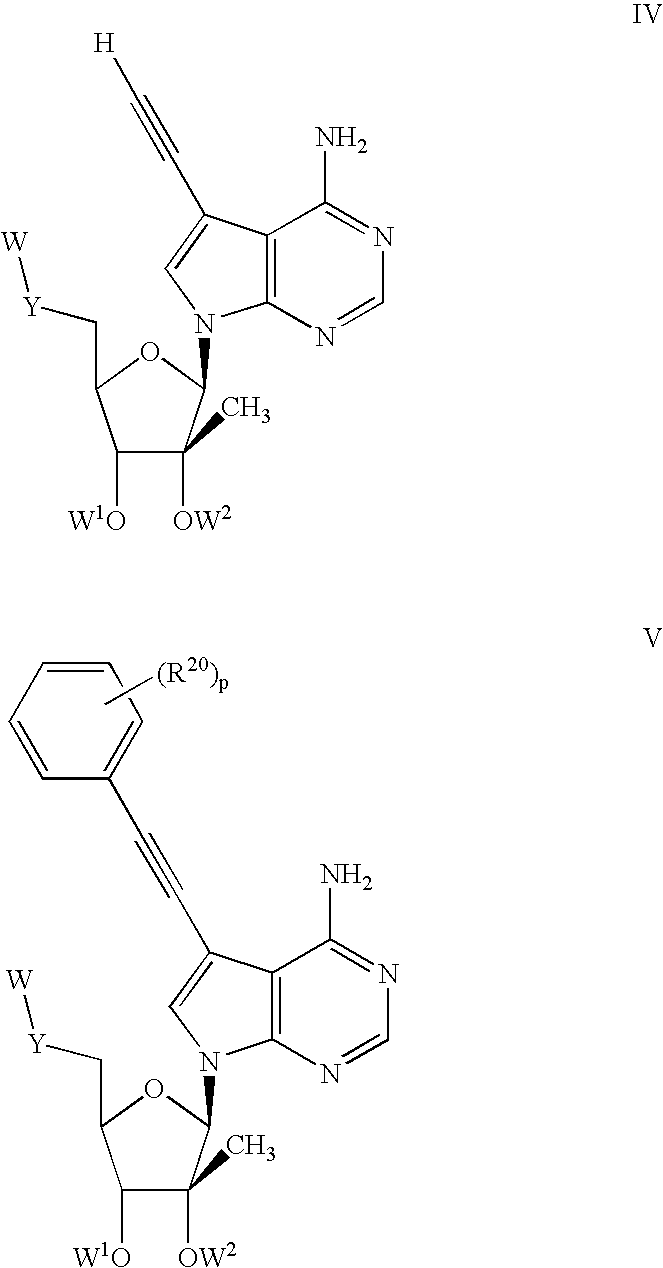
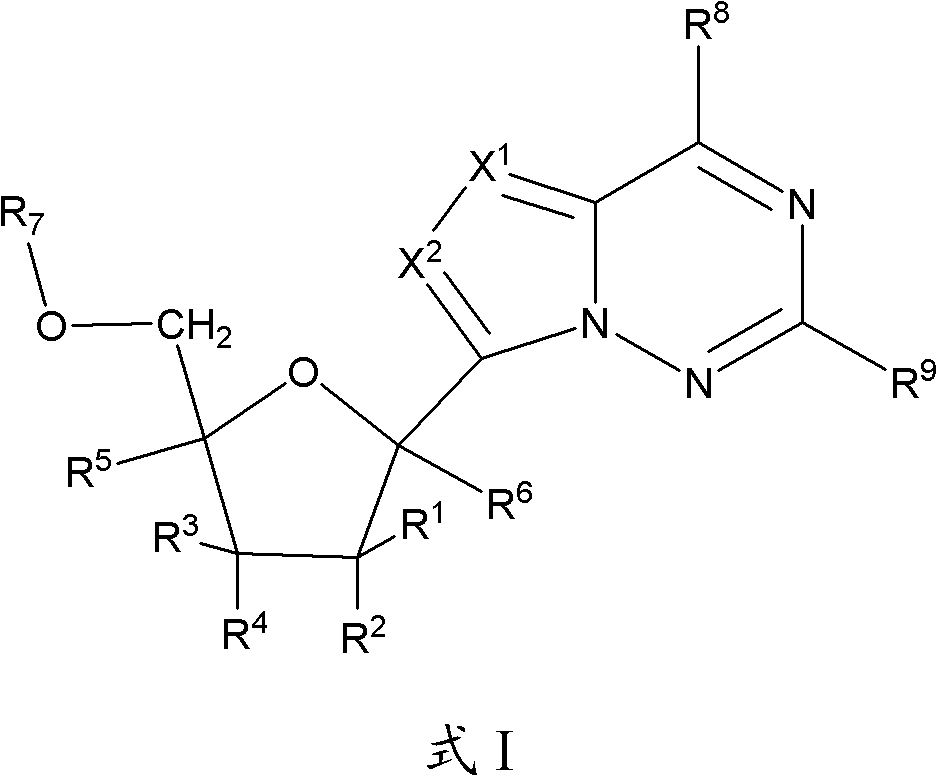
1'-substituted pyrimidine n-nucleoside analogs for antiviral treatment
ActiveUS20120263678A1Improve cell selectivityInhibition of replicationBiocideSugar derivativesPyrimidineNucleoside Analogs
Provided are compounds of Formula I:nucleosides, nucleoside phosphates and prodrugs thereof, wherein R6 is CN, ethenyl, 2-haloethen-1-yl, or (C2-C8)-alkyn-1-yl. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections.
Owner:GILEAD SCI INC
Compounds with the bicyclo[4.2.1]nonane system for the treatment of Flaviviridae infections
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Antisense antiviral compound and method for treating ssRNA viral infection
The invention provides antisense antiviral compounds and methods of their use and production in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Arteriviridae, Coronaviridae, Astroviridae and Hepeviridae families in the treatment of a viral infection. The antisense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of 12-40 subunits, including at least 12 subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 5′-terminal end 40 bases of the positive-sense RNA strand of the virus.
Owner:SAREPTA THERAPEUTICS INC
Application of nucleoside analogue or combined preparation containing nucleoside analogue in virus resistance
PendingCN112778310ASaccharide with heterocyclic radicalsGroup 5/15 element organic compoundsDisease causePorcine epidemic diarrhea virus
The invention relates to an application of nucleoside analogs in virus resistance. Specifically, the invention relates to the use of nucleoside analogs and pharmaceutical compositions thereof as (a) inhibitors for inhibiting the replication of coronavirus, influenza virus, respiratory syncytial virus, flaviviridae virus, filoviridae virus and / or porcine epidemic diarrhea virus (PEDV); and / or (b) medicines for treating and / or preventing and relieving related diseases caused by infection of coronavirus, influenza virus, respiratory syncytial virus, flaviviridae virus, filoviridae virus and / or porcine epidemic diarrhea virus (PEDV). The nucleoside analogue disclosed by the invention can be used for treating and / or preventing and relieving related diseases such as respiratory tract infection, pneumonia (COVID-19) and the like caused by 2019 novel coronavirus infection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +3
Triazines And Related Compounds Having Antiviral Activity, Compositions And Methods Thereof
Disclosed herein are novel triazines and related compounds, the synthesis thereof, and compositions, including pharmaceutical compositions, comprising the novel triazines and related compounds. Such novel triazines and related compounds function to inhibit or block entry of viruses of the Flaviviridae family, including Hepatitis C virus (HCV), into cells that are susceptible to virus infection. These compounds are useful for the treatment, therapy and / or prophylaxis of viral diseases and infection, including HCV infection.
Owner:PROGENICS PHARMA INC
Nucleoside compounds for treating viral infections
Disclosed are compounds, compositions and methods for treating viral infections caused by a flaviviridae family virus, such as hepatitis C virus.
Owner:SMITHKLINE BECKMAN CORP
Inhibitors of Flaviviridae viruses
Provided are compounds of Formula I:and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
Sense Antiviral Compound and Method for Treating Ssrna Viral Infection
InactiveUS20080311556A1Disruption of secondary structureInhibition of replicationOrganic active ingredientsBiocideSsRNA virusesViral infection
The invention provides sense antiviral compounds and methods of their use in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Coronaviridae families and hepatitis E virus in the treatment of a viral infection. The sense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of (12-40) subunits, including at least (12) subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 3′-terminal end (40) bases of the negative-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
Bacteriophage or lytic protein derived from the bacteriophage which effective for the treatment of staphylococcus aureus biofilm
ActiveUS20100254950A1Eliminate effectiveGood treatment effectAntibacterial agentsBiocideDiseaseBiofilm
The present invention relates to compositions for removing a biofilm formed by Staphylococcus aureus, comprising a bacteriophage, such as Myoviridae family T4-like phage genus bacteriophage (Accession No: KCTC 11153BP, SAP-I) or Podoviridae family φ29-like virus genus bacteriophage (Accession No: KCTC11154BP, SAP-2), and lytic protein derived therefrom, that destroys the biofilm. Also disclosed are pharmaceutical compositions for the treatment of diseases caused by Staphylococcus aureus capable of forming biofilm.
Owner:INTRON BIOTECHNOLOGY INC
N-(6-membered aromatic ring)-amido anti-viral compounds
Owner:SMITHKLINE BECKMAN CORP
Anti-viral compounds, compositions, and methods of use
Disclosed are compounds and compositions of Formula (I), pharmaceutically acceptable salts and solvates thereof, and their preparation and uses for treating viral infections mediated at least in part by a virus in the Flaviviridae family of viruses.
Owner:SMITHKLINE BECKMAN CORP
Viral replication inhibitors
ActiveUS20140213586A1Efficiently inhibit proliferationPromote efficient proliferationBiocideOrganic chemistryRNA Virus InfectionsMedicine
The present invention relates to a series of novel compounds, methods to prevent or treat viral infections in animals by using the novel compounds and to said novel compounds for use as a medicine, more preferably for use as a medicine to treat or prevent viral infections, particularly infections with RNA viruses, more particularly infections with viruses belonging to the family of the Flaviviridae, and yet more particularly infections with the Dengue virus. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the novel compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of viral infections. The invention also relates to processes for preparation of the compounds.
