Patents
Literature
30results about How to "Satisfactory treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
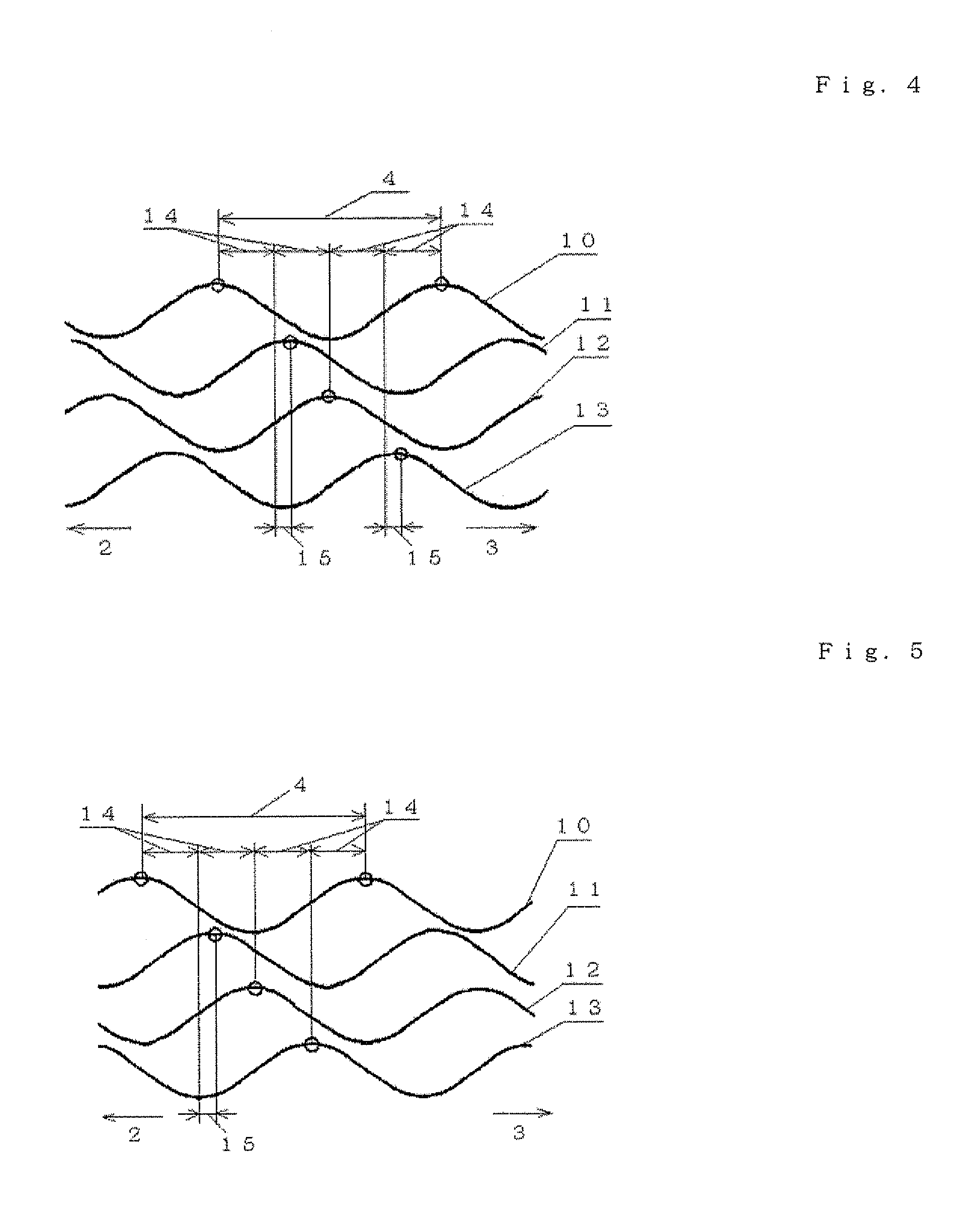
Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease
InactiveUS20050021104A1Reducing time and number of interactionSatisfactory treatmentHead electrodesExternal electrodesDiseaseNervous system
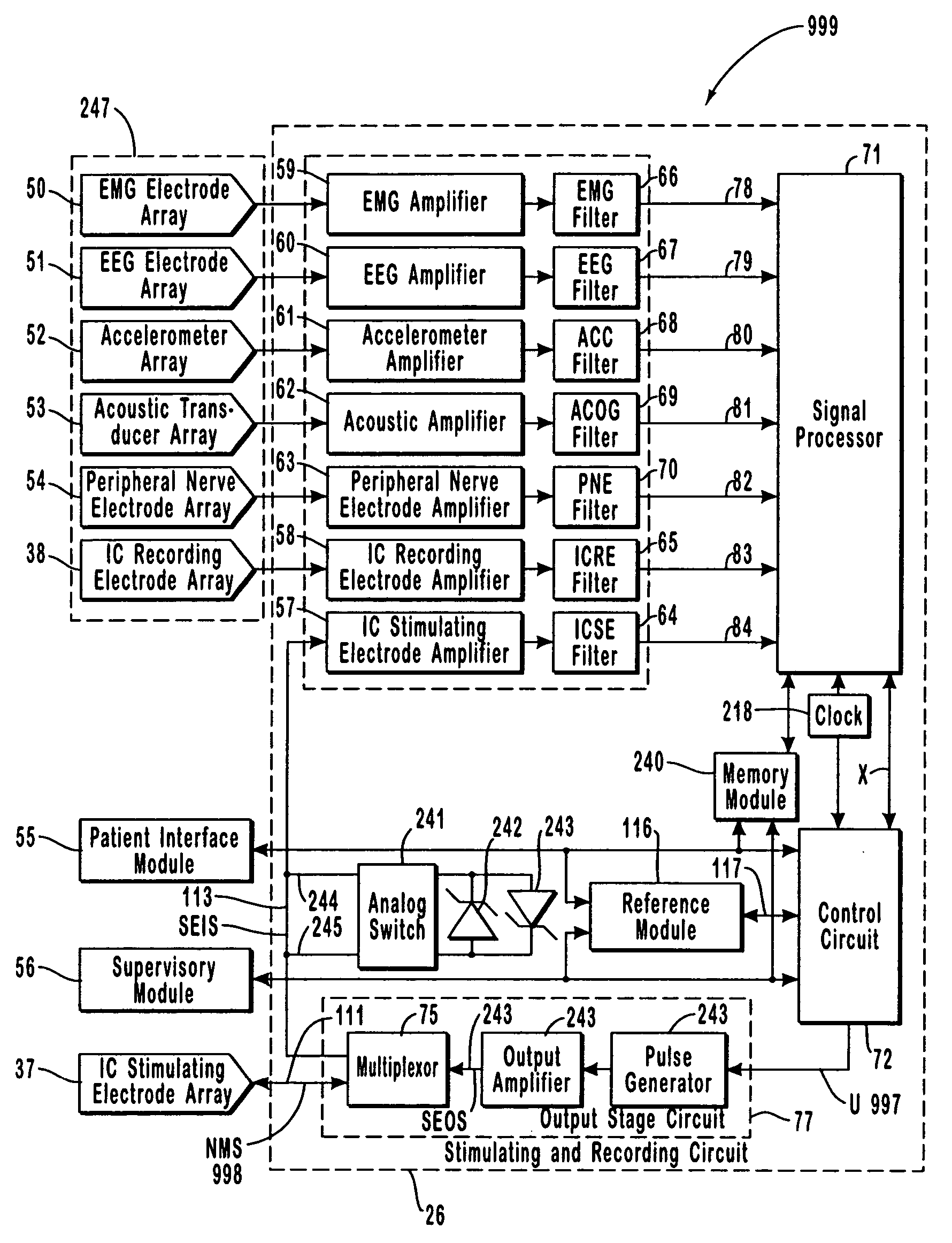
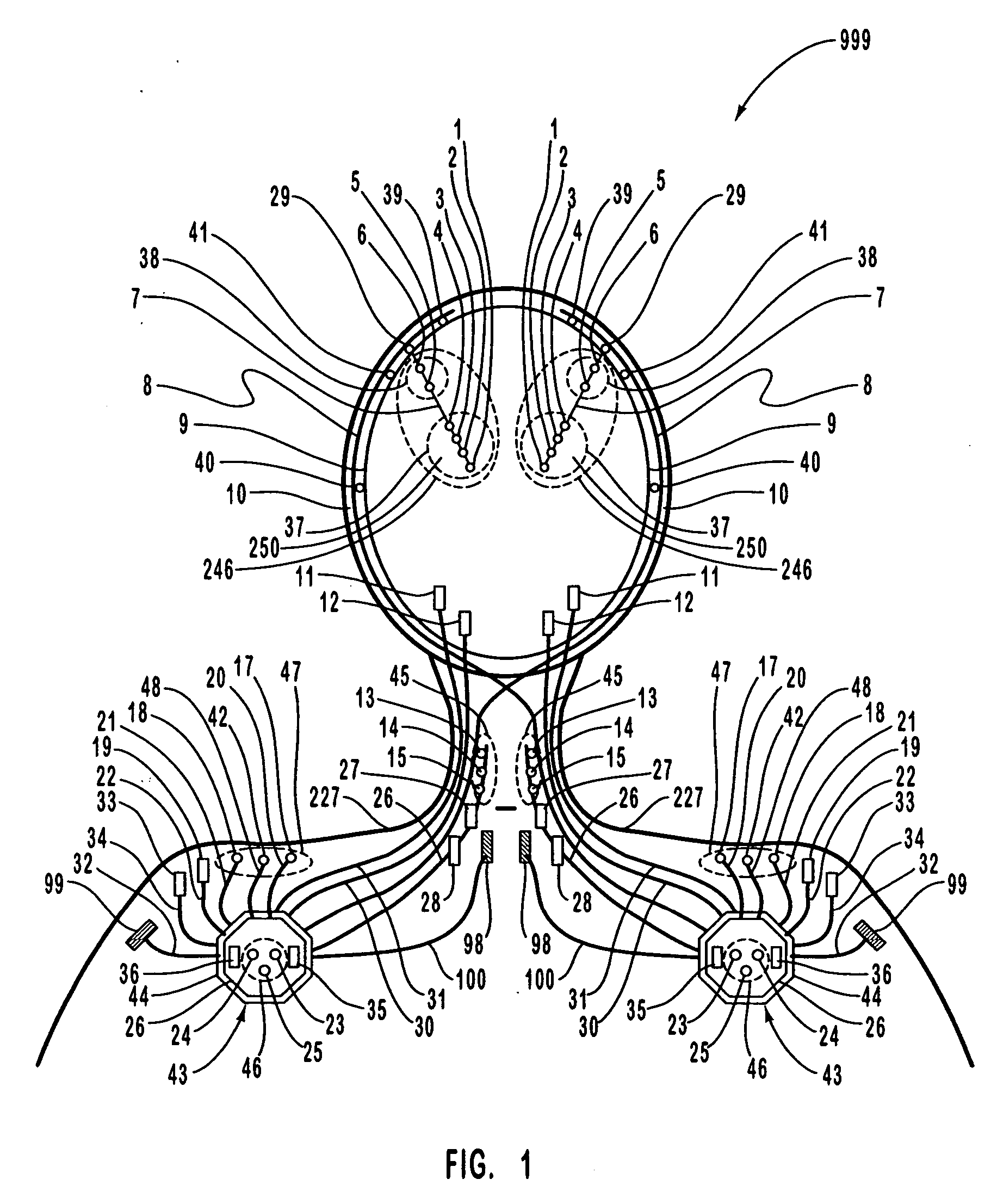
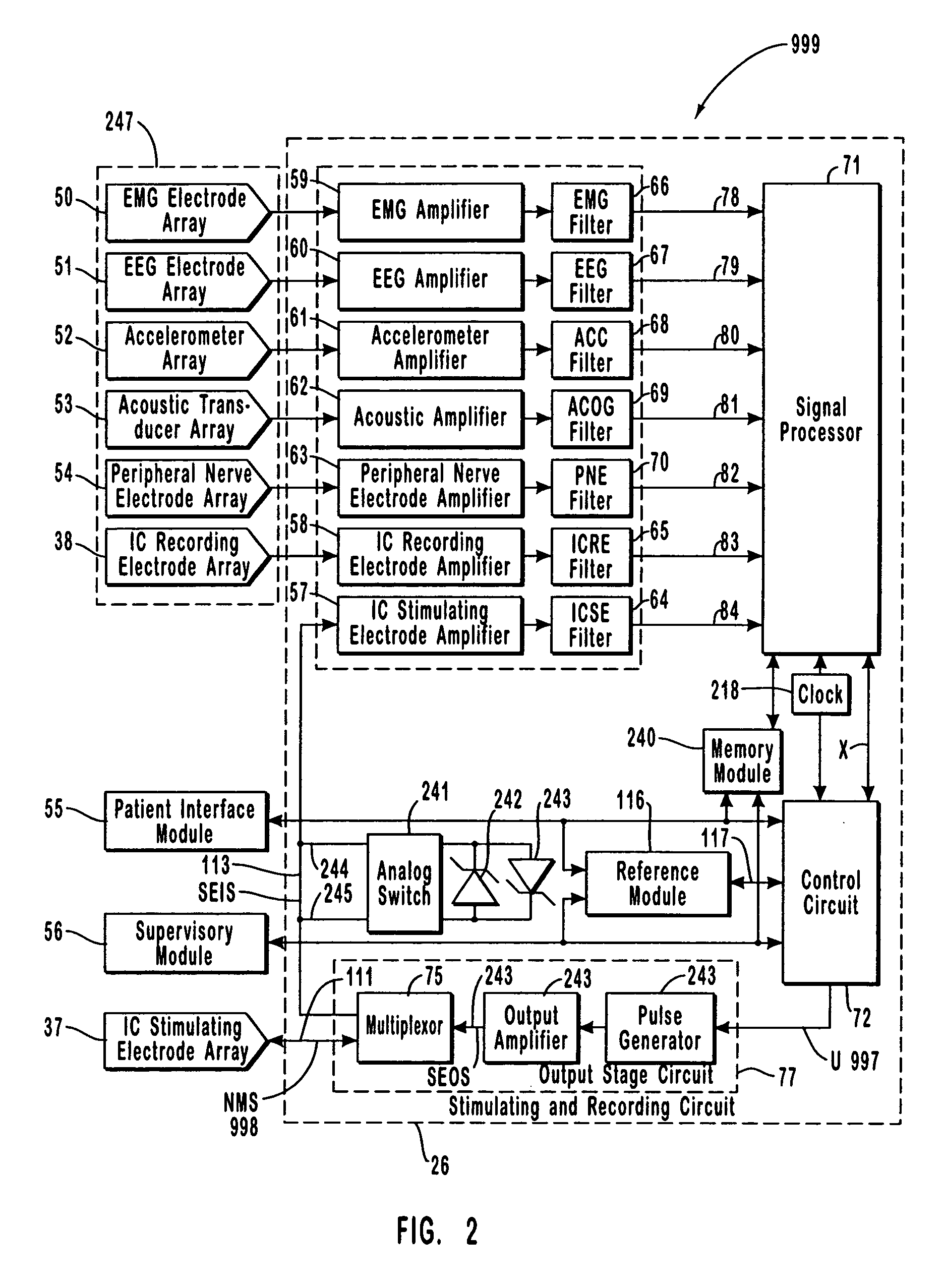
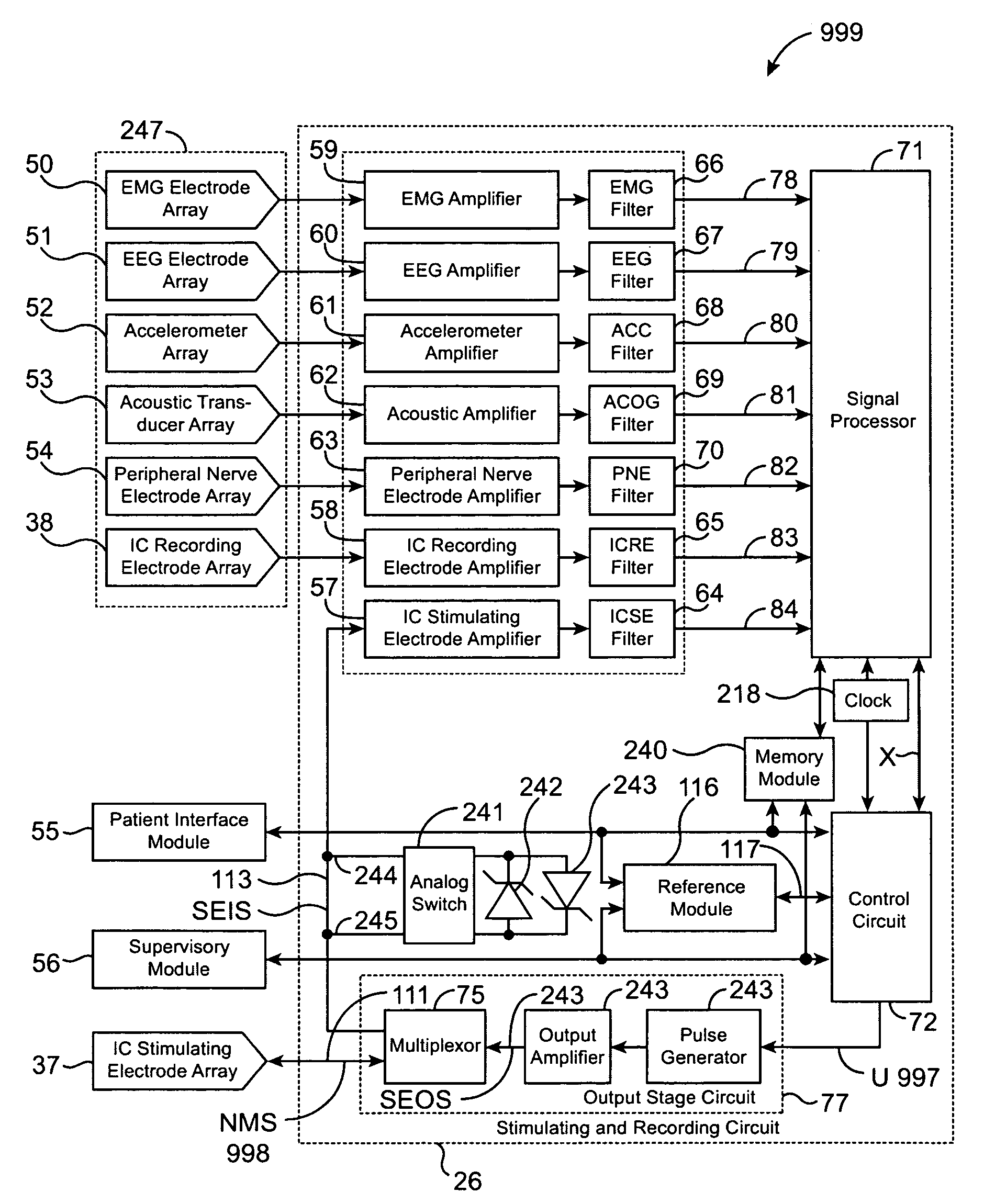
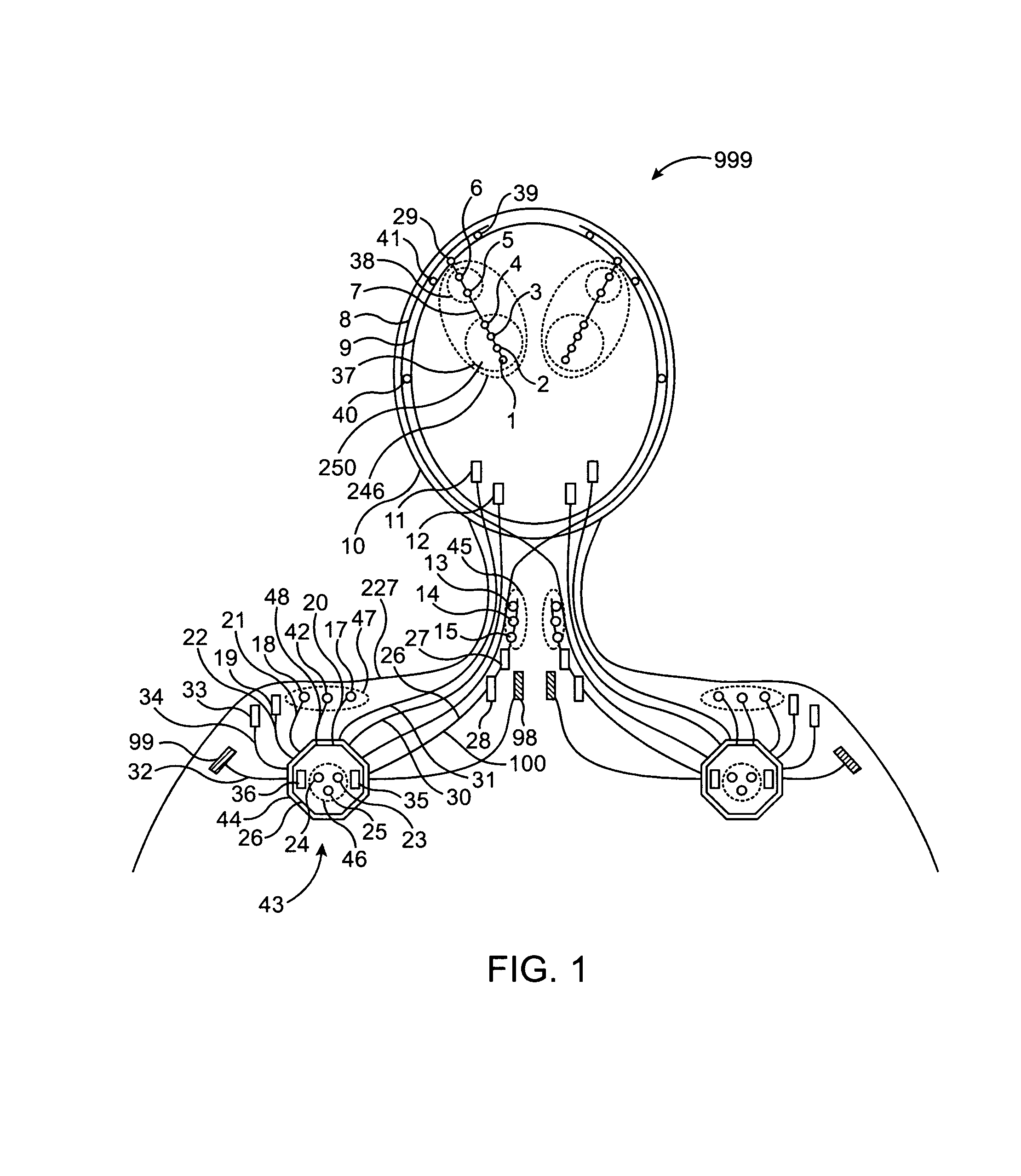
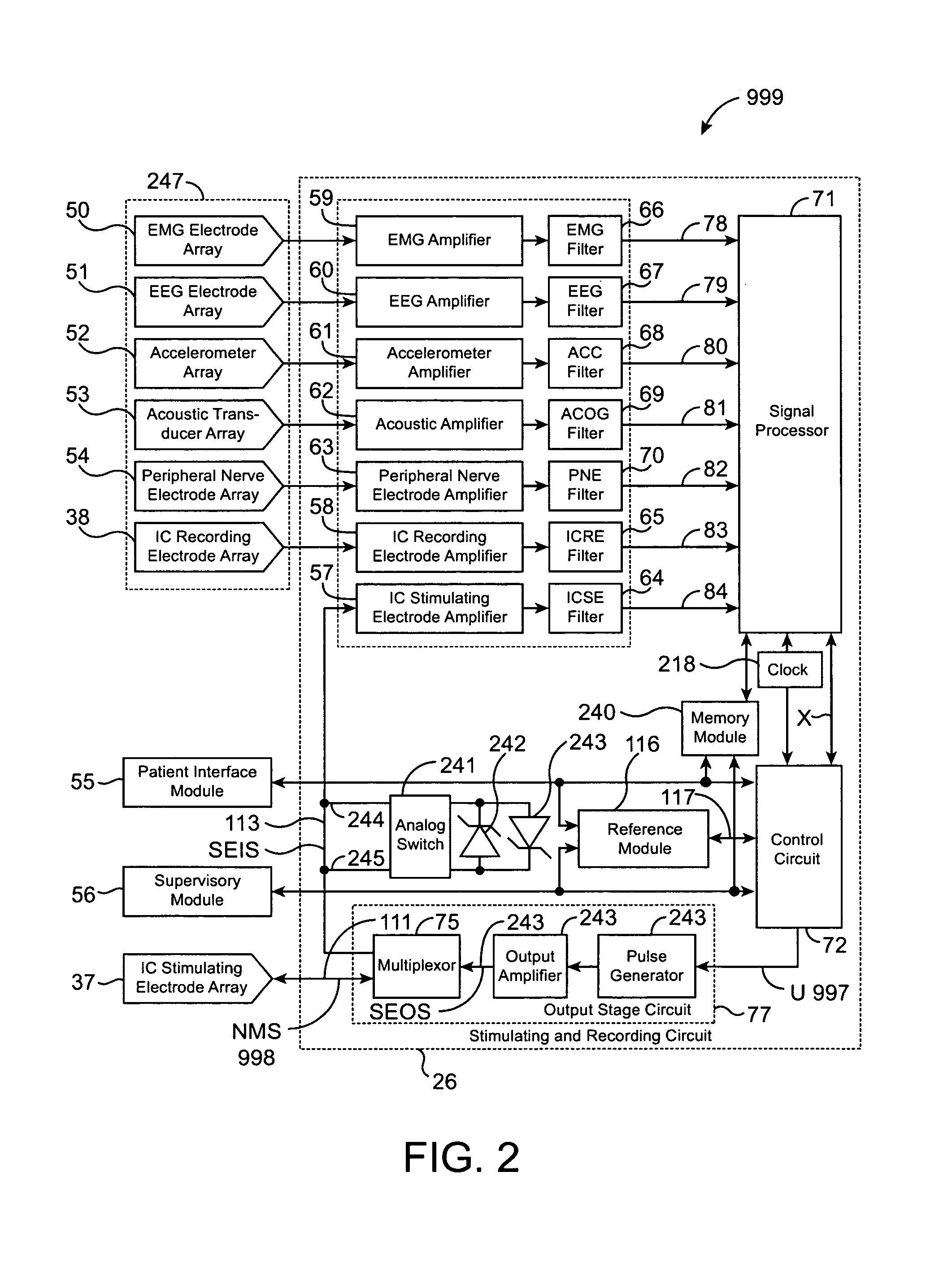
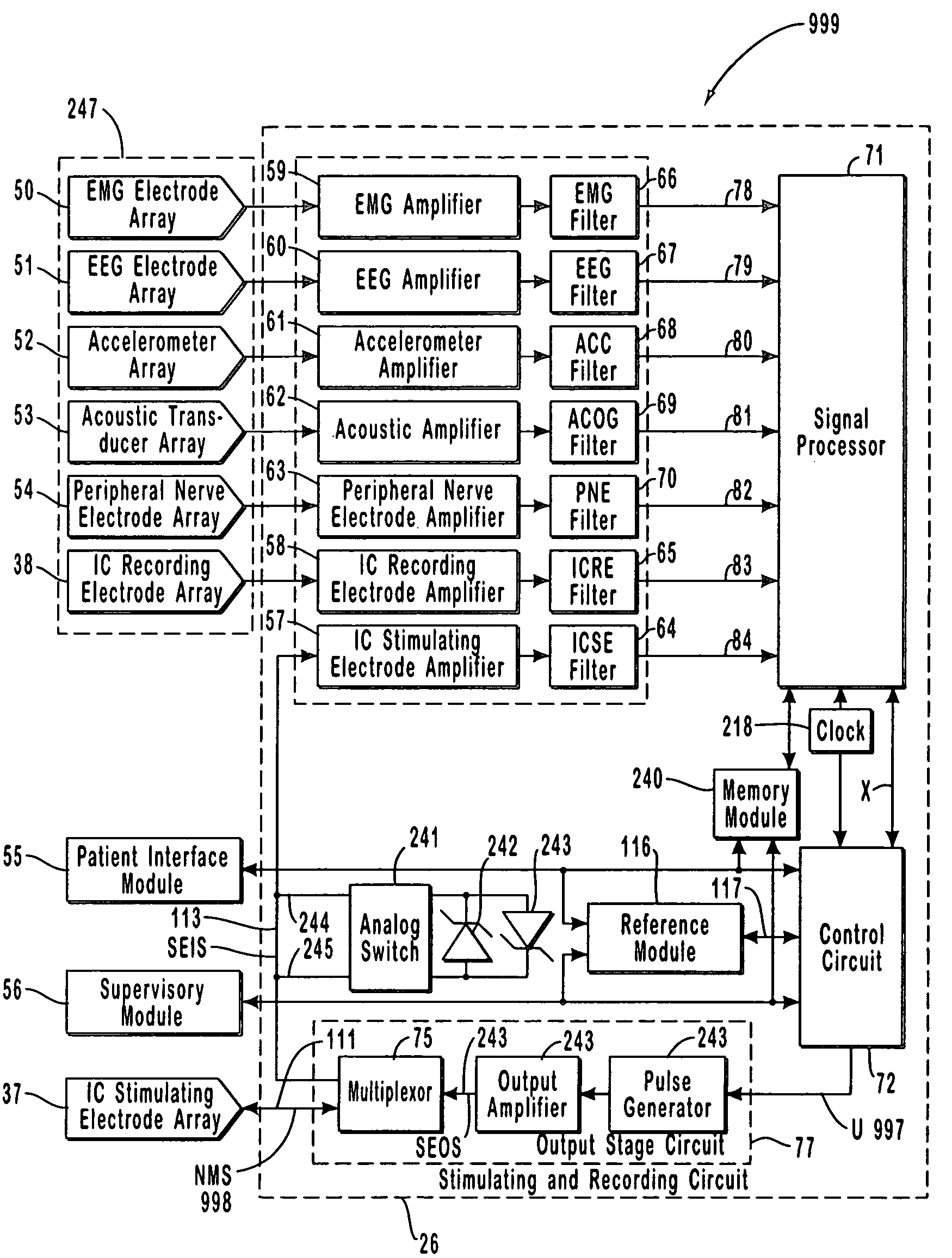
