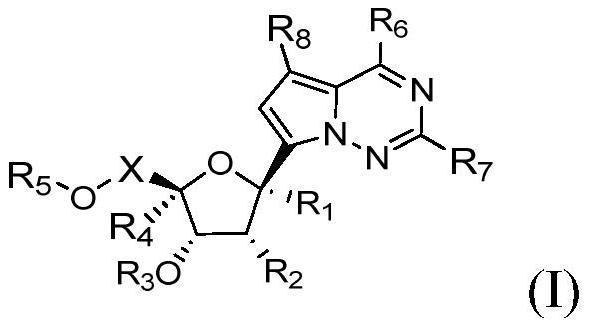
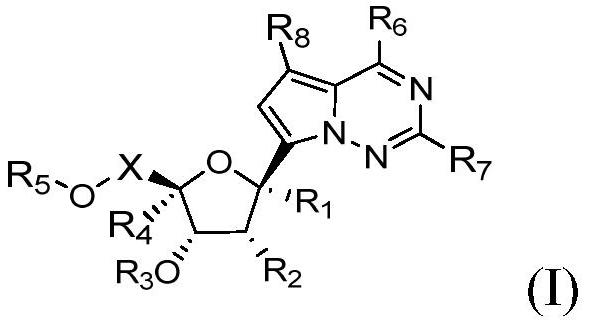
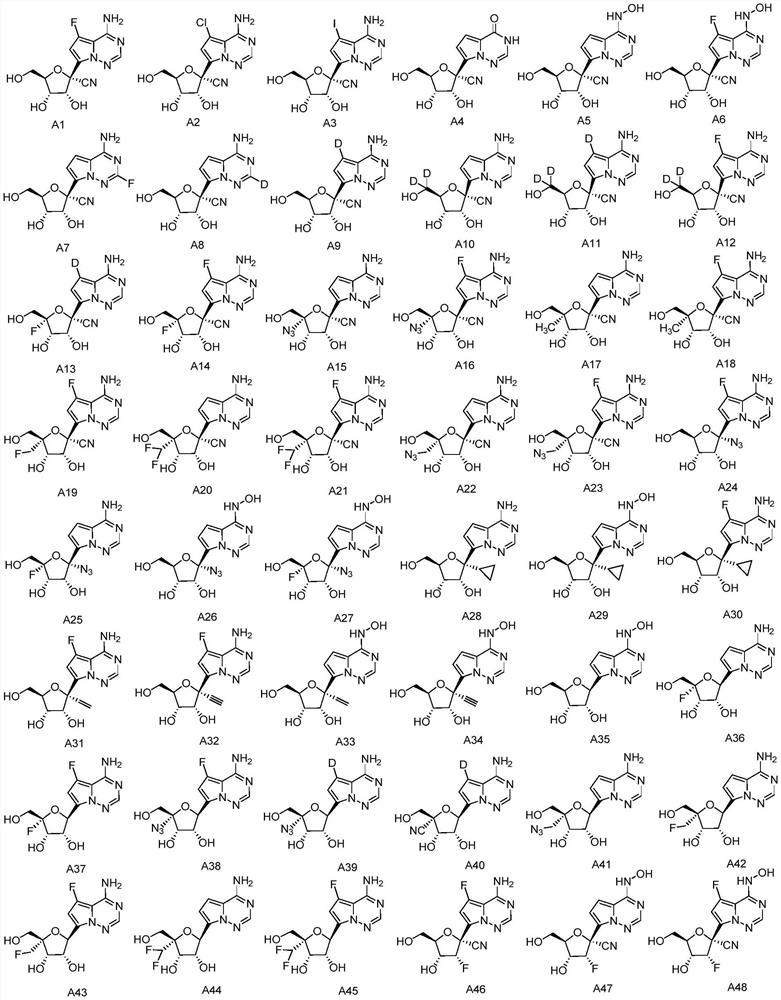
Application of nucleoside analogue or combined preparation containing nucleoside analogue in virus resistance
A compound and solvate technology, which is applied in the field of antiviral application of combined preparations, can solve the problems of vaccines and antiviral drugs without specific effects for severe pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0292] Preparation Example 1: Synthesis of Compound A1
[0293]
[0294] Compound 1-1 was synthesized by the method reported in the literature (Nature.2016, 531, 381-385). Compound 1-1 (1.5 g, 2.67 mmol) was added to acetonitrile (30 mL), followed by the addition of 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octanebis(tetra Fluoboric acid) salt (Selectfluor, 1.13g, 3.2mmol, 1.2eq) and sodium bicarbonate (0.67g, 8.0mmol, 3eq) were added and reacted at room temperature for 3-4 hours. TLC showed that the reaction was complete. The reaction solution was added to water (120 mL), extracted with ethyl acetate, the organic layer was separated, dried, concentrated, and separated by silica gel column chromatography to obtain compound 1-2, 0.43 g of off-white solid, with a yield of 28%. 1 H NMR (500MHz, DMSO-d 6)δ8.12(brs,1H),7.87(s,1H),7.50–7.20(m,16H),6.59(s,1H),4.92(d,J=11.7Hz,1H),4.85–4.78(m ,2H),4.57–4.46(m,4H),4.42–4.35(m,1H),4.12(t,J=5.7Hz,1H),3.73(dd,J=11.2,3.2Hz,1H),3....
preparation Embodiment 2
[0296] Preparation Example 2: Synthesis of Compound A2
[0297]
[0298] Compound 2-1 was synthesized according to the method reported in the literature (Nature.2016, 531, 381-385). Compound 2-1 (291mg, 1.0mmol) was added to N,N-dimethylformamide (5mL), N-chlorosuccinimide (245mg, 1.1mmol, 1.1eq) and trifluoroacetic acid ( 24mg, 0.2mmol, 0.2eq), reacted at 50°C for 1 hour, TLC showed that the reaction was complete. The reaction solution was added into a mixed solution of sodium sulfite and sodium carbonate, and compound A2 was obtained by filtration, 185 mg of white solid, with a yield of 57%. 1 H NMR (500MHz, DMSO-d 6 )δ8.26(brs,1H),7.95(s,1H),7.09(brs,1H),6.98(s,1H),6.23(d,J=5.7Hz,1H),5.18(d,J=5.6 Hz,1H),4.92(t,J=5.6Hz,1H),4.52(t,J=5.0Hz,1H),4.06–4.00(m,1H),3.96–3.88(m,1H),3.69–3.61 (m,1H),3.54–3.45(m,1H). MS m / z=326.0[M+1] + .
preparation Embodiment 3
[0299] Preparation Example 3: Synthesis of Compound A3
[0300]
[0301] Compound 2-1 (291mg, 1.0mmol) was added to N,N-dimethylformamide (5mL), N-iodosuccinimide (245mg, 1.1mmol, 1.1eq) and trifluoroacetic acid ( 24mg, 0.2mmol), reacted at 50°C for 1 hour, TLC showed that the reaction was complete. The reaction liquid was added into a mixed solution of sodium sulfite and sodium carbonate, and compound A3 was obtained by filtration, 200 mg of white solid, with a yield of 48%. 1 H NMR (500MHz, DMSO-d 6 )δ7.98(s,1H),7.12(s,1H),6.21(brs,1H),5.20(brs,1H),4.91(s,1H),4.52(d,J=4.6Hz,1H), 4.11–3.99 (m, 1H), 3.97–3.85 (m, 1H), 3.64 (d, J=11.3Hz, 1H), 3.49 (d, J=10.9Hz, 1H). MS m / z=418.0[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com