Owner:KATHOLIEKE UNIV LEUVEN
2' -fluoro substituted CARBA-nucleoside analogs for antiviral treatment
InactiveCN102596958AGroup 5/15 element organic compoundsMulticolor photographic processingPyrroleHalogen
Provided are pyrrolo[1,2-f][1,2,4]triazinyl, imidazo[1,5-f][1,2,4]triazinyl, imidazo[1,2-f][ 1,2,4]triazinyl, and [1,2,4]triazolo[4,3-f][1,2,4]triazinyl nucleosides, nucleoside phosphates and prodrugs thereof, wherein the 2' position of the nucleoside sugar is substituted with halogen and carbon substitutents. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections caused by both wild type and mutant strains of HCV.
Owner:GILEAD SCI INC
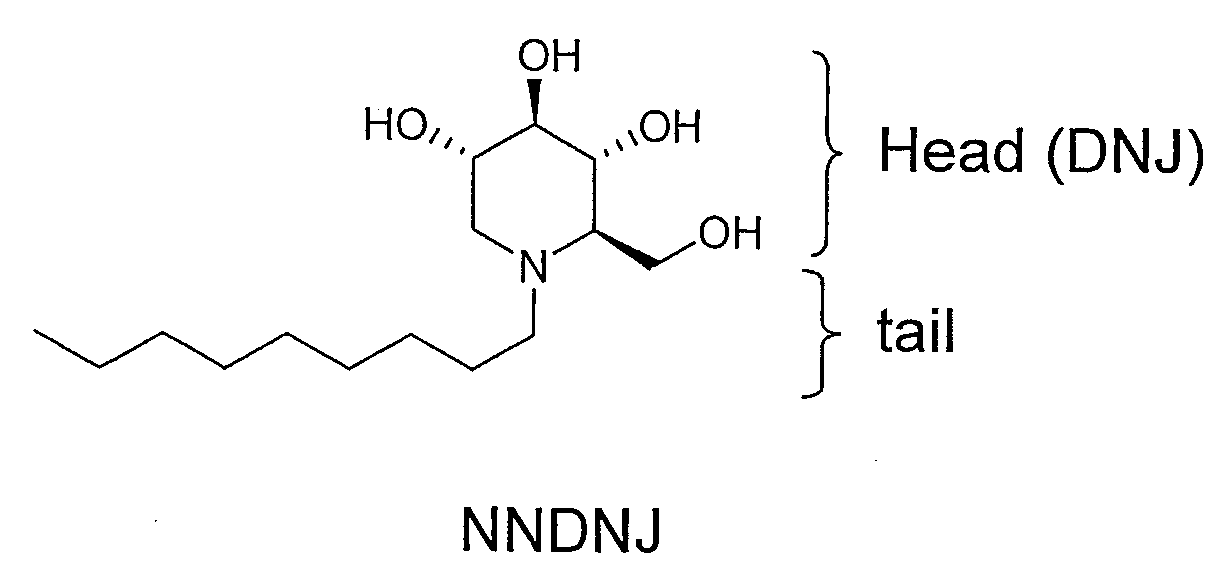
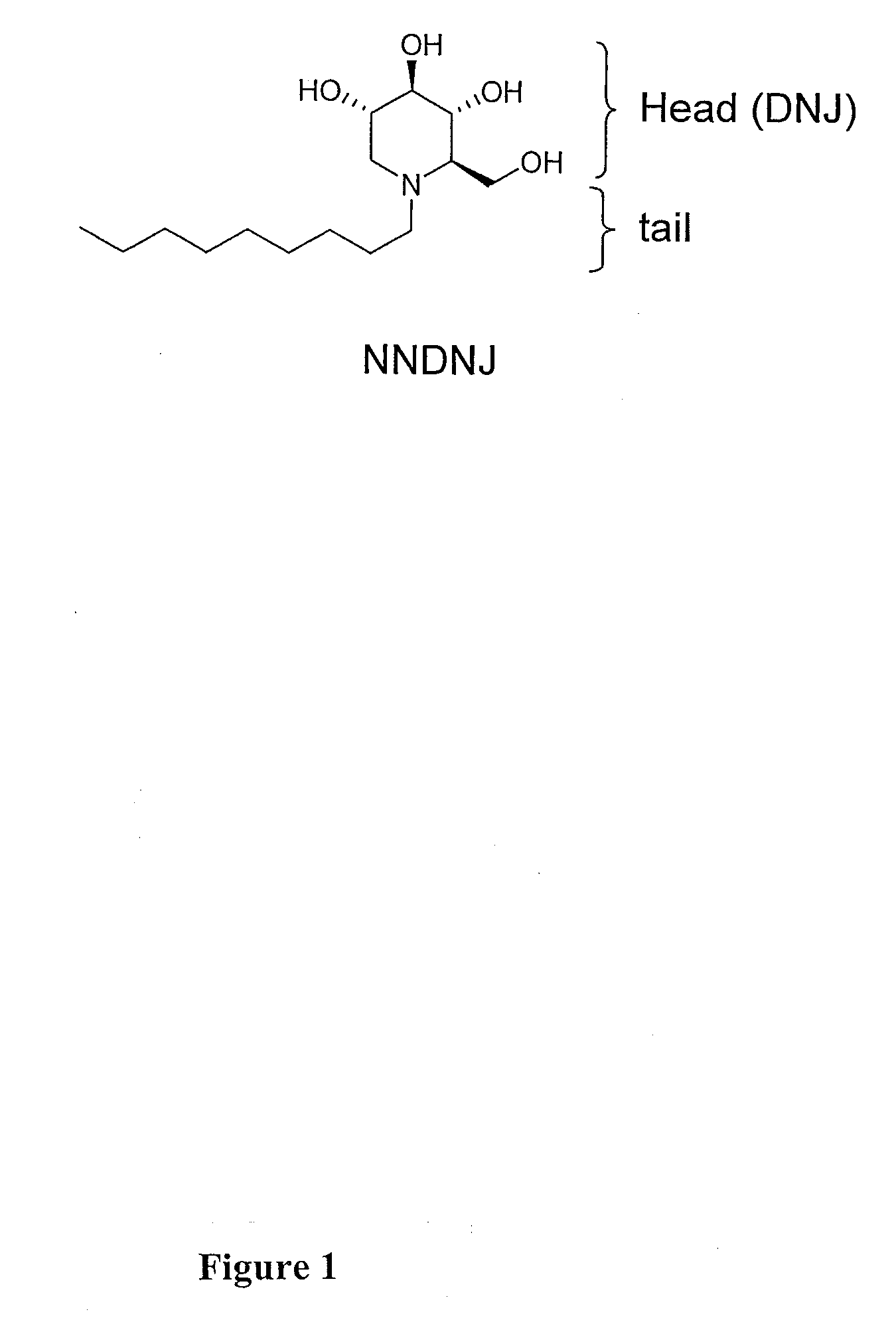
Iminosugar compounds with antiflavirus activity
An anti-viral compounds effective against viruses belonging to the Flaviviridae family, wherein the anti-viral compounds are 1,5-dideoxy-1,5-imino-D-glucitol derivative compounds having the general formula (I)wherein R2, R3, R4 and R5 are the same or different and are selected from the group consisting of hydrogen, acyl, benzyl, alkyl, aryl, sulfonyl, phosphonyl, silyl, R6 is at least one of alkyl or branched alkyl, heteroalkyl or aryl, R6′ is a bridging group selected from at least one of bicycle[2.2.1]heptyl, bicycle[3.2.1]octyl, oxa analogs, admonyl and cubyl, n′=2-10, n″=1-10, enantiomers and stereoisomers of said compounds and physiologically acceptable salts or solvates of said compounds, enantiomer or stereoisomer.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
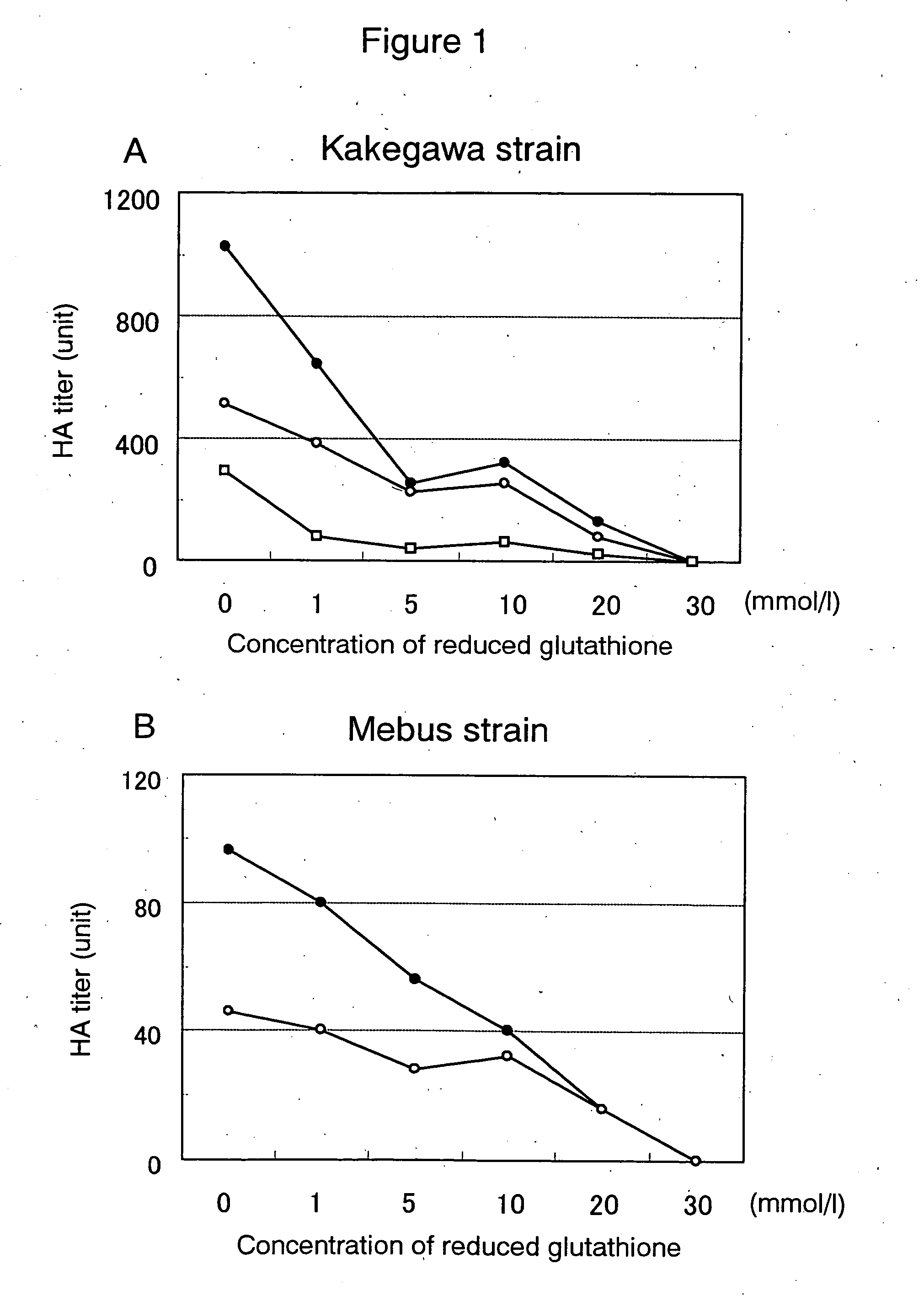
Preventive or therapeutic composition for viral infectious disease
InactiveUS20060189542A1Preventing flavivirus infectious diseasesOrganic active ingredientsBiocideOxidized GlutathioneFlaviviridae
The invention provides a composition for preventing or treating infectious diseases of a virus belonging to the Coronavirus family or Flavivirus family, the composition containing one or more substances selected from reduced glutathione or oxidized glutathione or pharmaceutically acceptable salts thereof and catechin, and a composition for preventing or treating infectious diseases of a virus belonging to the Coronavirus family or Flavivirus family, the composition containing reduced glutathione or oxidized glutathione or pharmaceutically acceptable salts thereof and catechin.