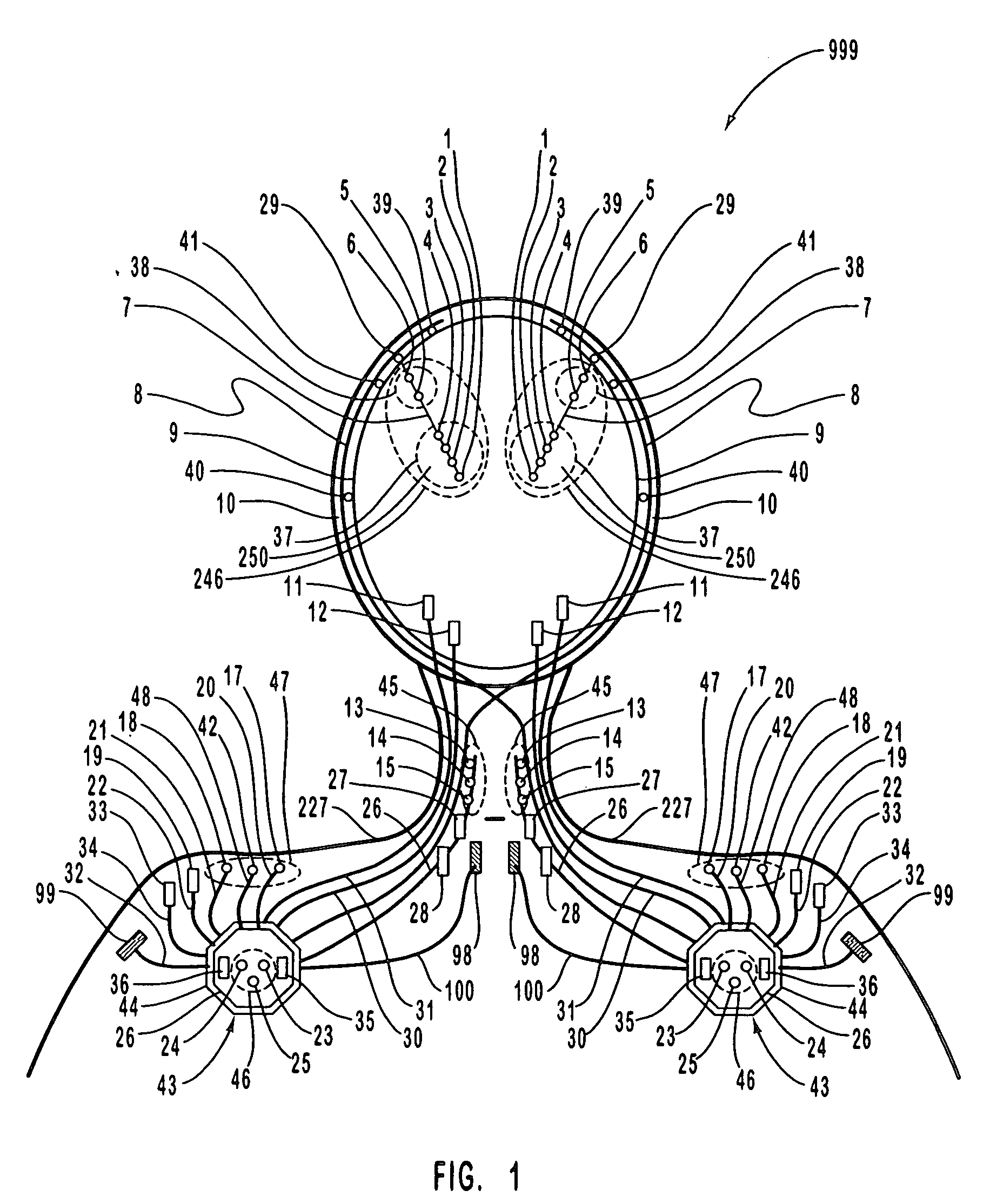
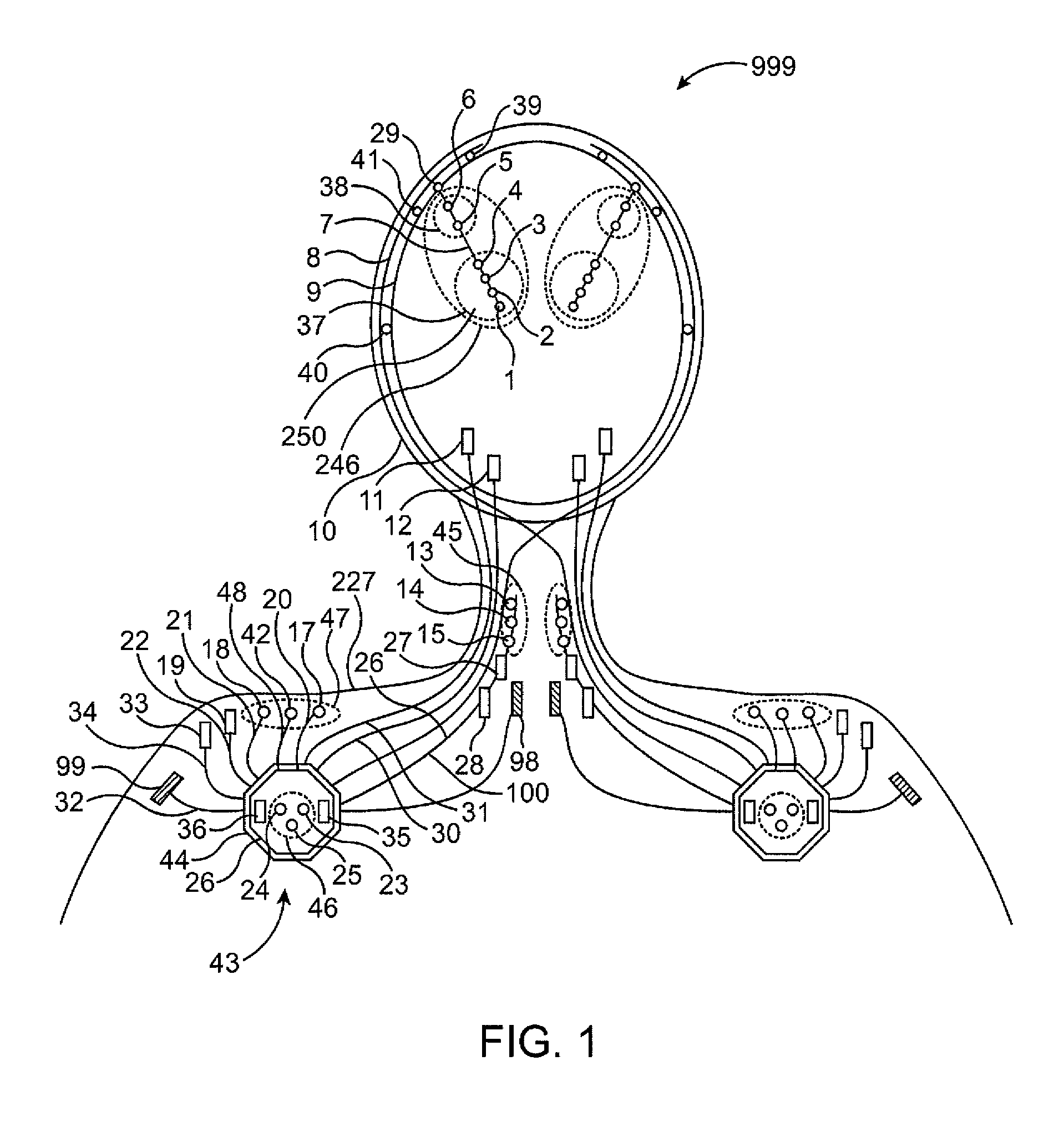
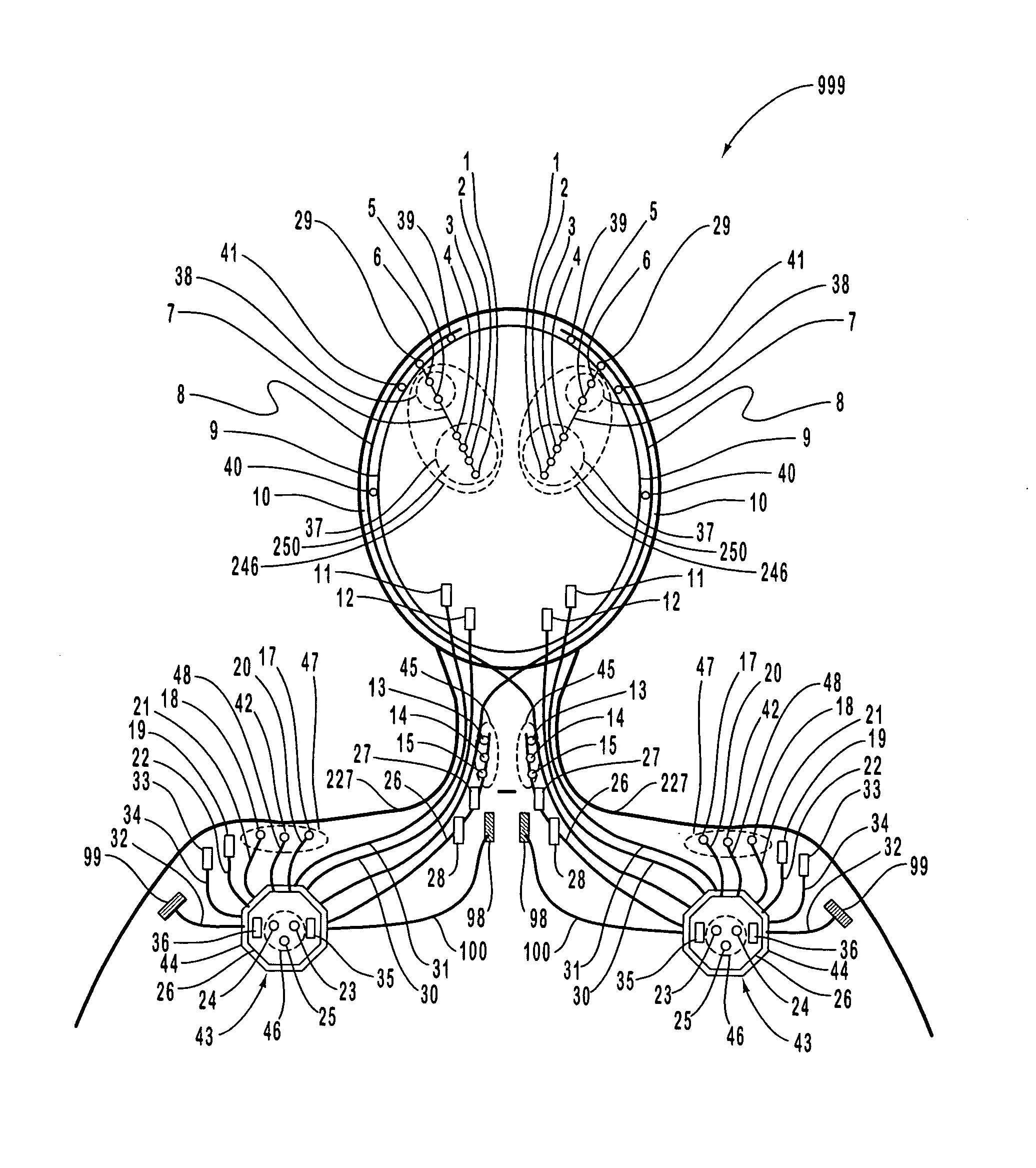
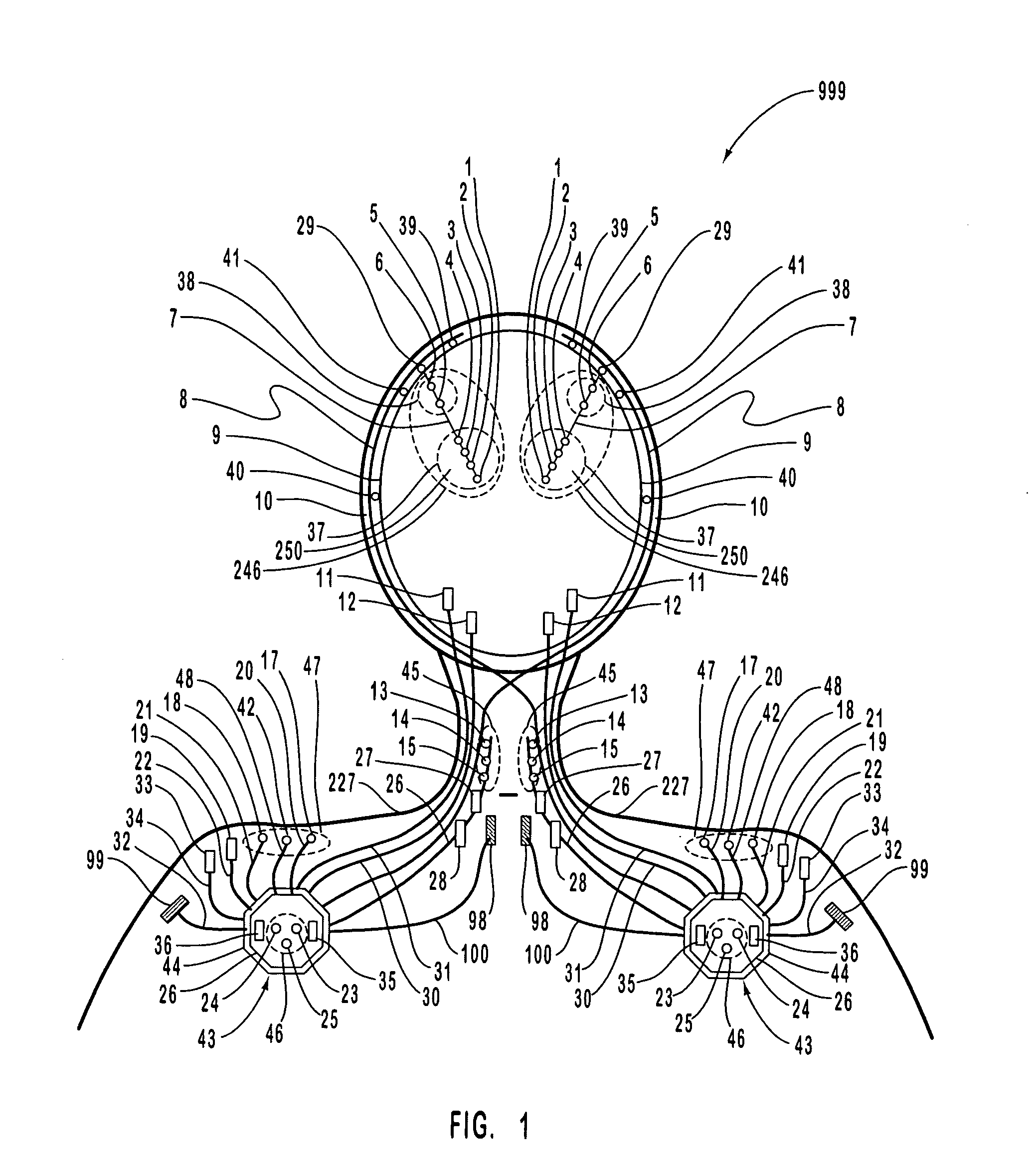
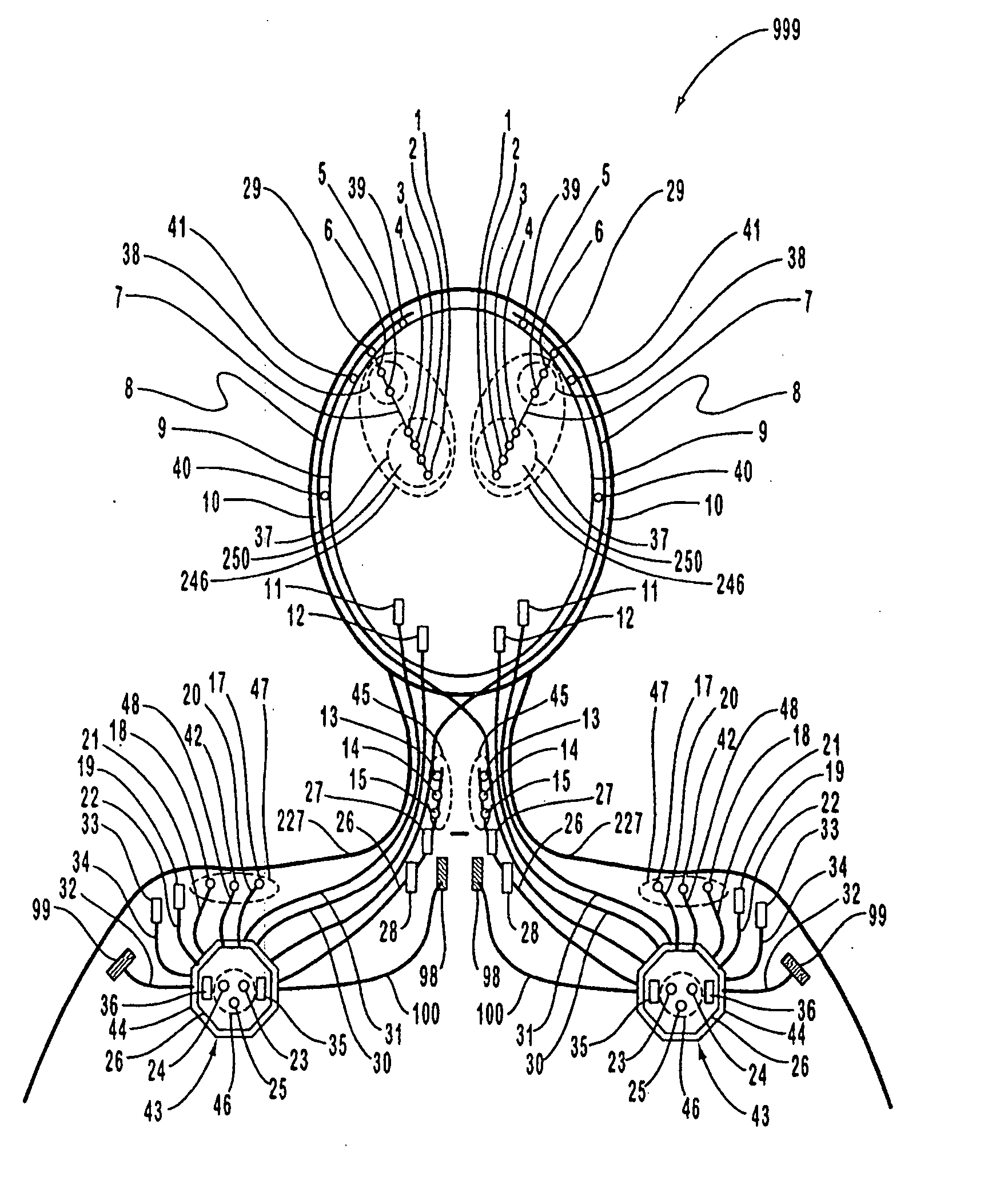
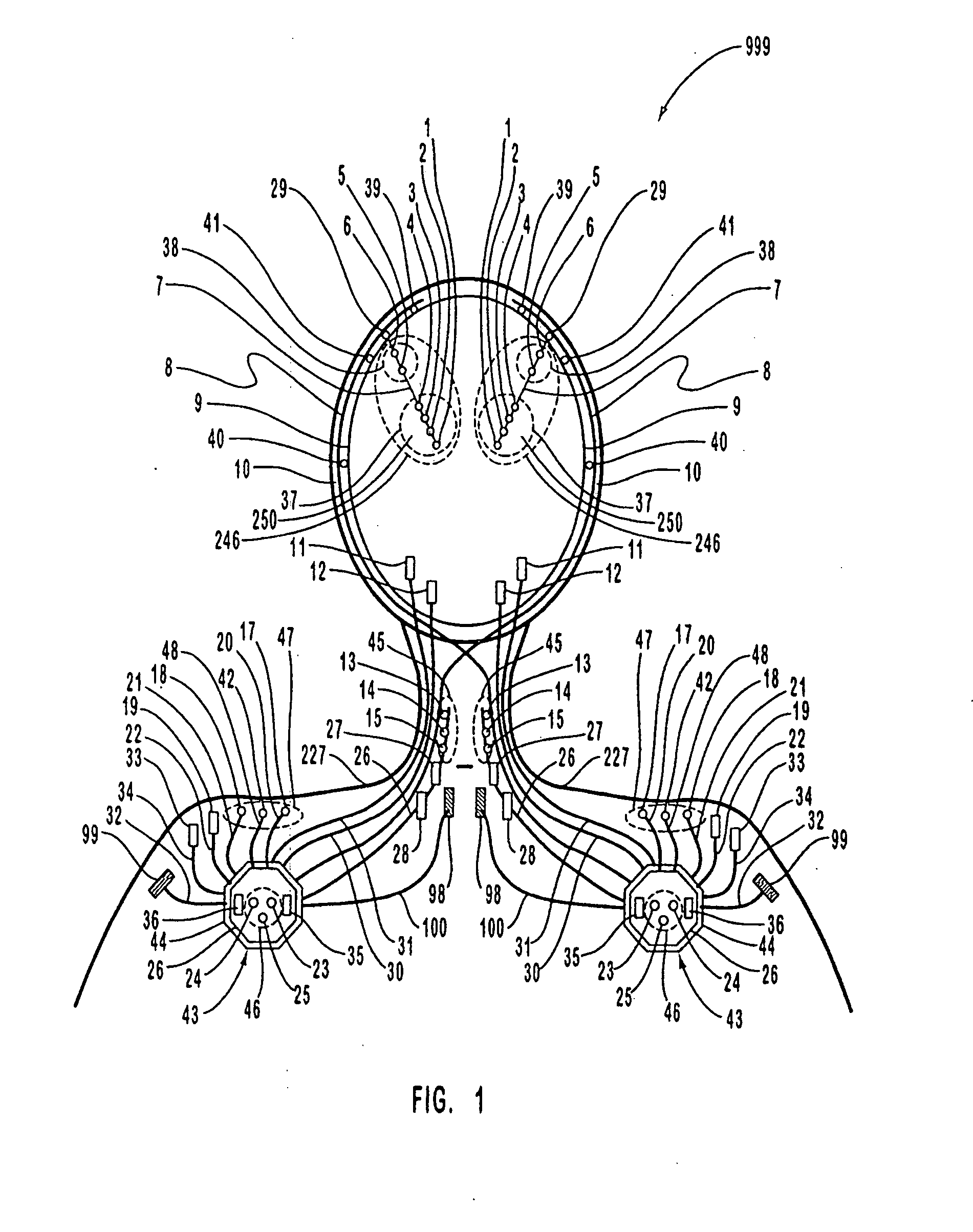
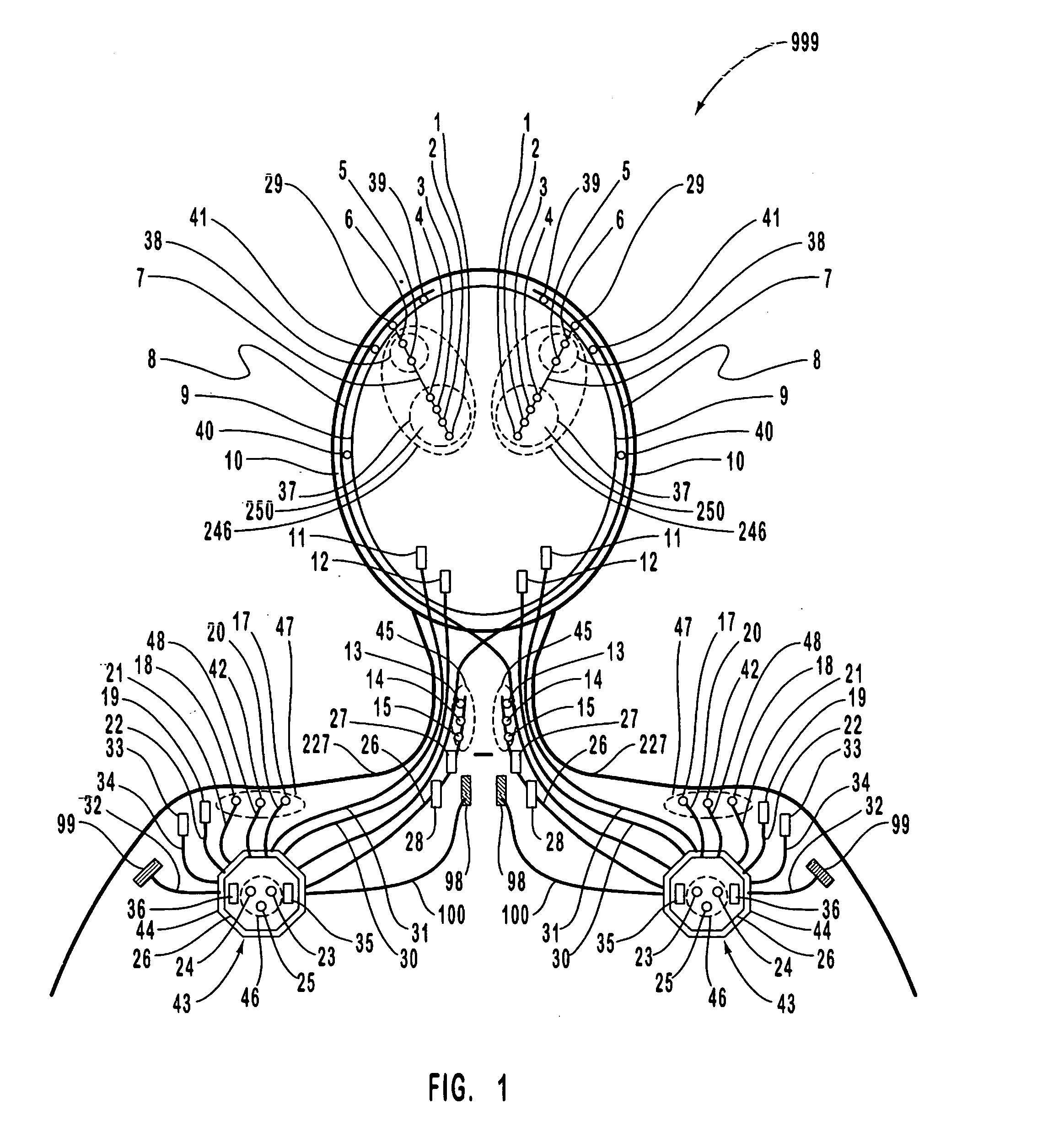
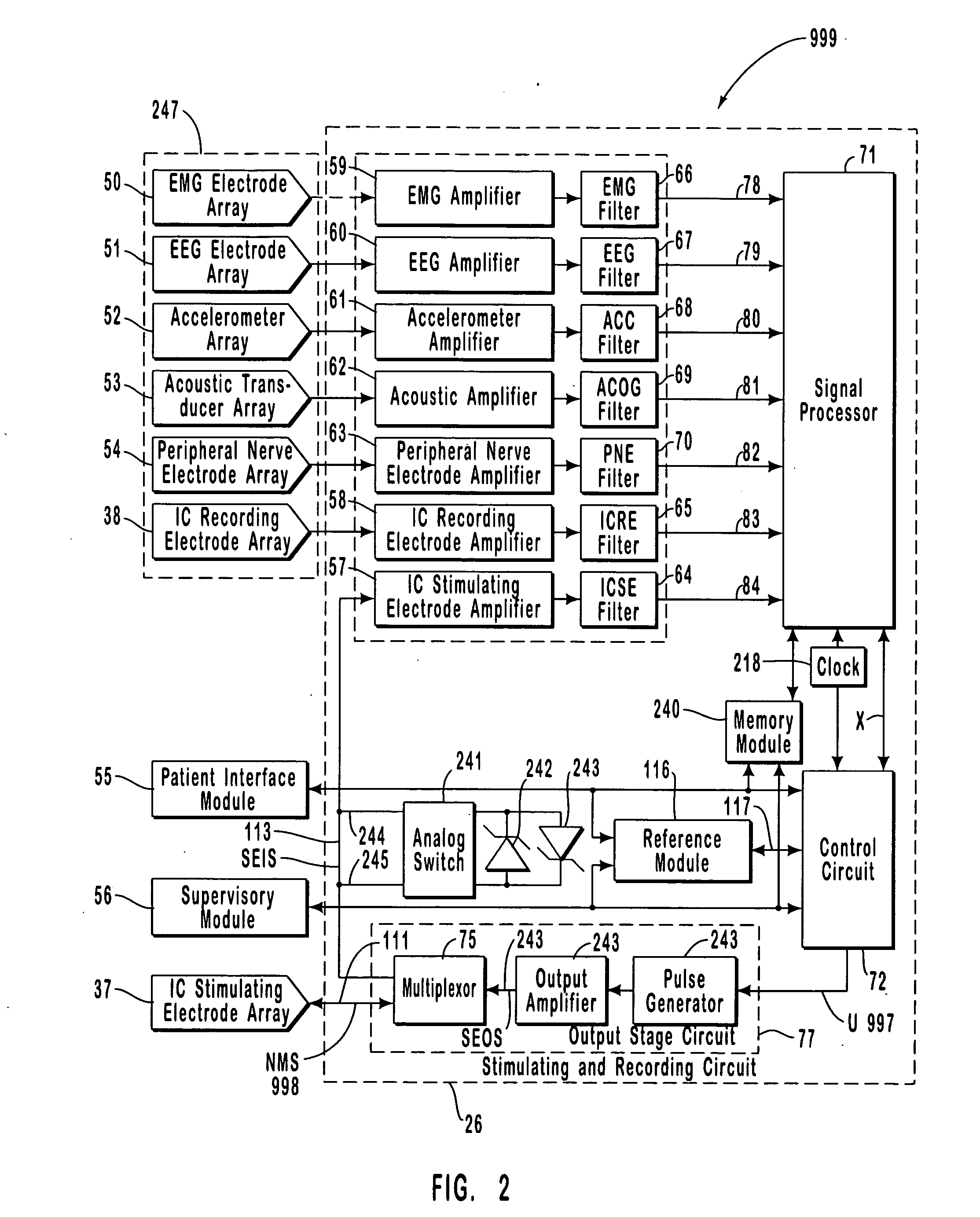
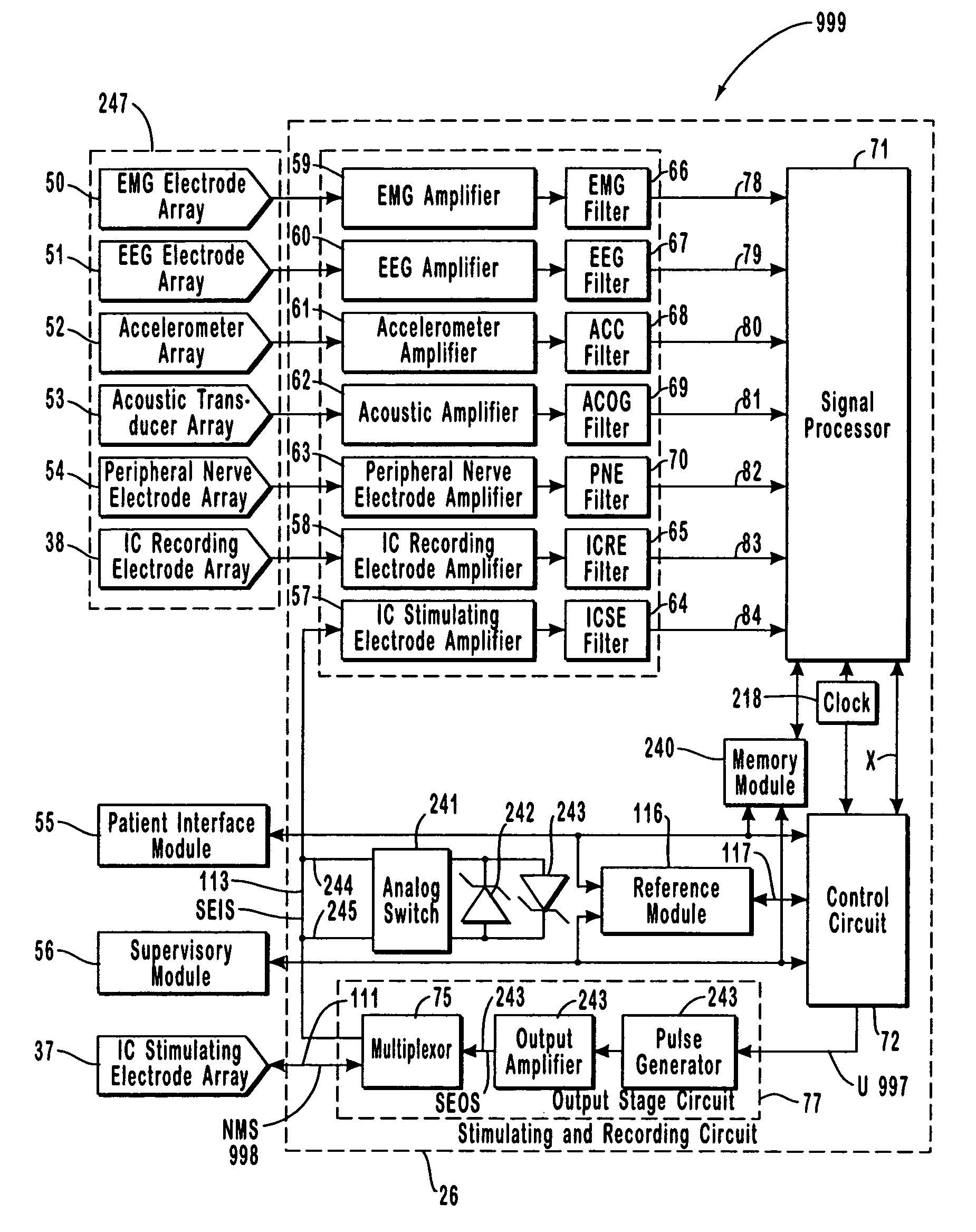
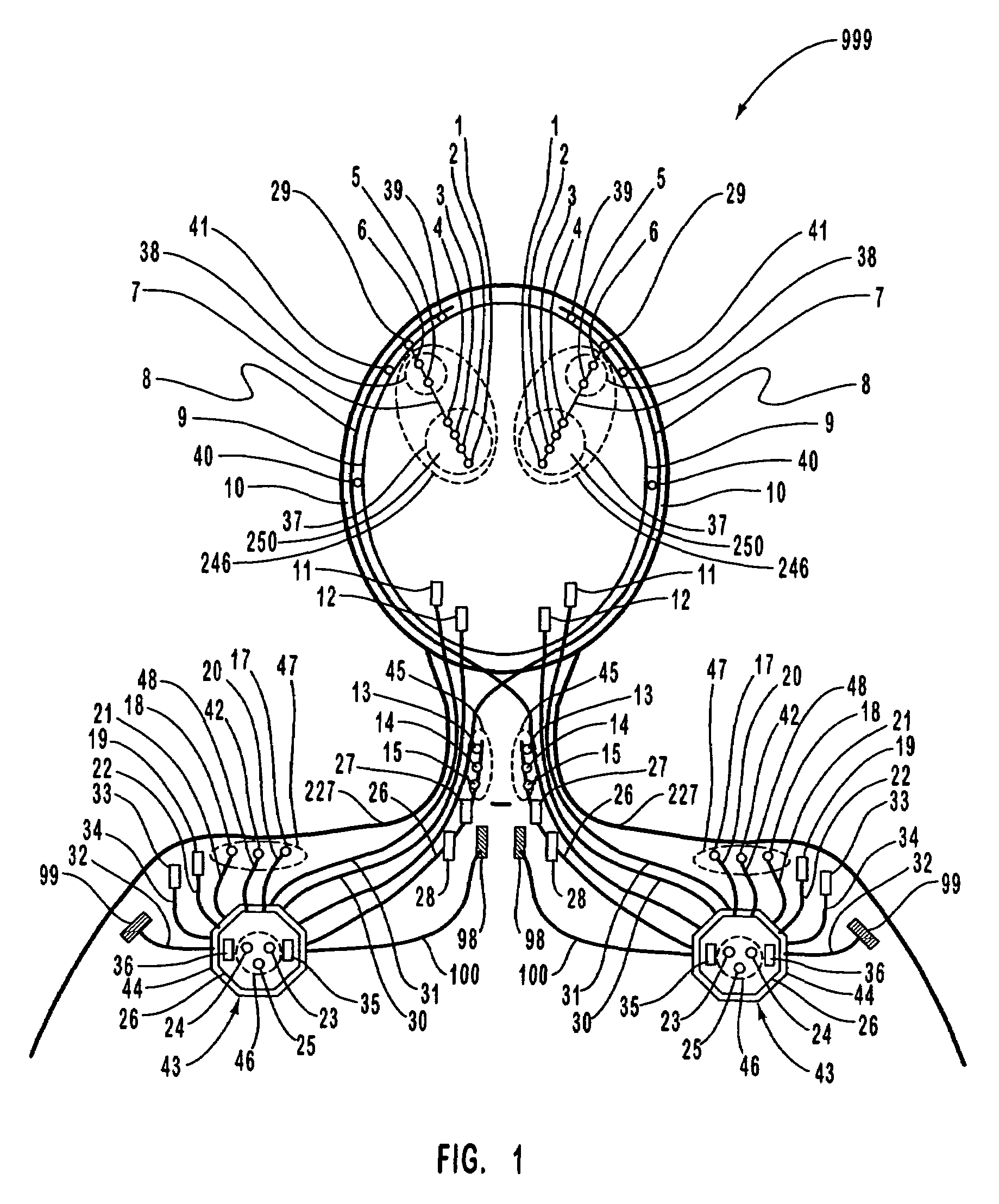
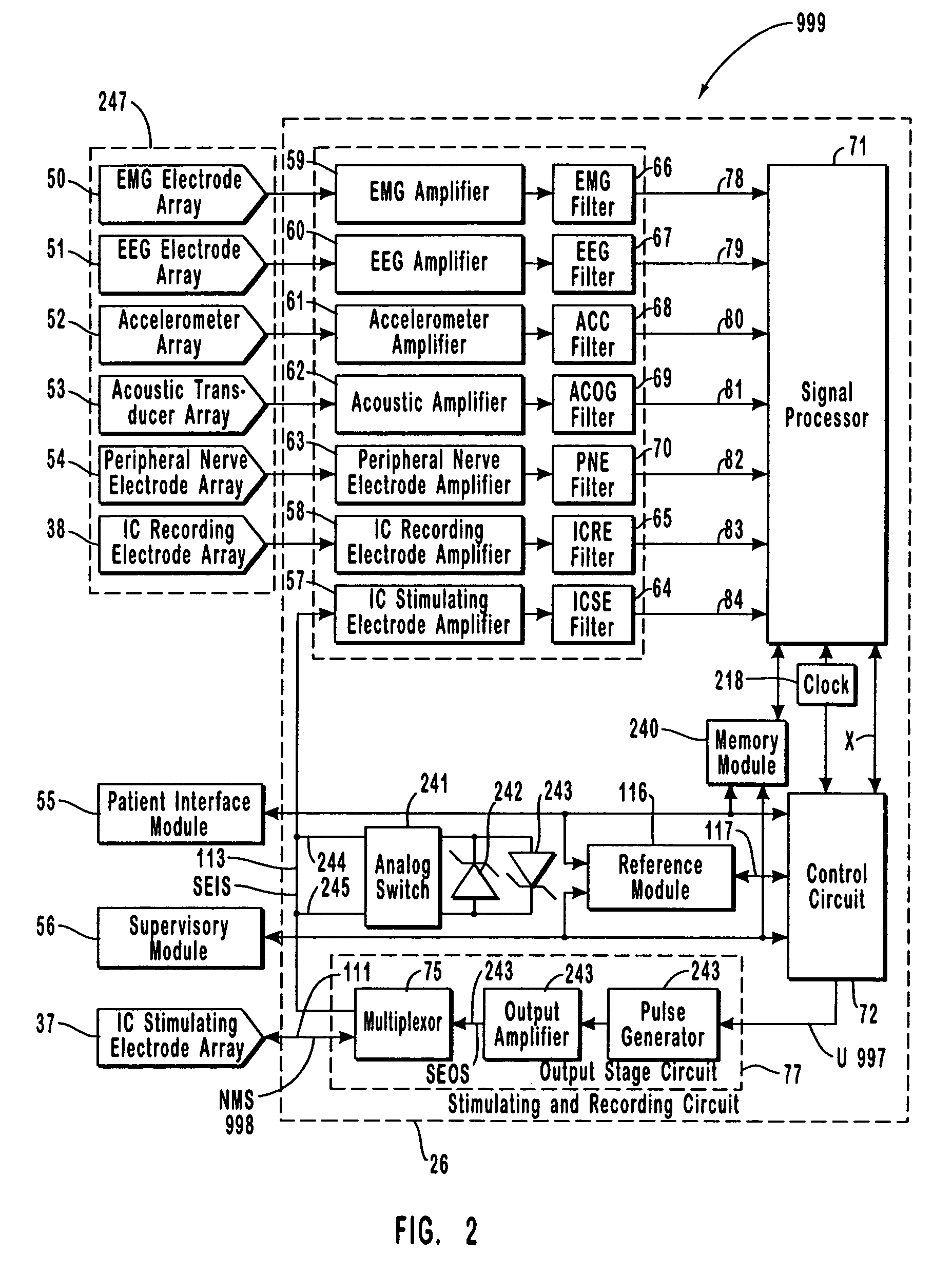
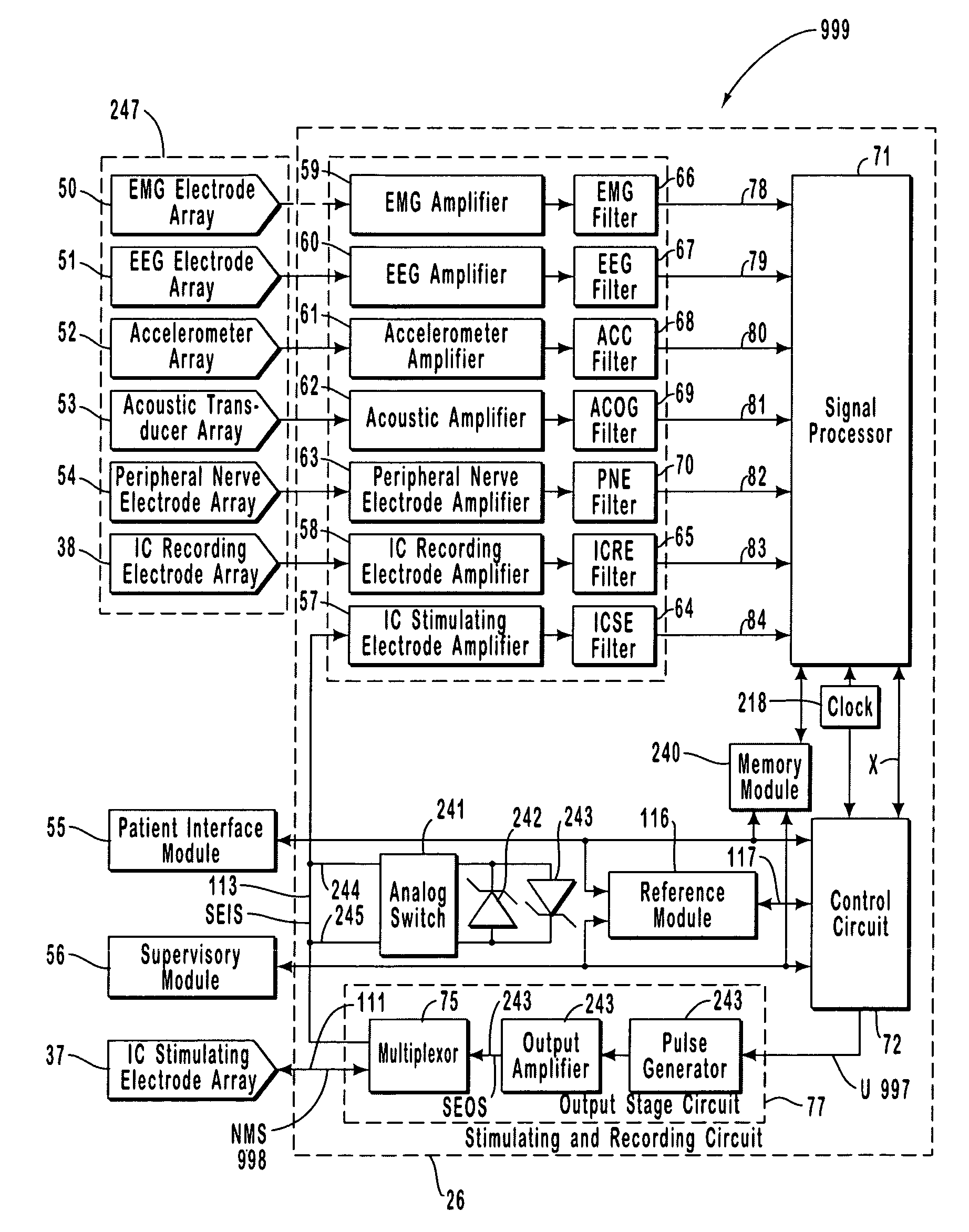
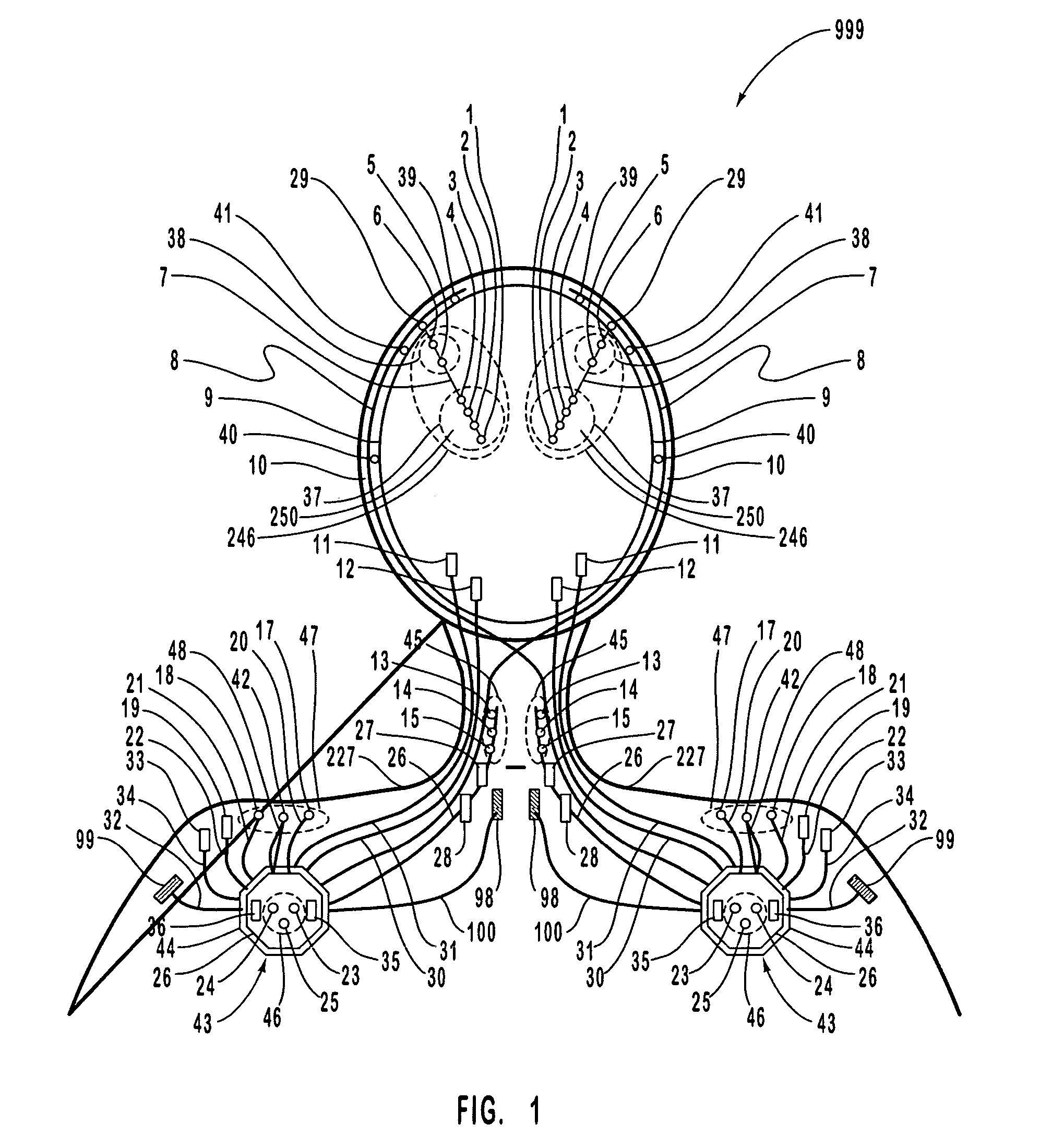
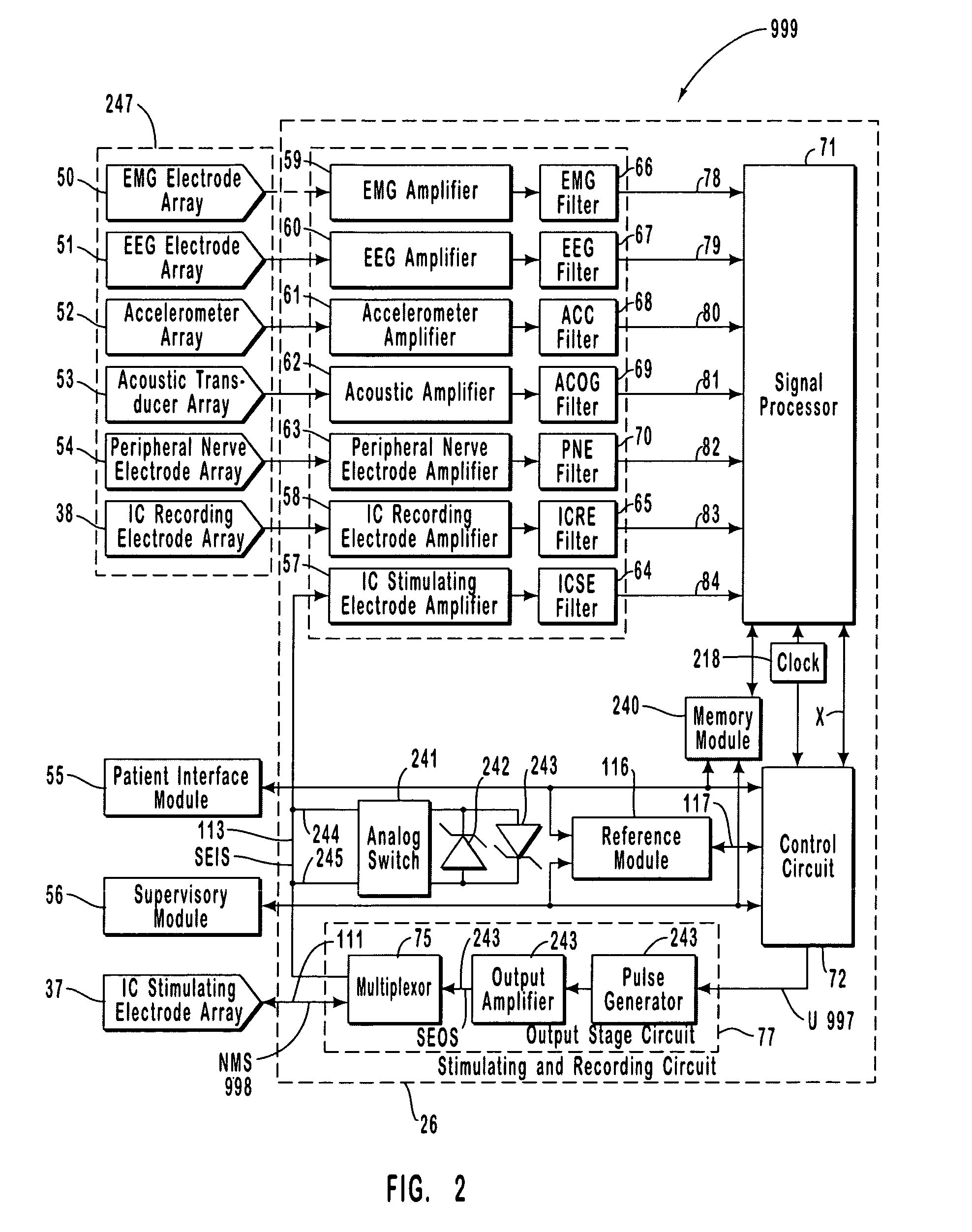
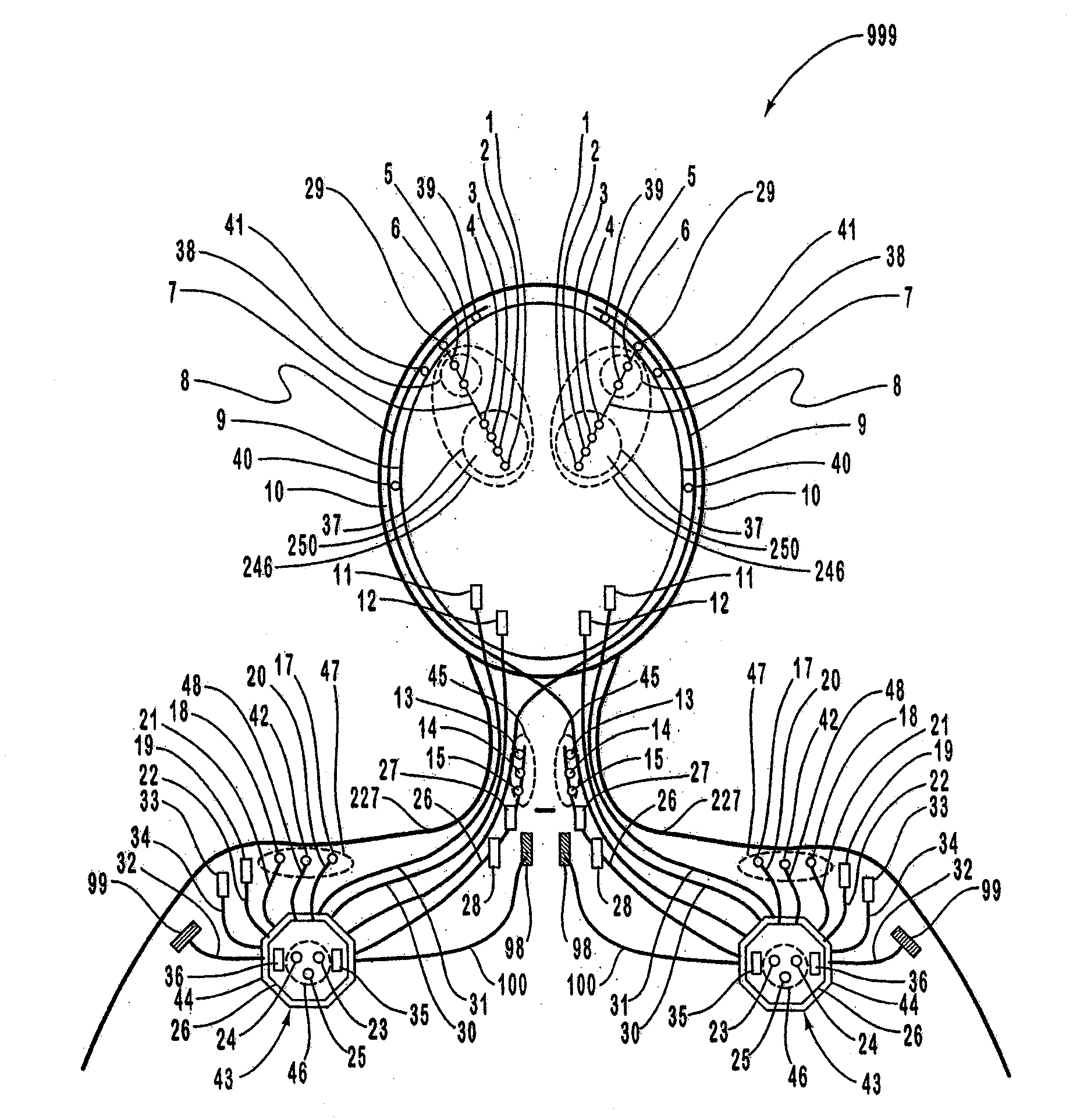