Owner:KYOWA HAKKO BIO CO LTD
Inhibitors of flaviviridae viruses
Provided are compounds of Formula I:and pharmaceutically acceptable salts and esters thereof. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections.
Owner:GILEAD SCI INC
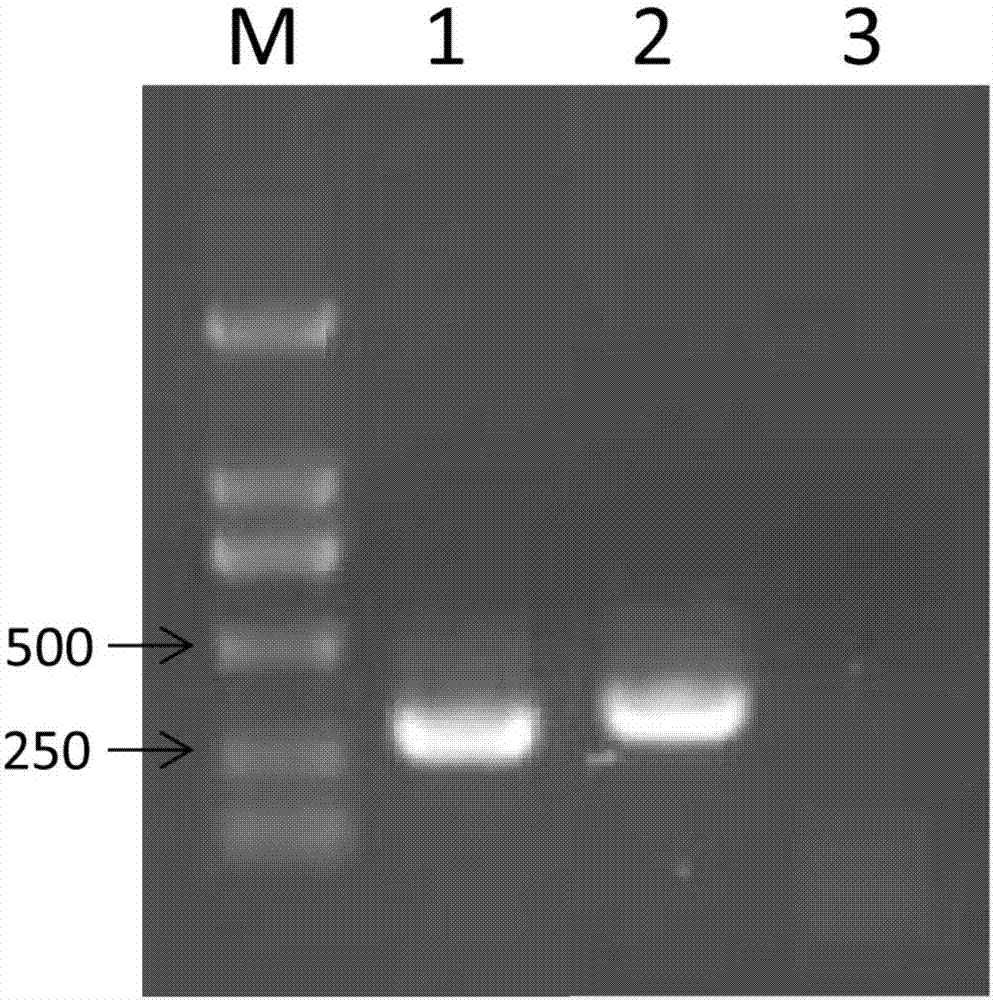
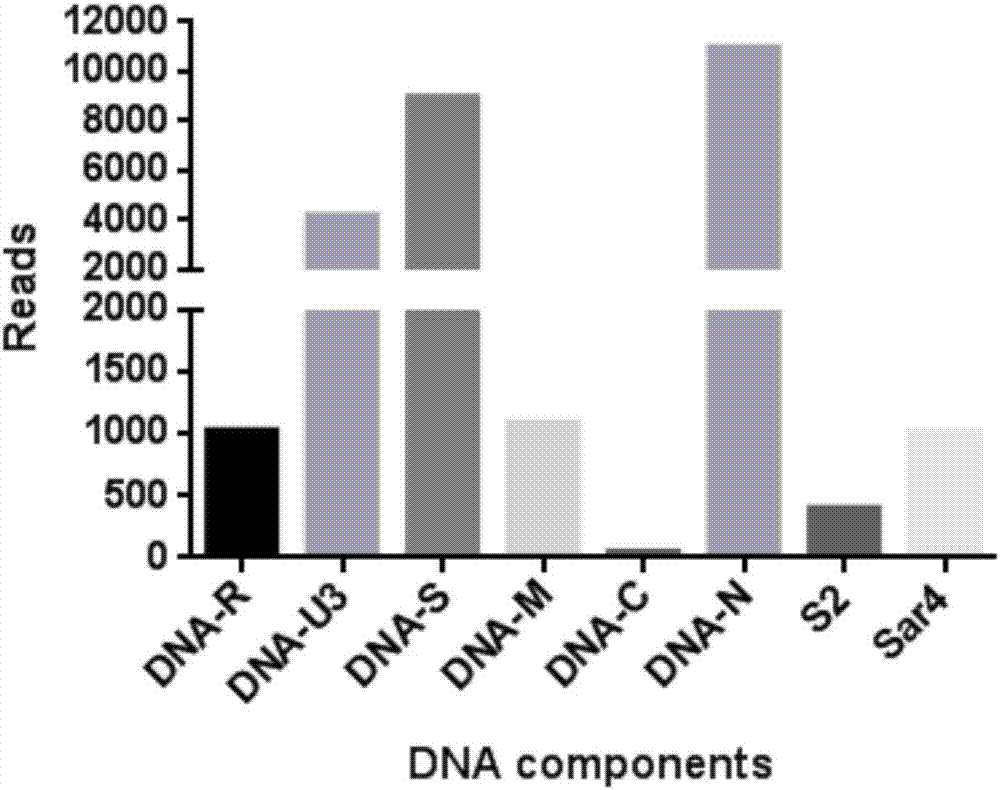
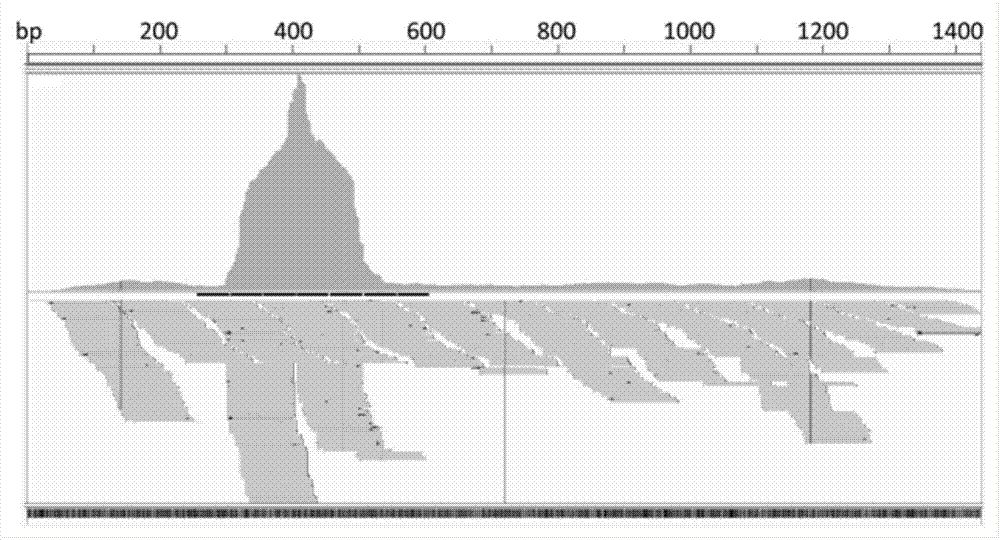
Transcriptome sequencing method for microviridae and geminivirus viral genome splice
PendingCN107475449AAccurate identificationAutomate quicklyMicrobiological testing/measurementMicroorganism based processesDna viralTranscriptome Sequencing
The invention provides a transcriptome sequencing method for microviridae and geminivirus viral genome splice. The method comprises the three steps of performing transcriptome sequencing on an infected DNA virus sample, comparing and splicing viral sequences and filling a blank area of the viral genome sequence. According to the method provided by the invention, the whole genome sequence of the banana bunchy top virus can be acquired and the other microviridae or geminivirus viral genomes can be spliced so as to acquire the whole genome sequence of the virus. The invention firstly reports the method for sequencing the spliced DNA viral genome in high throughput by utilizing transcriptome at home and abroad and fills the blank of the field at home and abroad. The method provided by the invention can be used for identifying and detecting DNA virus.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
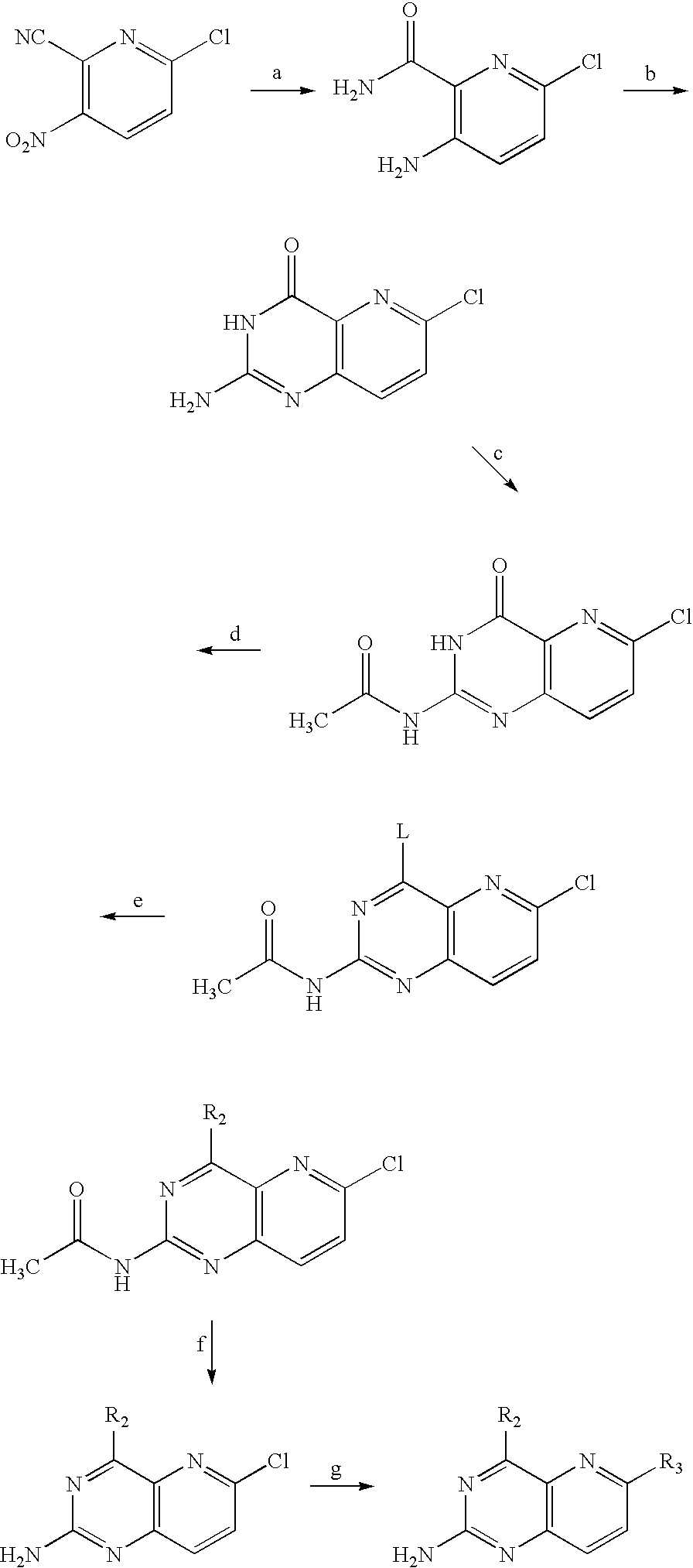
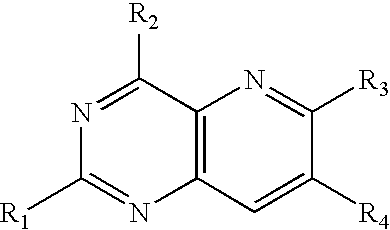
PYRIDO(3,2-d)PYRIMIDINES USEFUL FOR TREATING VIRAL INFECTIONS
ActiveUS20090324543A1Significant HCV replication inhibiting activityDesirable propertyBiocideOrganic chemistryFamily FlaviviridaePyrimidine
2-amino-pyrido(3,2-d)pyrimidine derivatives with a specific substitution pattern on positions 4 and 6 of the core structure are useful in the treatment or prevention of an infection due to a virus from the Flaviviridae family, especially HCV, when administered to a patient in a therapeutically effective amount.
Owner:GILEAD SCI INC
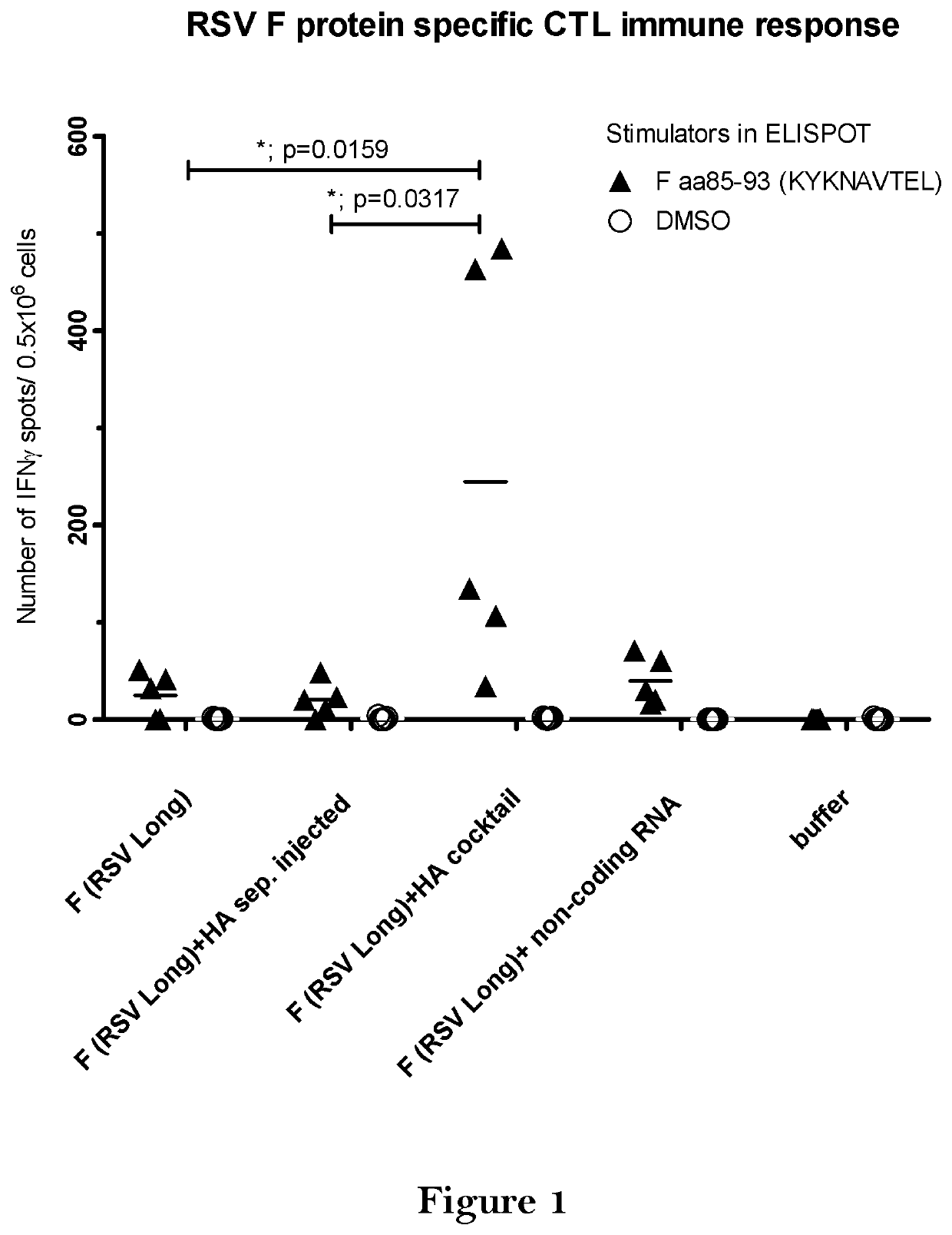