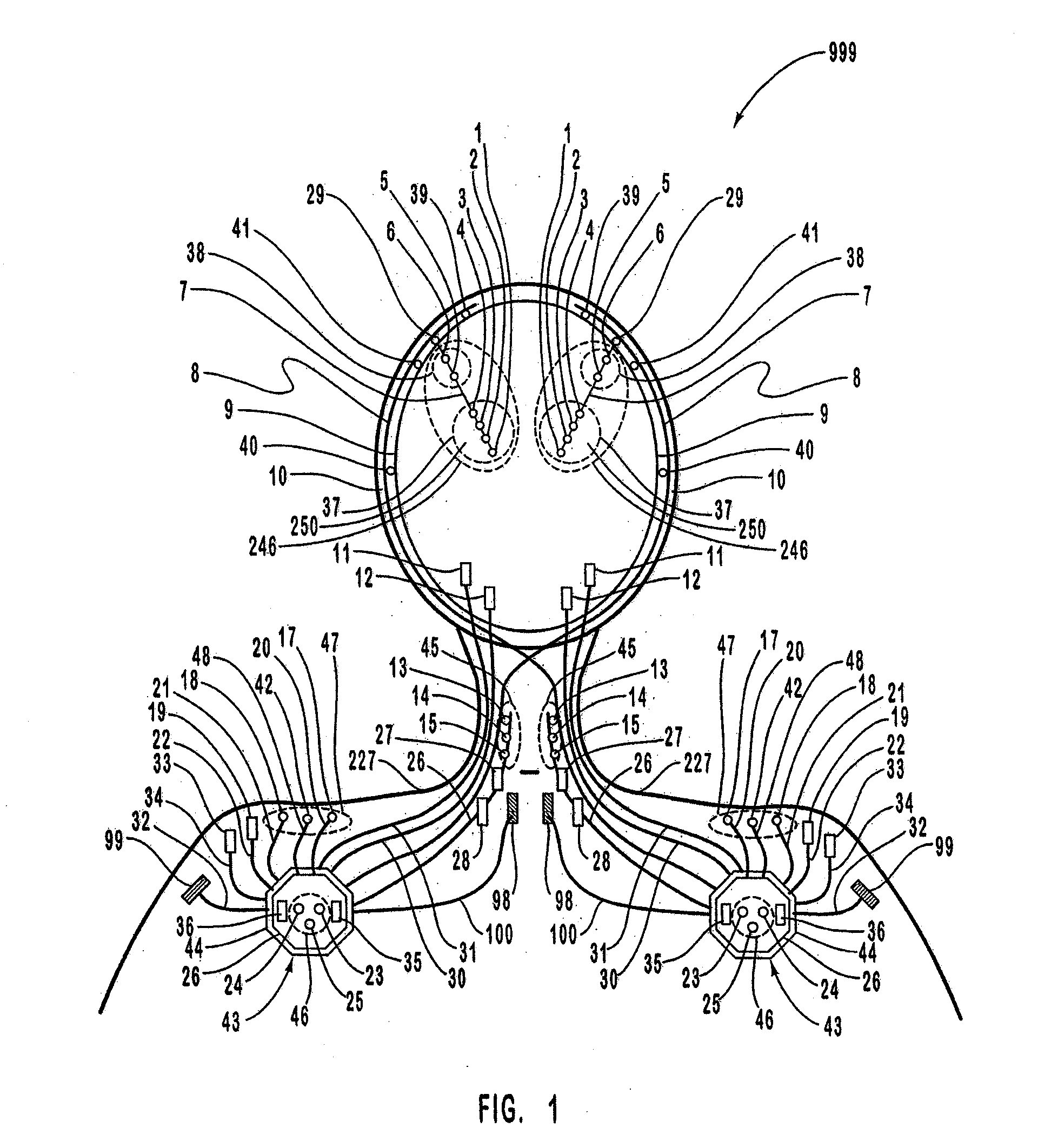
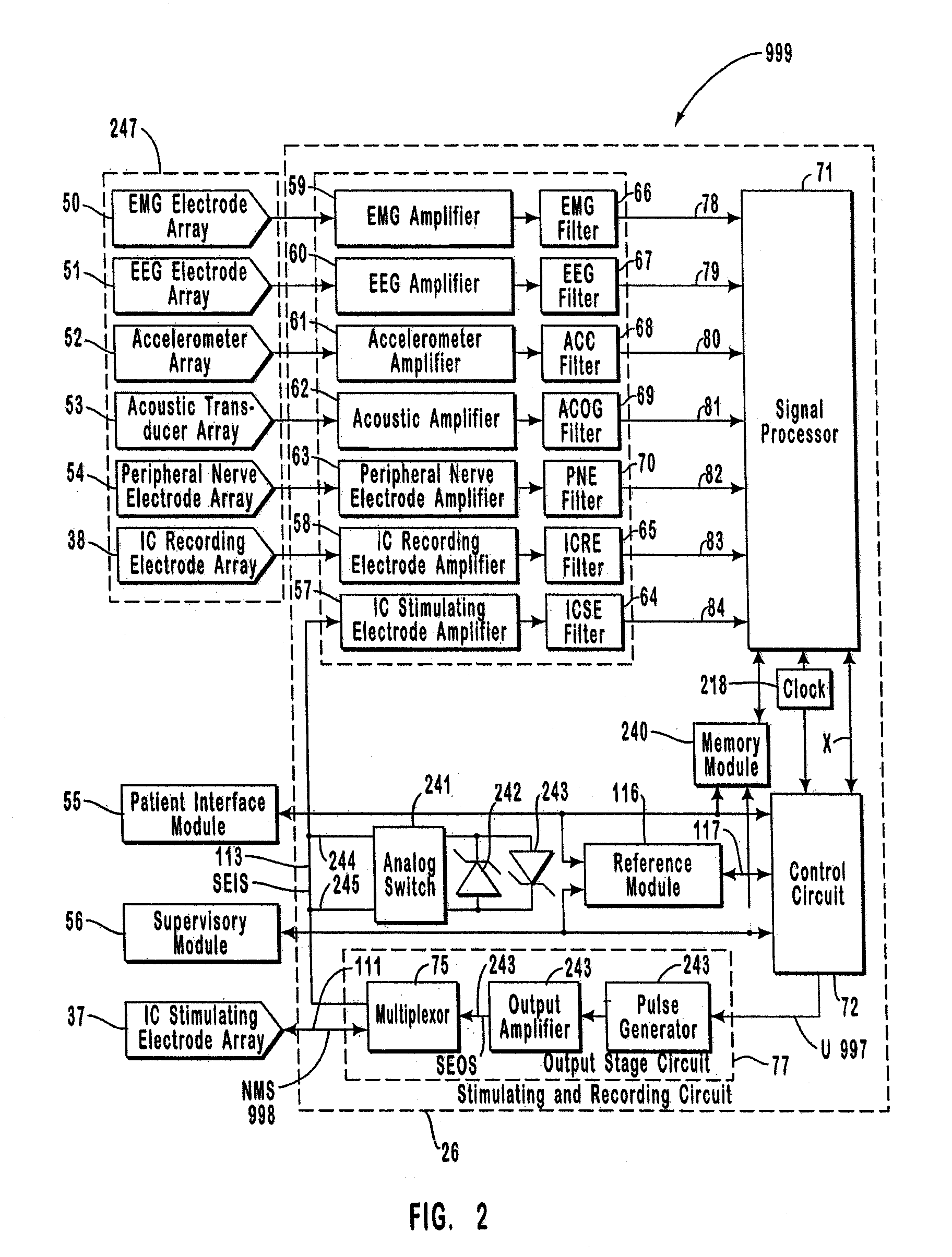
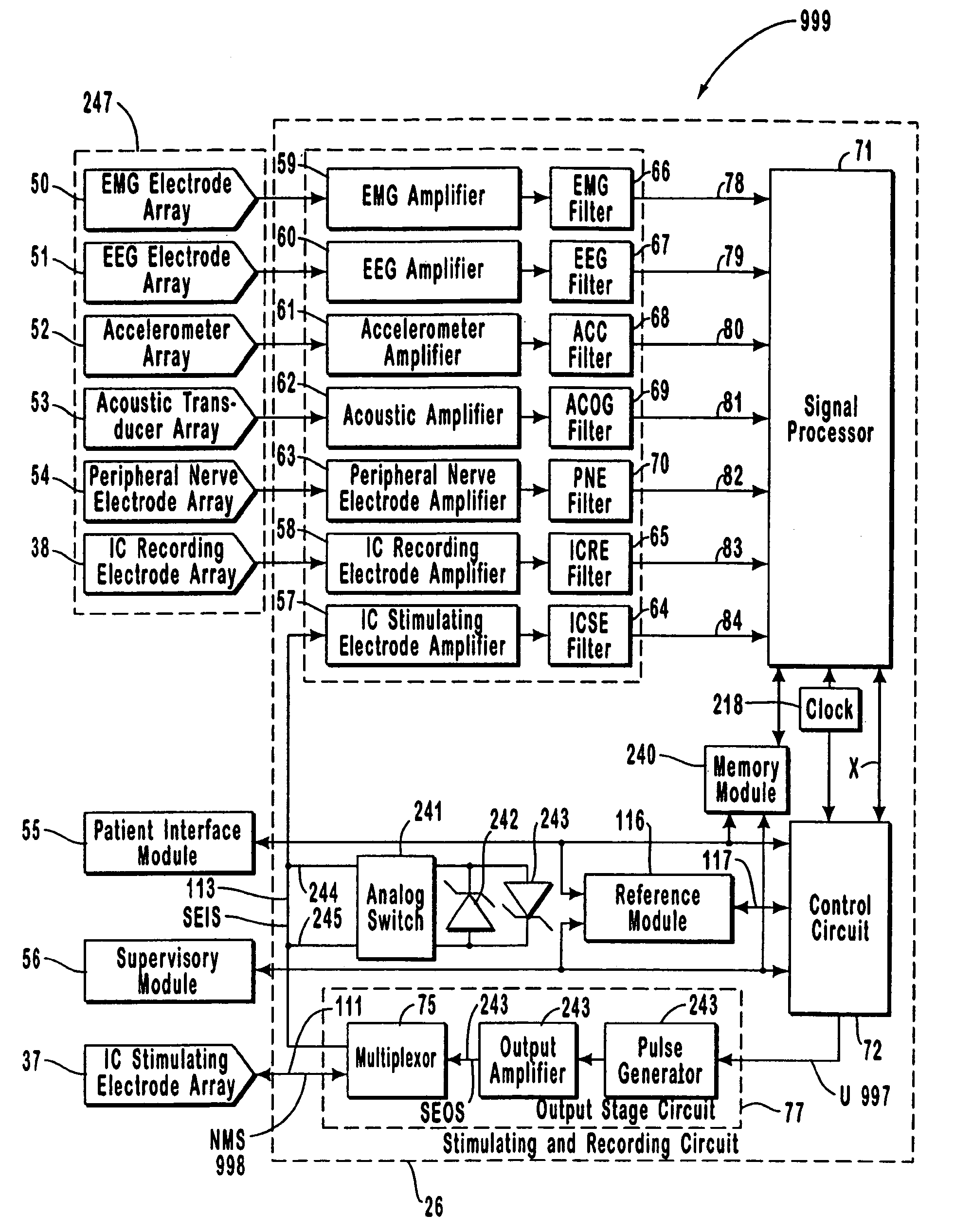
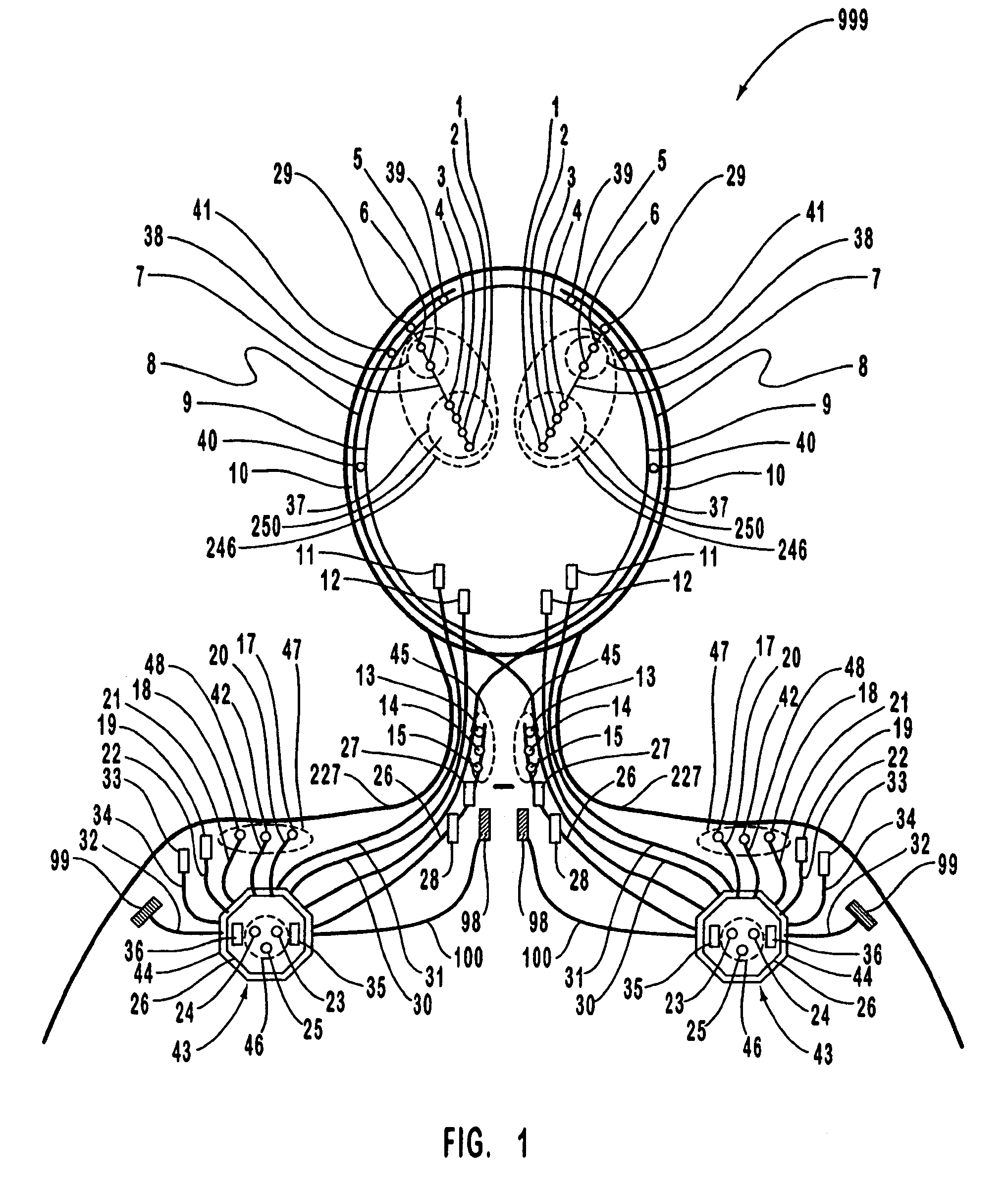
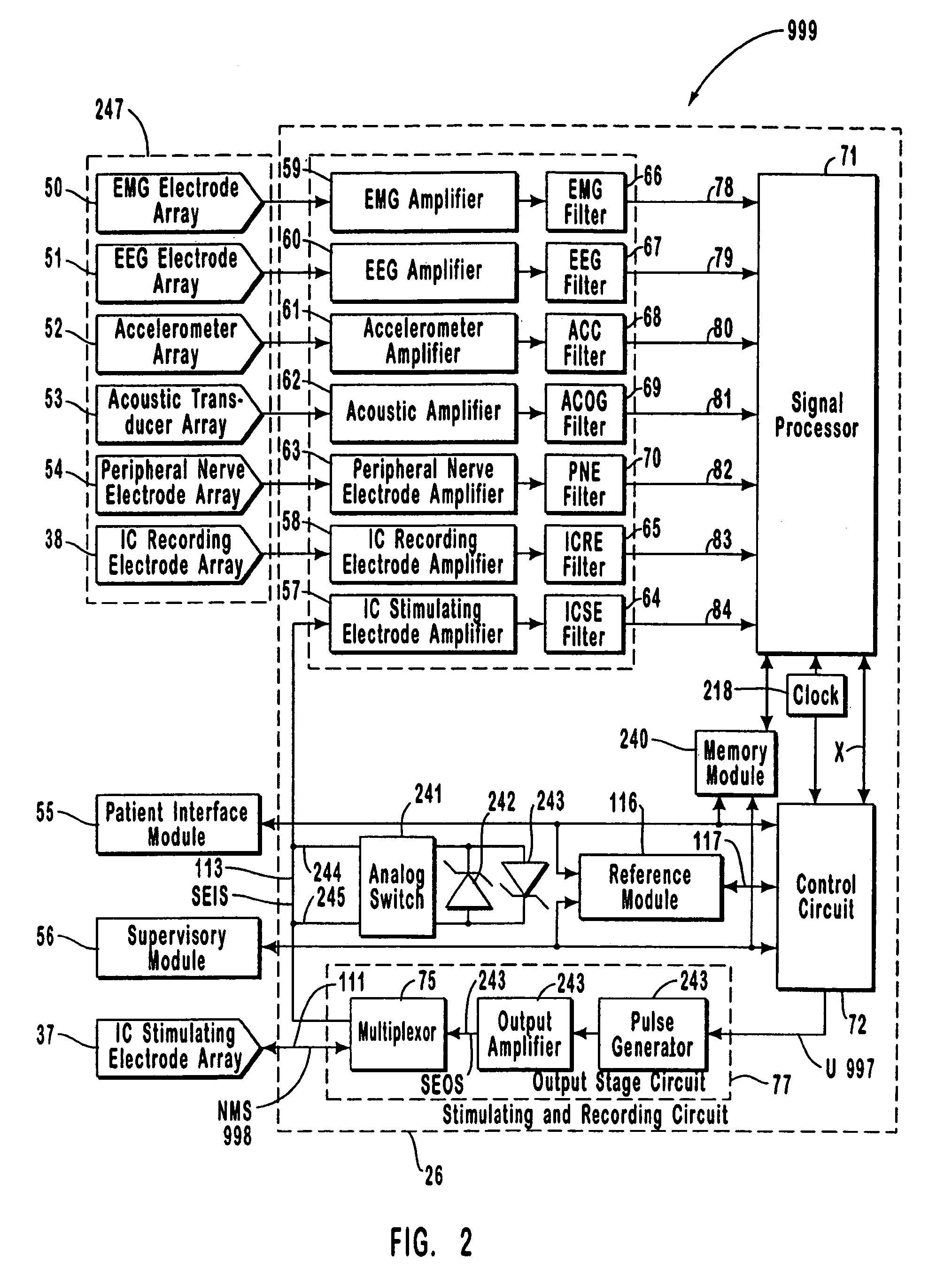
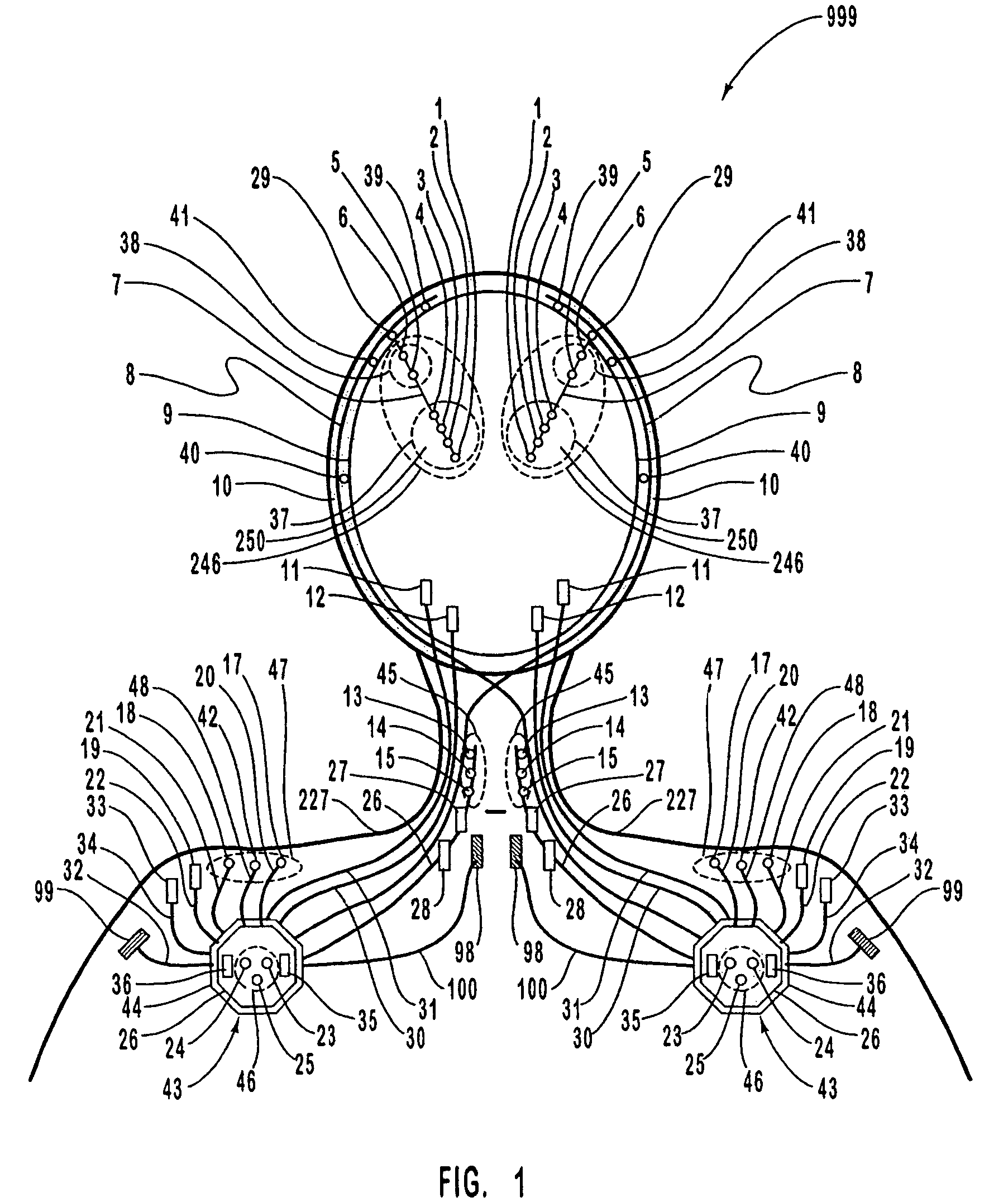
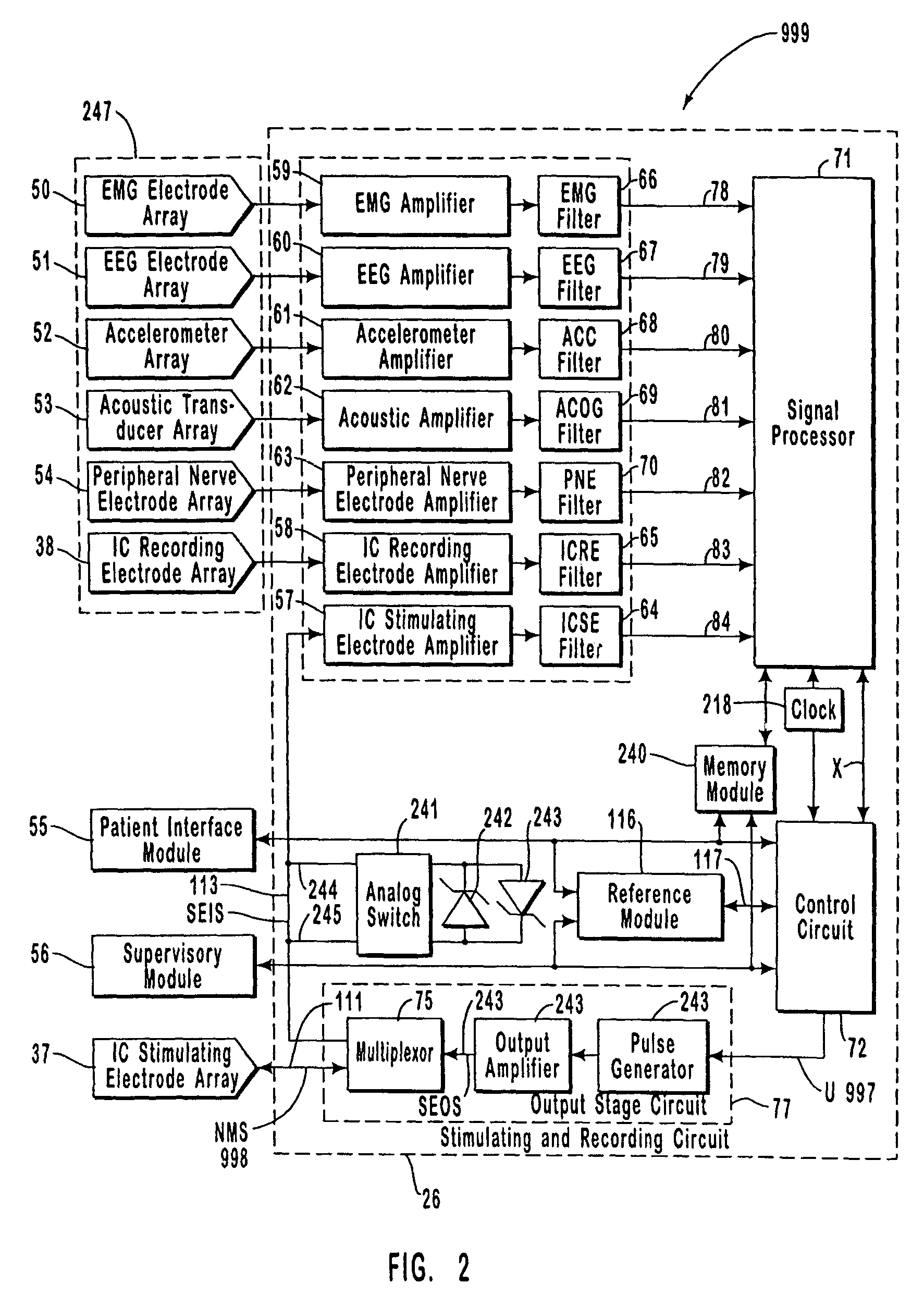
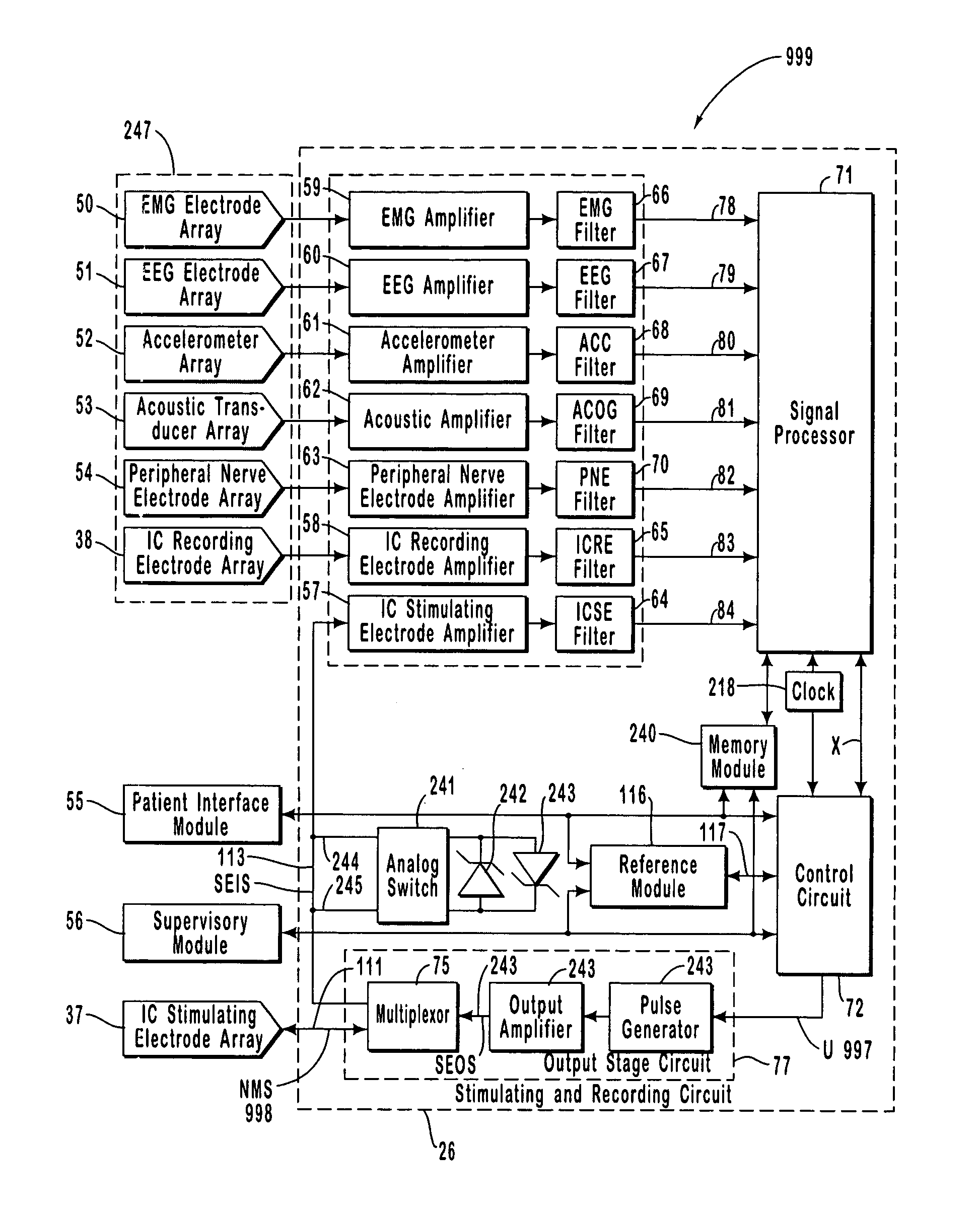
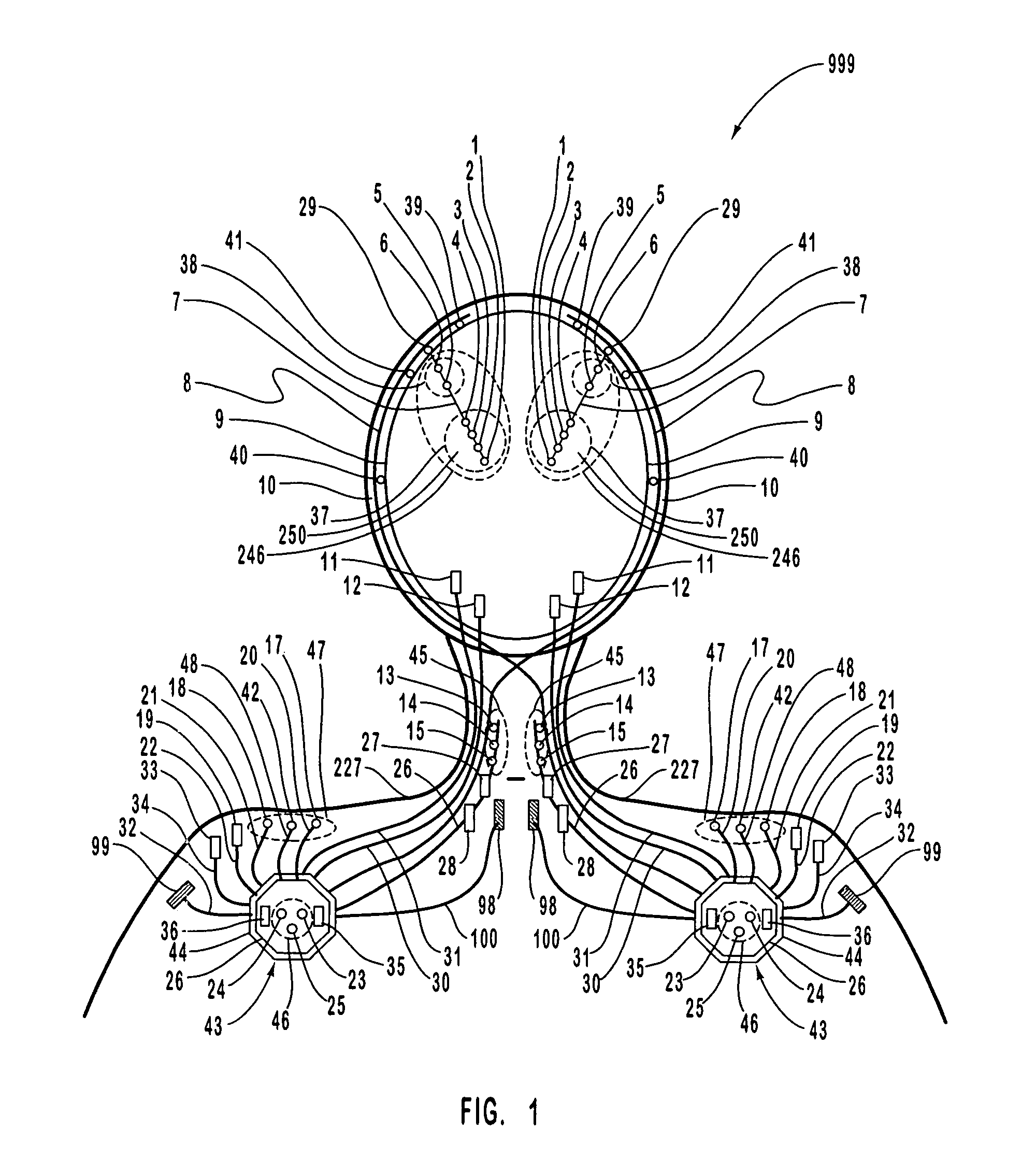
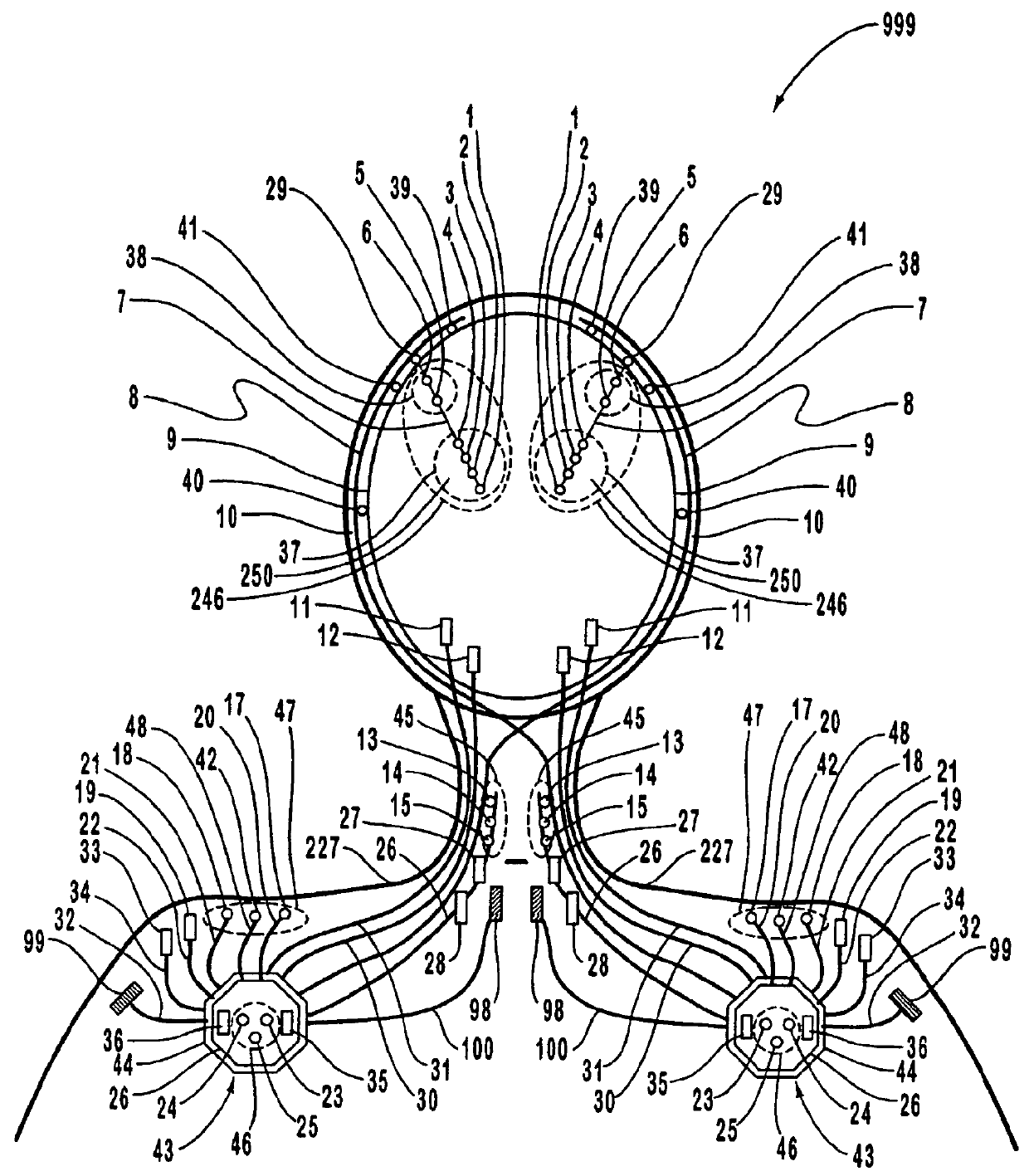
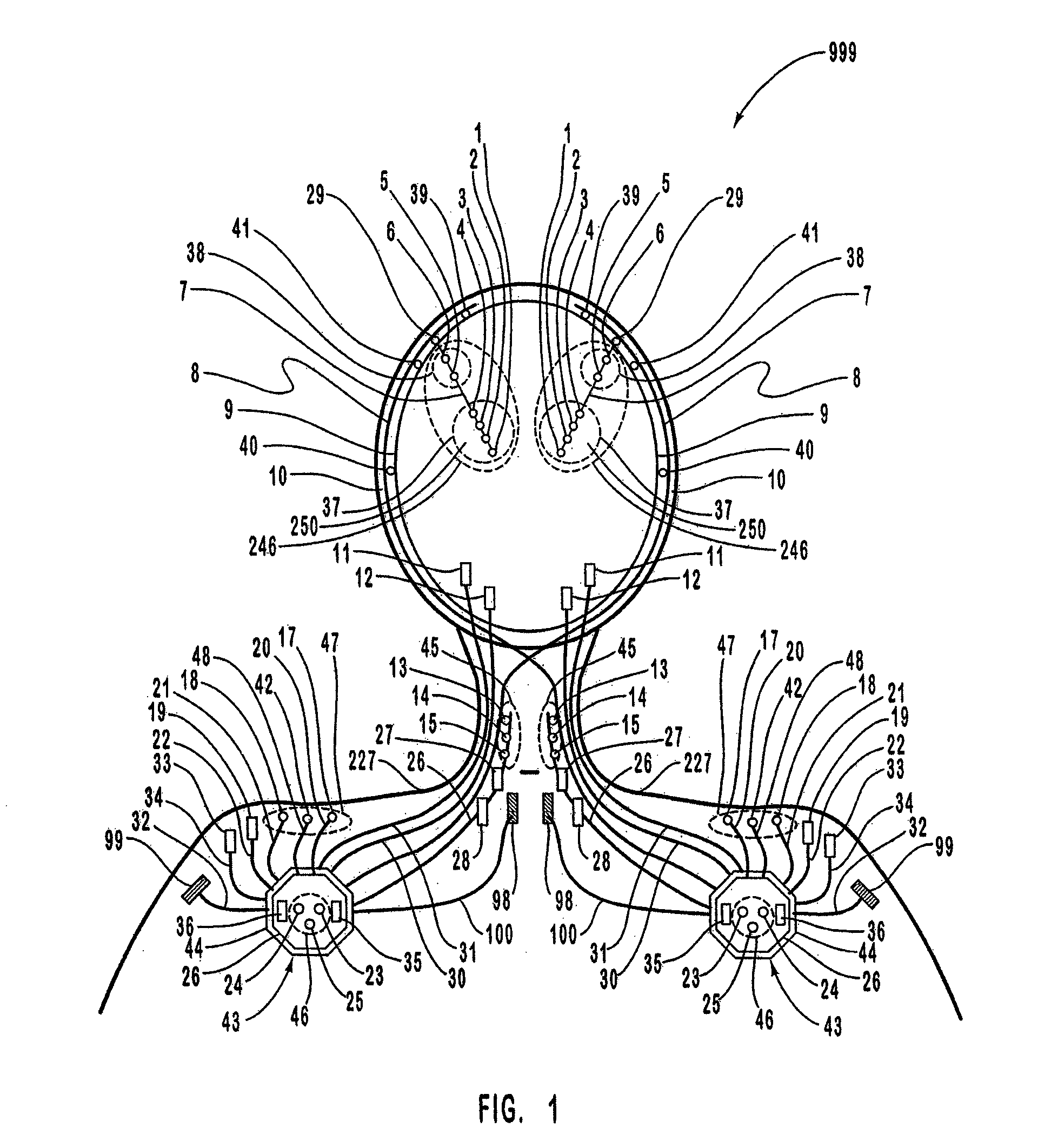
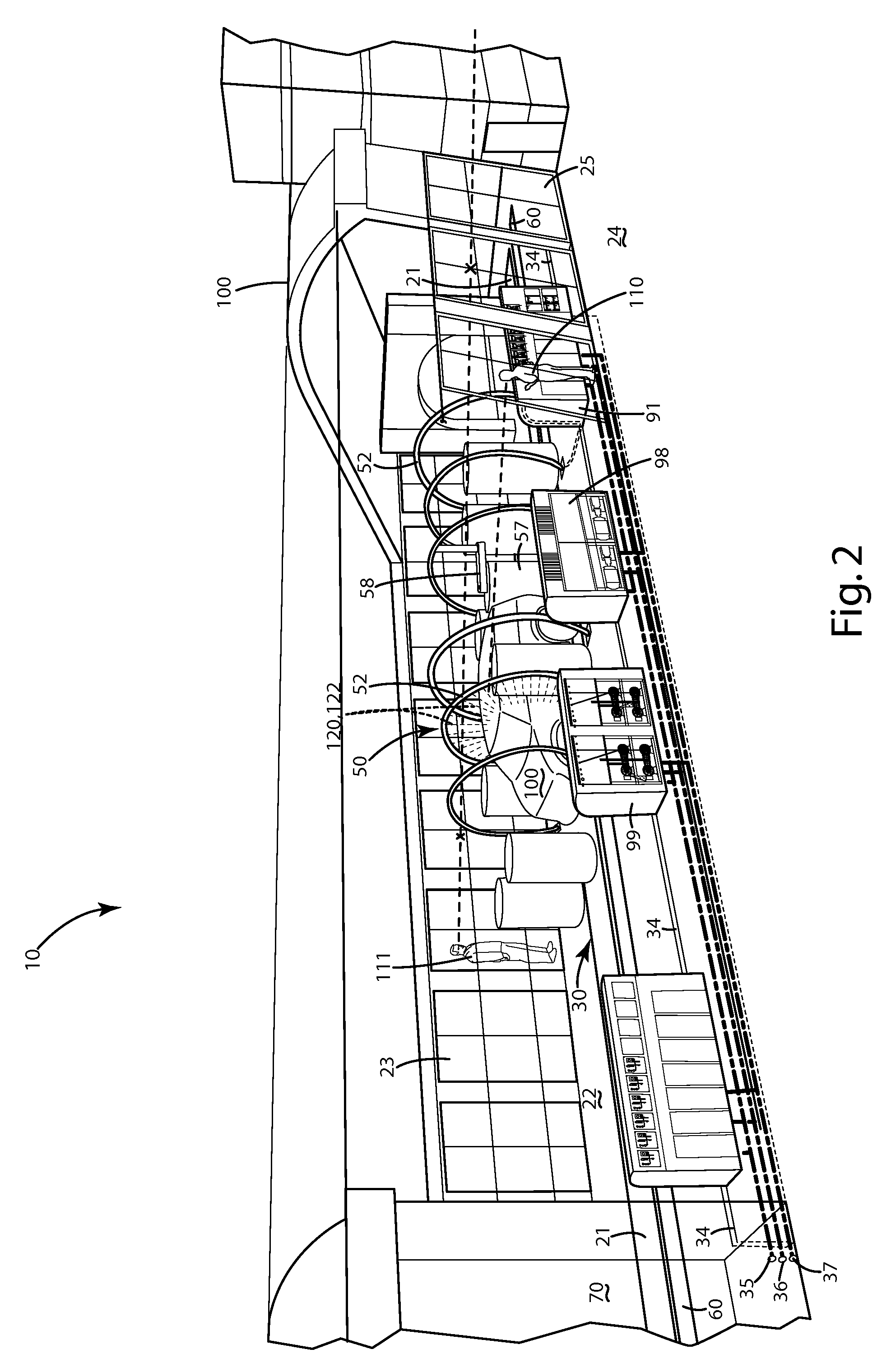
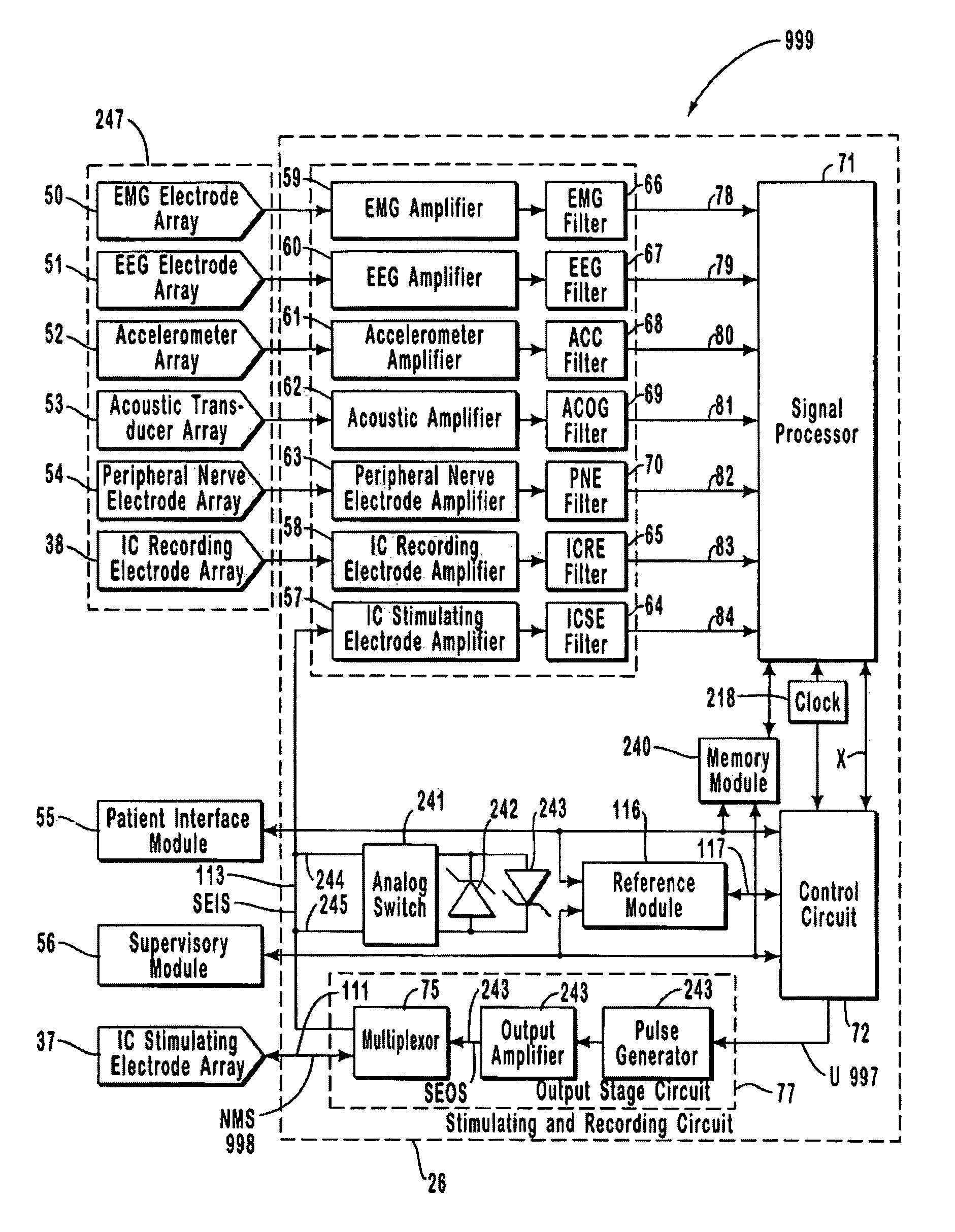
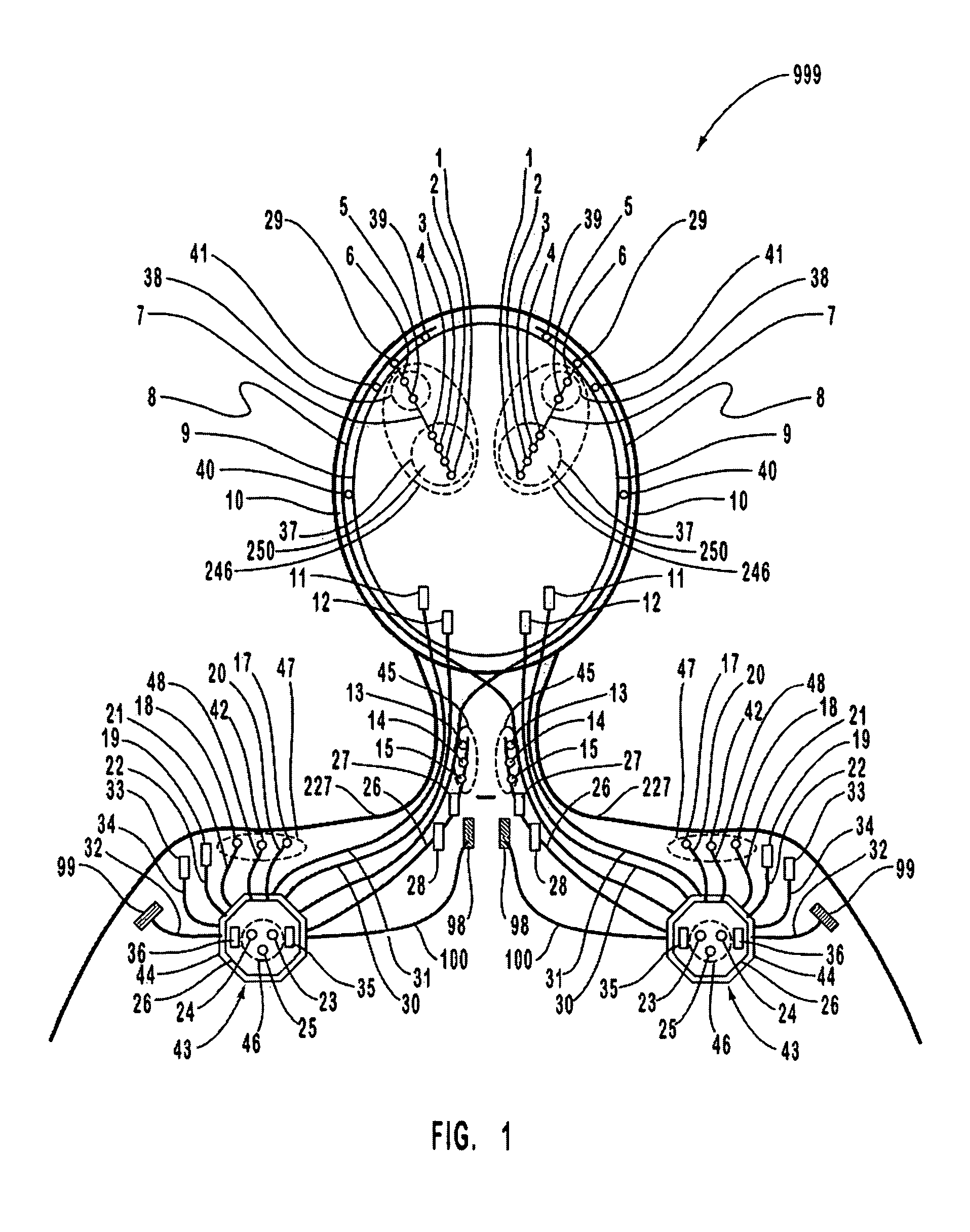
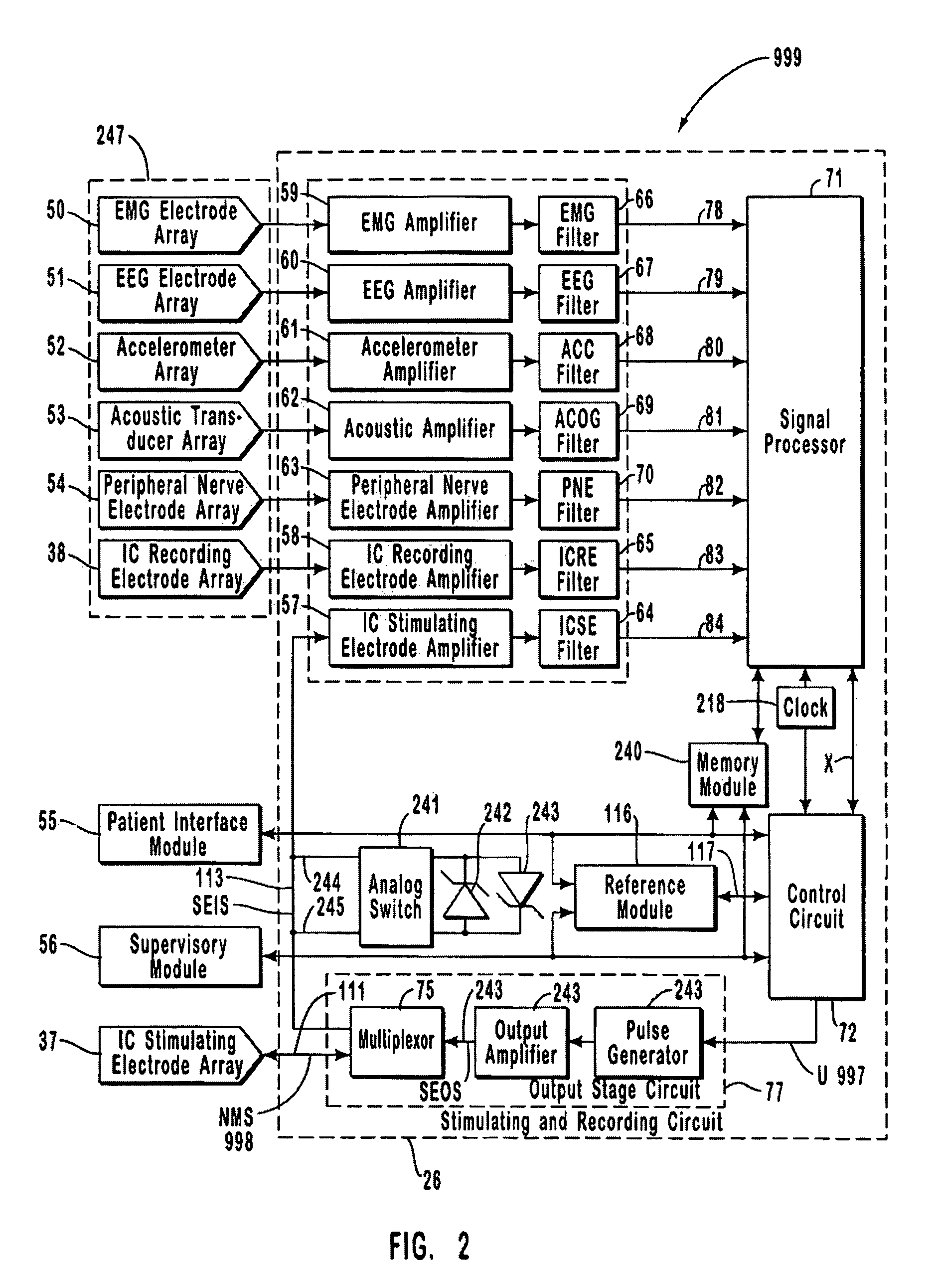