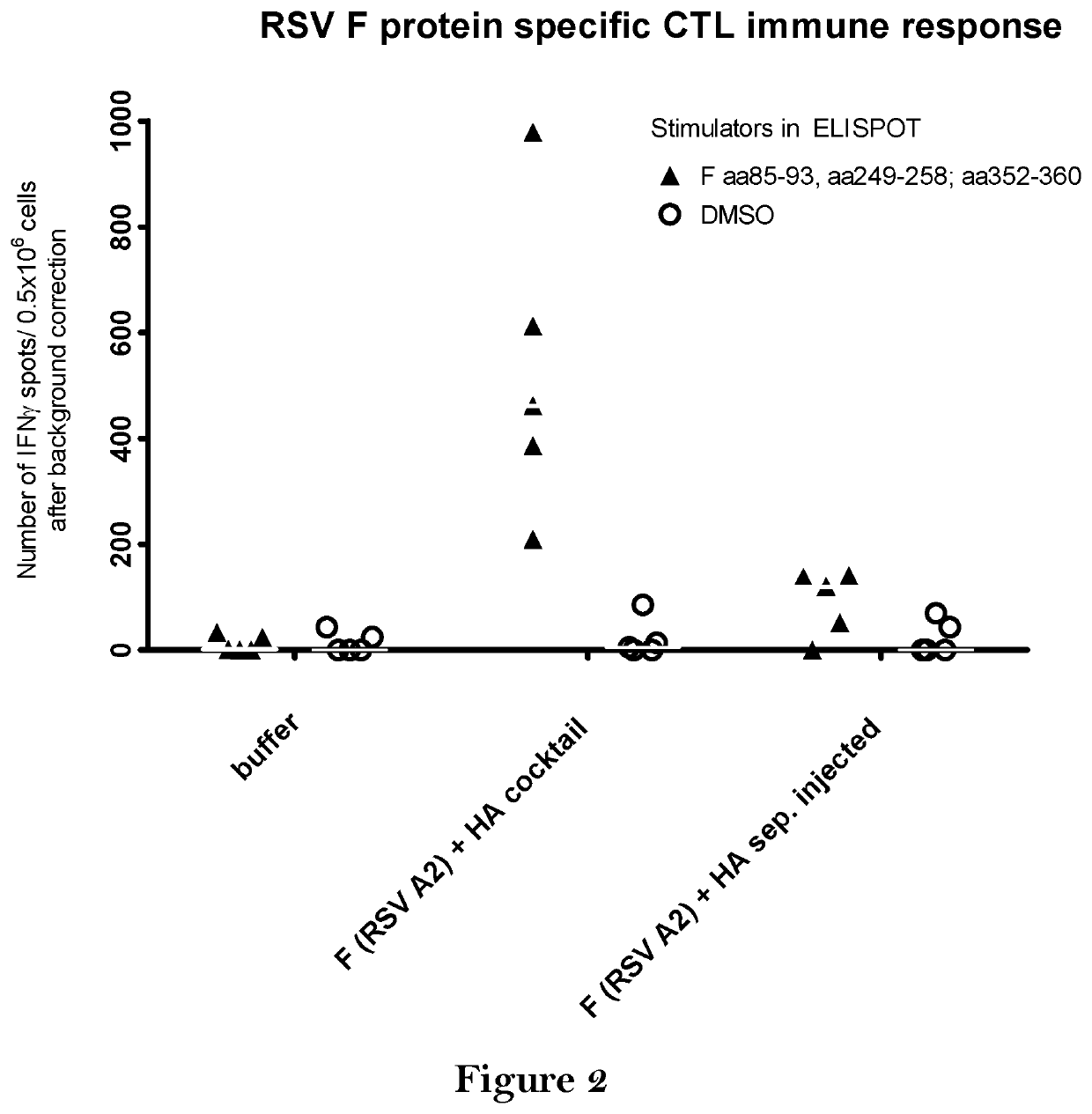
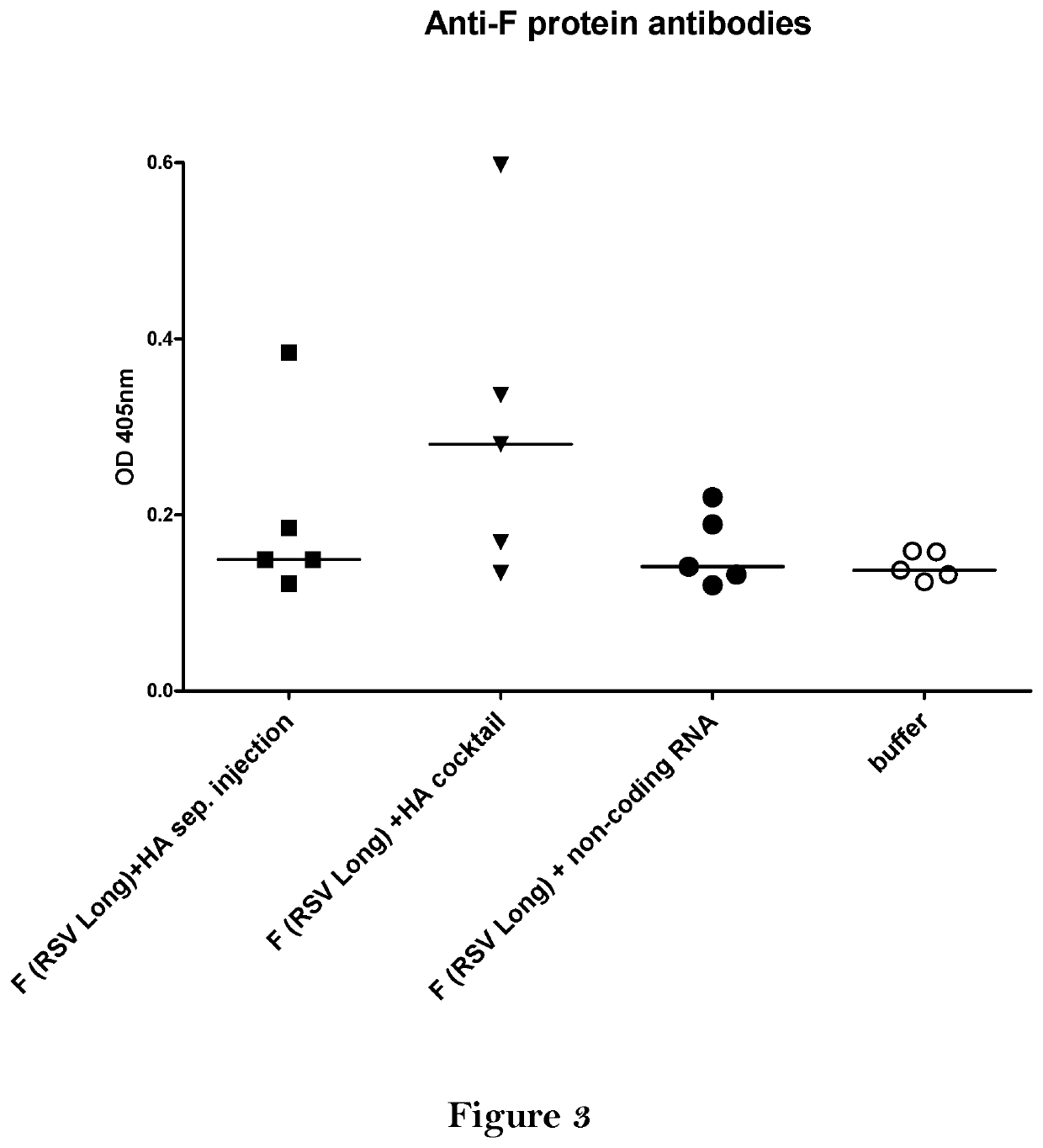
Combination vaccine
The present invention relates to a vaccine, especially a combination vaccine providing at least a first and a second antigenic function, the combination vaccine comprising at least one RNA encoding at least one or more proteins or fragments, variants or derivatives of proteins awarding antigenic function, wherein the first antigenic function being a Fusion (F) protein or a fragment, variant or derivative of a Fusion (F) protein derived from the virus family Paramyxoviridae and the second antigenic function being an Hemagglutinin (HA) protein or a fragment, variant or derivative of an Hemagglutinin (HA) protein derived from the virus family Orthomyxoviridae. Furthermore, the present invention is directed to a kit or kit of parts comprising the components of said combination vaccine and to said combination vaccine for use in a method of prophylactic or therapeutic treatment of diseases, particularly in the prevention or treatment of infectious diseases like RSV and influenza.
Owner:CUREVAC SE
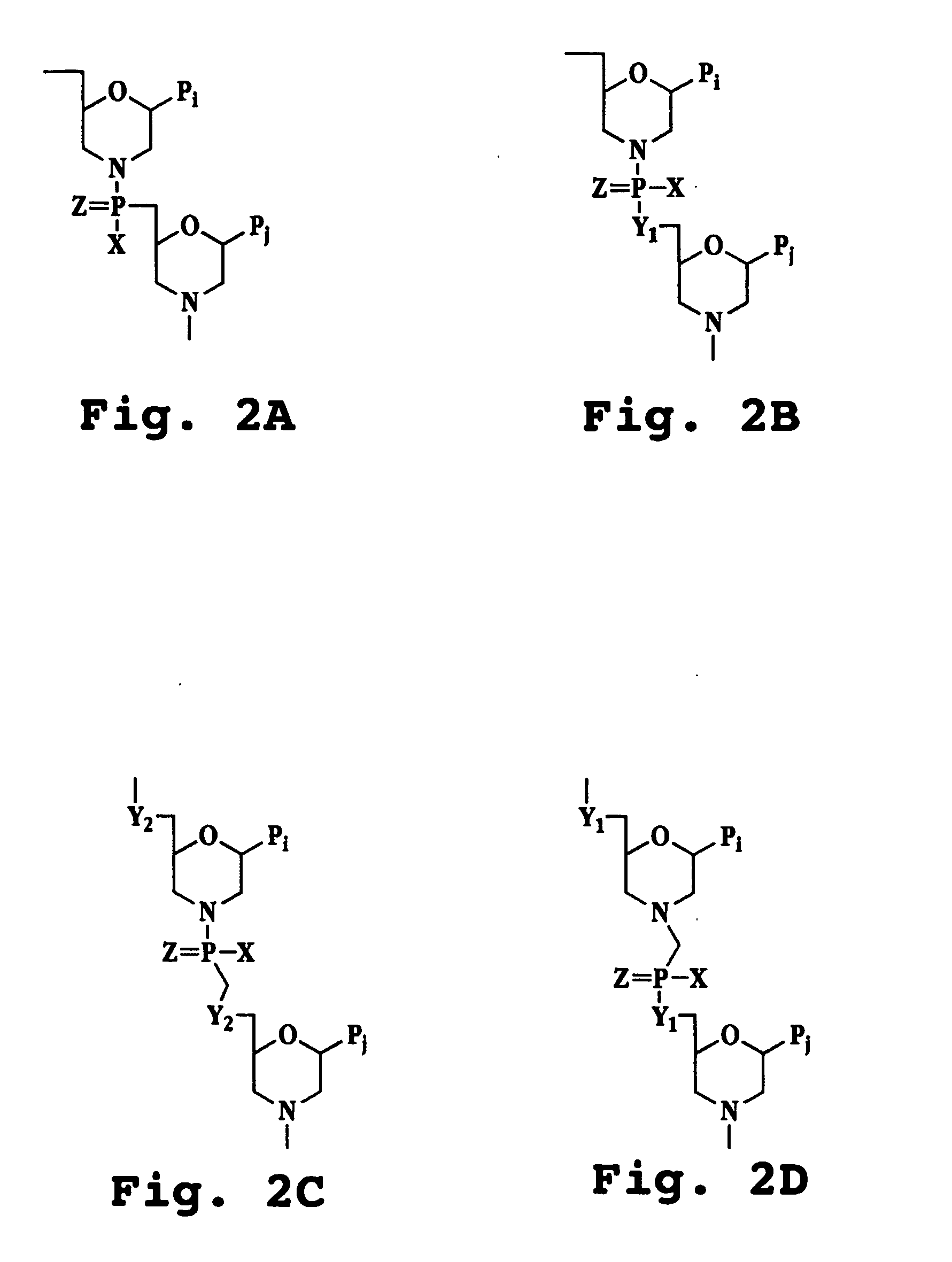
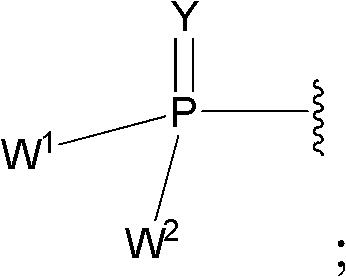
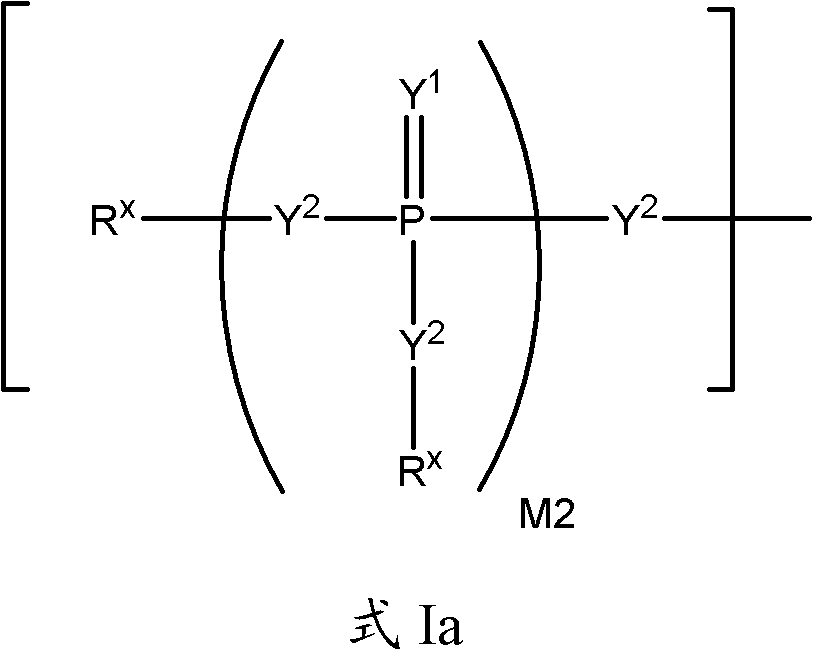
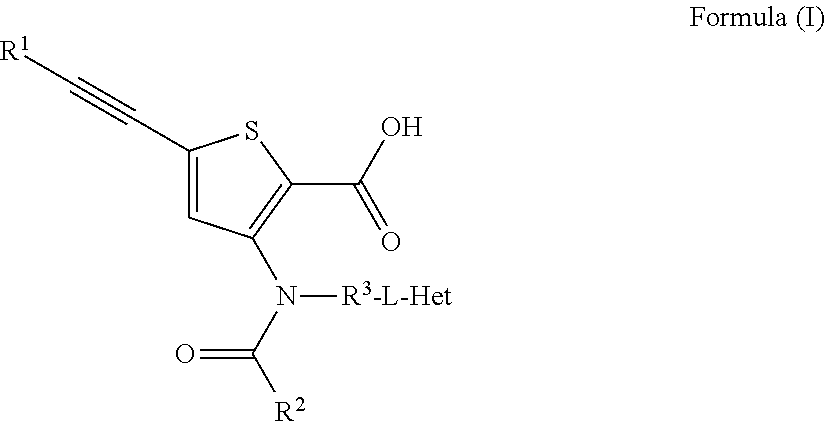
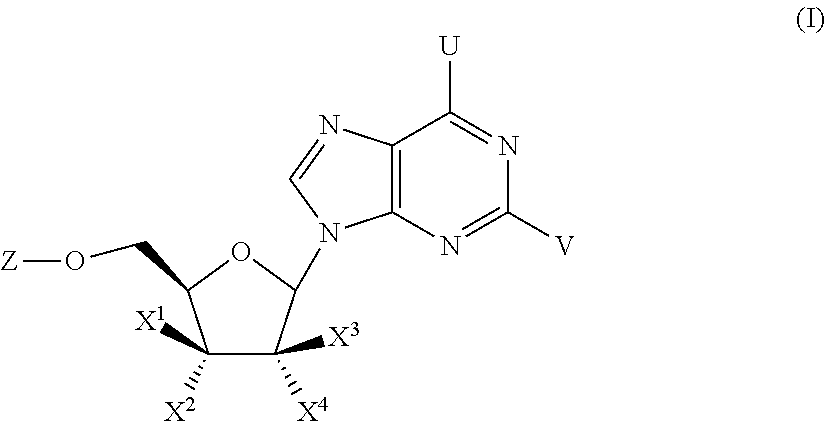
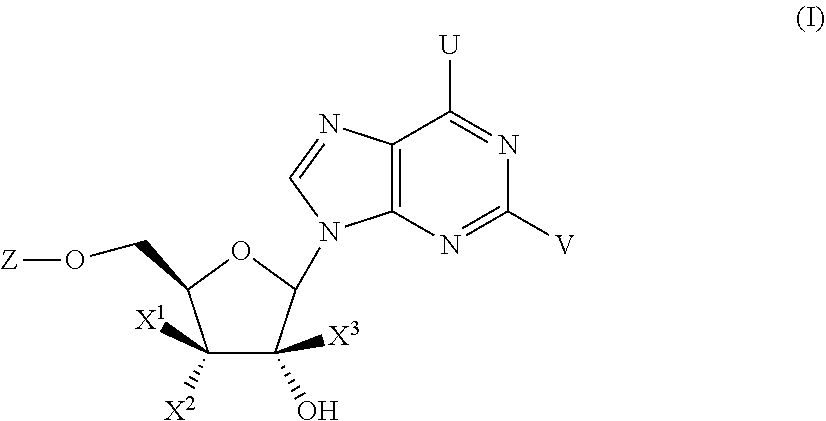
Substituted Purine Nucleosides, Phosphoramidate and Phosphordiamidate Derivatives for Treatment if Viral Infections
This invention is directed to compounds of Formula (I) having the structure that are useful in the treatment of viral infections in mammals, particularly in humans, mediated, at least in part, by a virus in the Flaviviridae family of viruses.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
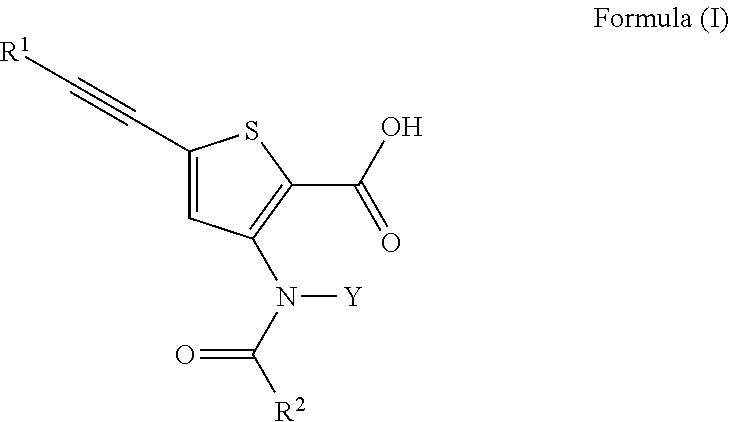
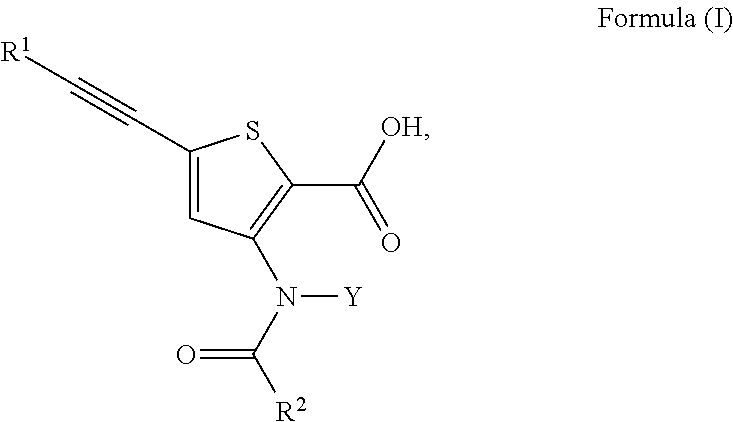
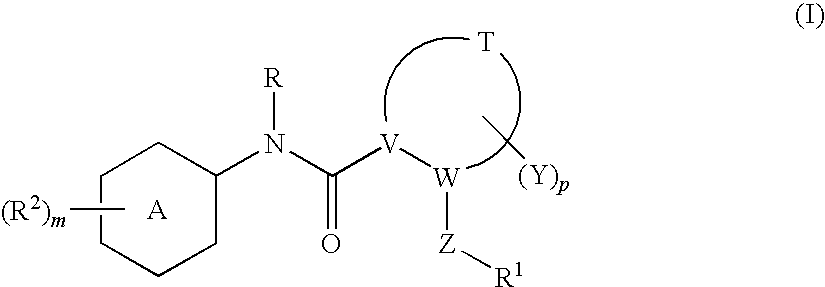
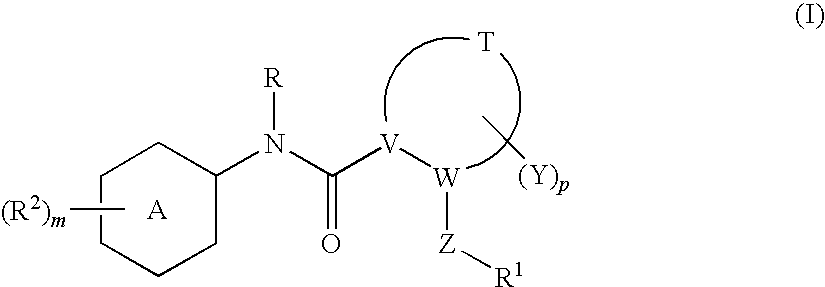
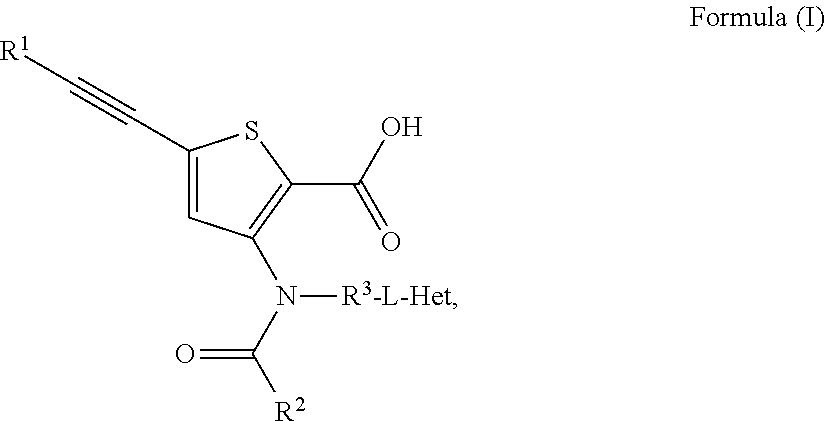
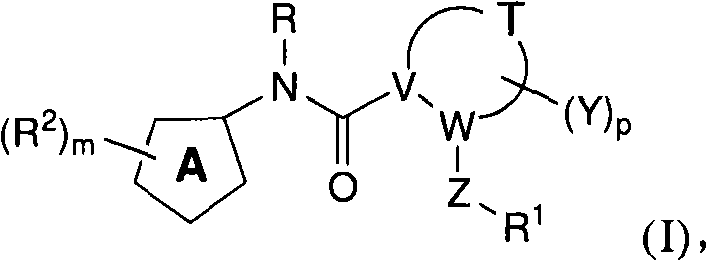
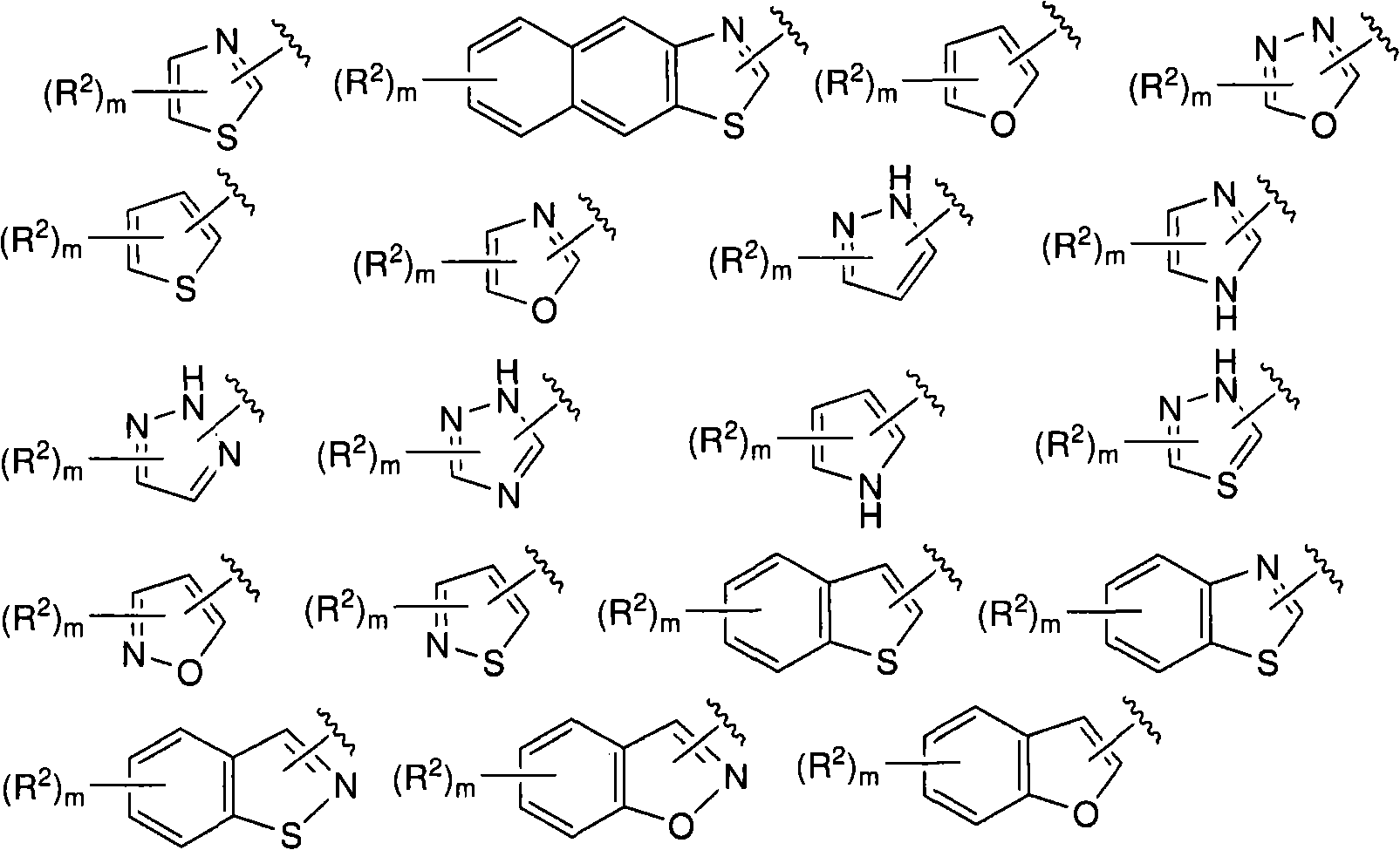
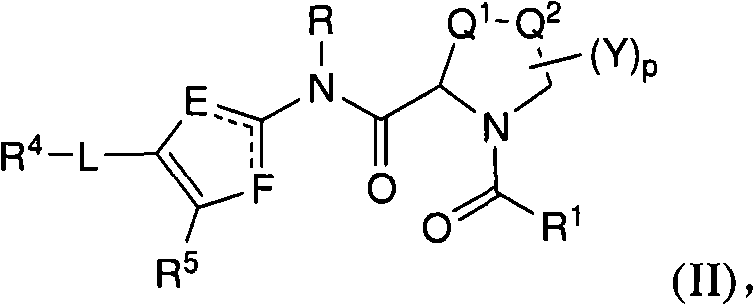
N-(5-membered aromatic ring)-amido anti-viral compounds
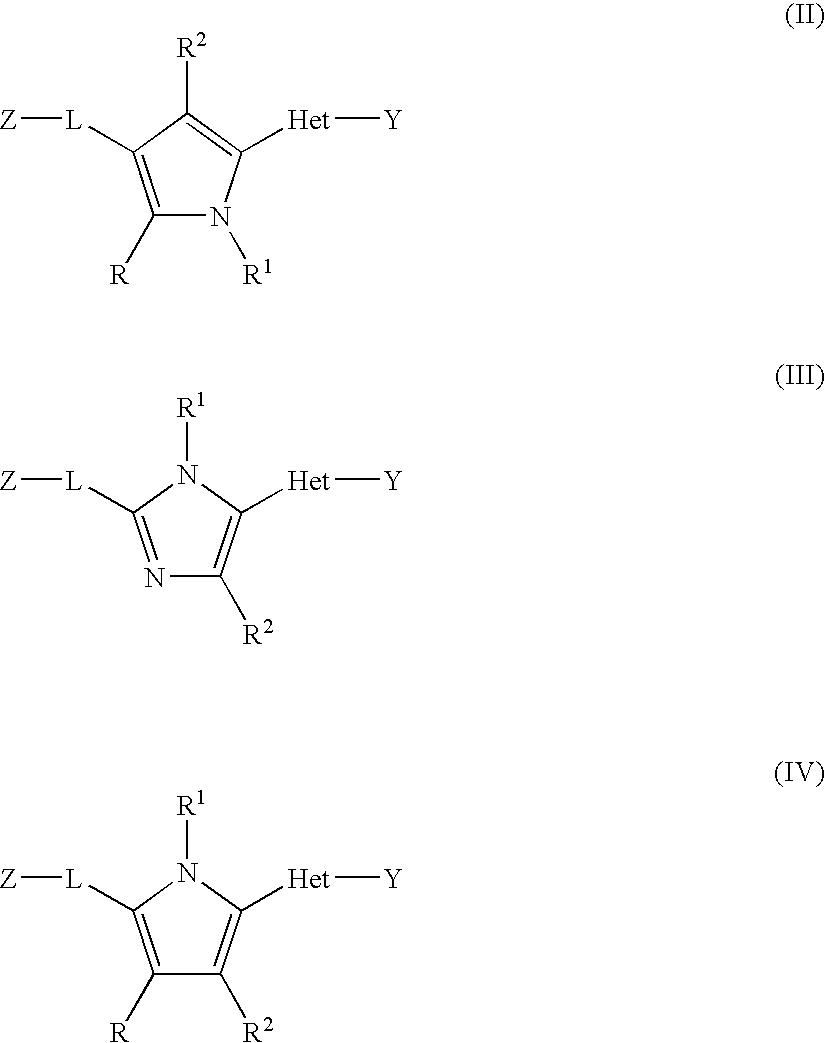
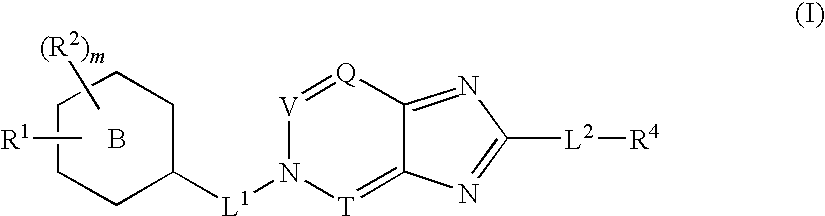
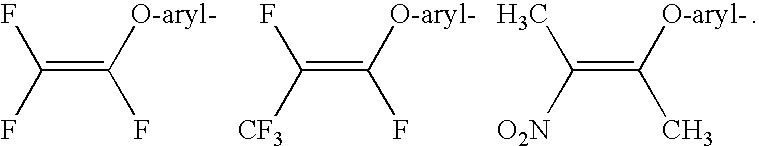
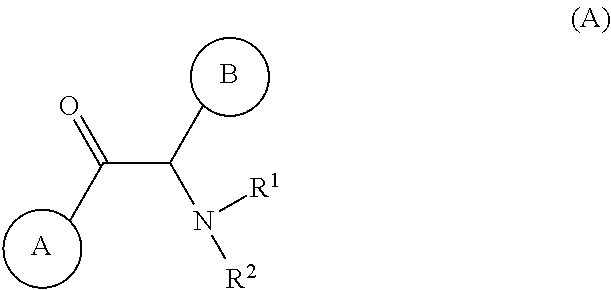
Disclosed are compounds having Formula (I) and the compositions and methods thereof for treating or preventing a viral infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, R<2>, m, R, V, W, T, Z, R<1>, Y and p are disclosed herein.
Owner:SMITHKLINE BECKMAN CORP
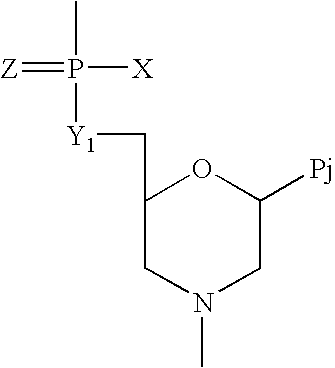
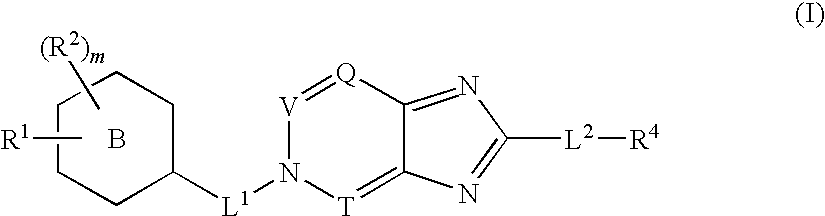
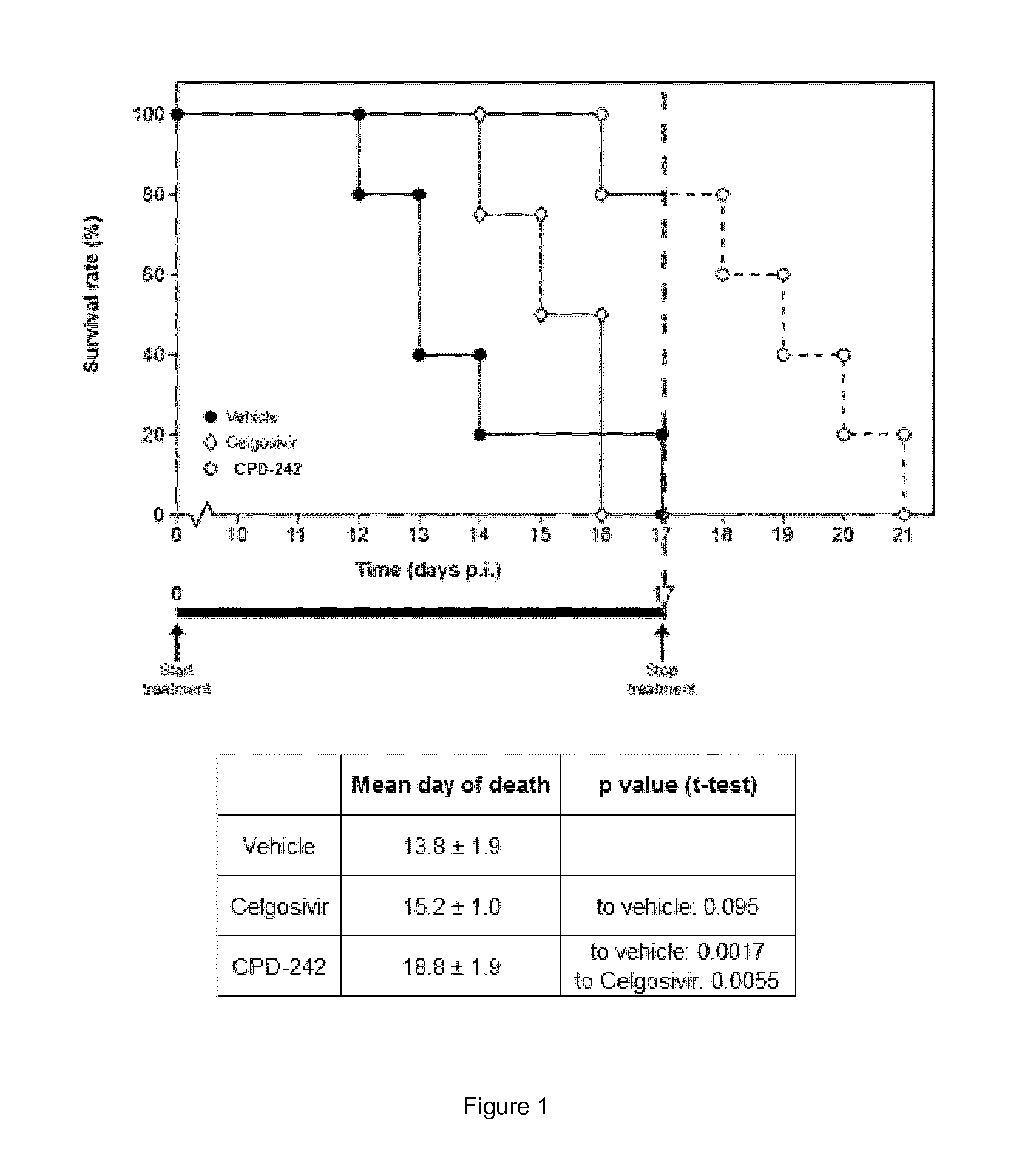
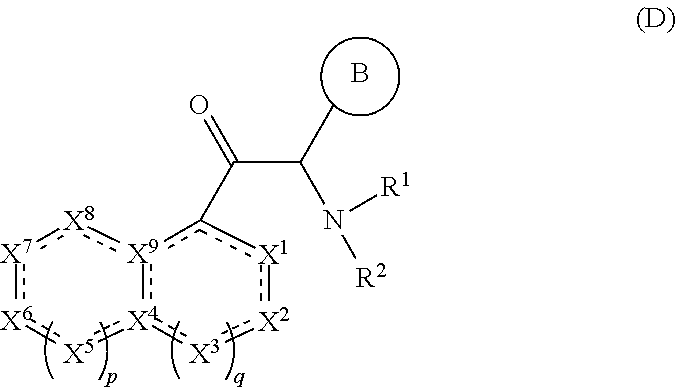
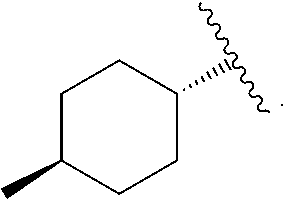
Imidazo [1, 2-a] pyrrolo [3, 2-c] pyridine compounds useful as pestivirus inhibitors
Owner:UNIV CLERMONT AUVERGNE +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00002.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00003.png)
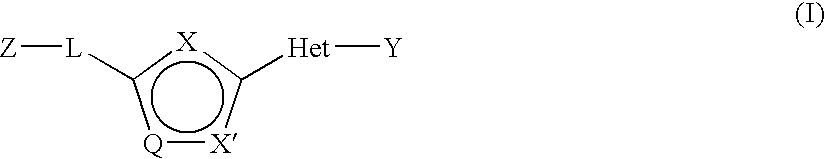

![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00000.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-D00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of <i>Flaviviridae </i>infections](https://images-eureka.patsnap.com/patent_img/9480776e-ac75-471c-a196-be25f1dad448/US08093380-20120110-C00001.png)
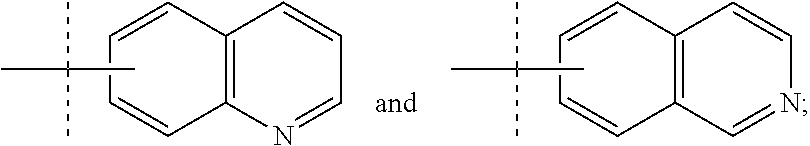
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00001.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00002.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00003.png)