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more intracranial (IC) stimulating electrodes in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
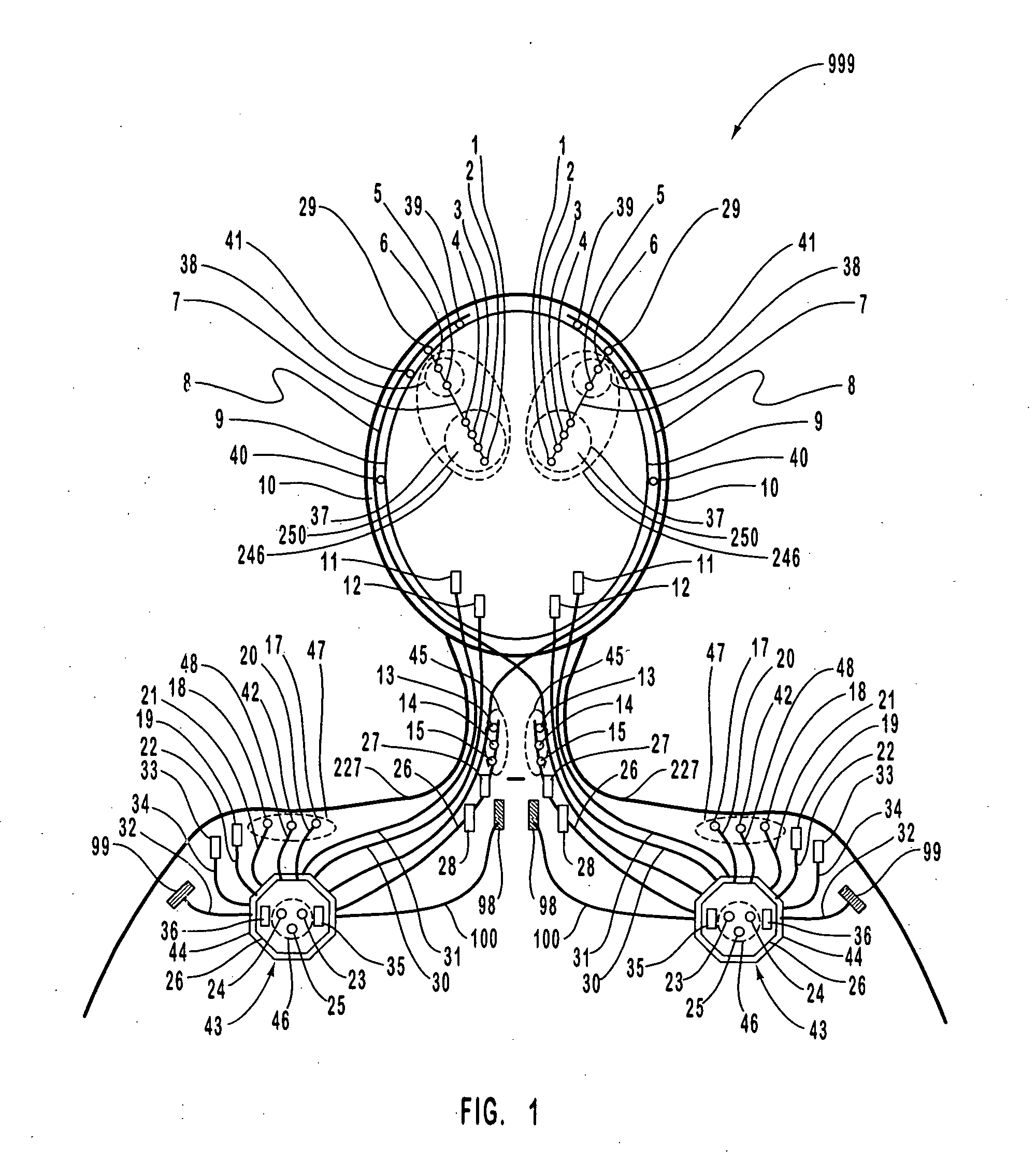
Closed-loop feedback-driven neuromodulation
InactiveUS7231254B2Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsNeurological signNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease
InactiveUS20050021103A1Shorten the timeReduce the numberHead electrodesImplantable neurostimulatorsNervous systemIntracranial stimulation
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more intracranial (IC) stimulating electrodes in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease
InactiveUS7242984B2Reducing time and number of interactionSatisfactory treatmentHead electrodesExternal electrodesDiseaseIntracranial stimulation
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more intracranial (IC) stimulating electrodes in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Closed-loop feedback-driven neuromodulation
InactiveUS7324851B1Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsNervous systemNEUROLOGIC REACTION
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a □nervous system component.□ The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators, comprising intracranial (IC) stimulating electrodes and other actuators, in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Apparatus and method for closed-loop intracranial stimulation for optimal control of neurological disease
InactiveUS20070073355A1Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsIntracranial stimulationNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more intracranial (IC) stimulating electrodes in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Closed-loop feedback-driven neuromodulation
InactiveUS20050240242A1Shorten the timeSatisfactory treatmentHead electrodesImplantable neurostimulatorsNeurological signNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Methods and systems for predicting future symptomatology in a patient suffering from a neurological or psychiatric disorder
InactiveUS7277758B2Reducing time and number of interactionSatisfactory treatmentHead electrodesExternal electrodesDiseaseNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more intracranial (IC) stimulating electrodes in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Closed-loop feedback-driven neuromodulation
InactiveUS7403820B2Reducing time and number of interactionSatisfactory treatmentHead electrodesAngle modulation detailsNEUROLOGIC REACTIONNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators, comprising intracranial (IC) stimulating electrodes and other actuators, in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Closed-Loop Feedback-Driven Neuromodulation
InactiveUS20090018609A1Shorten the timeSatisfactory treatmentHead electrodesAngle modulation detailsNervous systemMedicine
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators, comprising intracranial (IC) stimulating electrodes and other actuators, in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
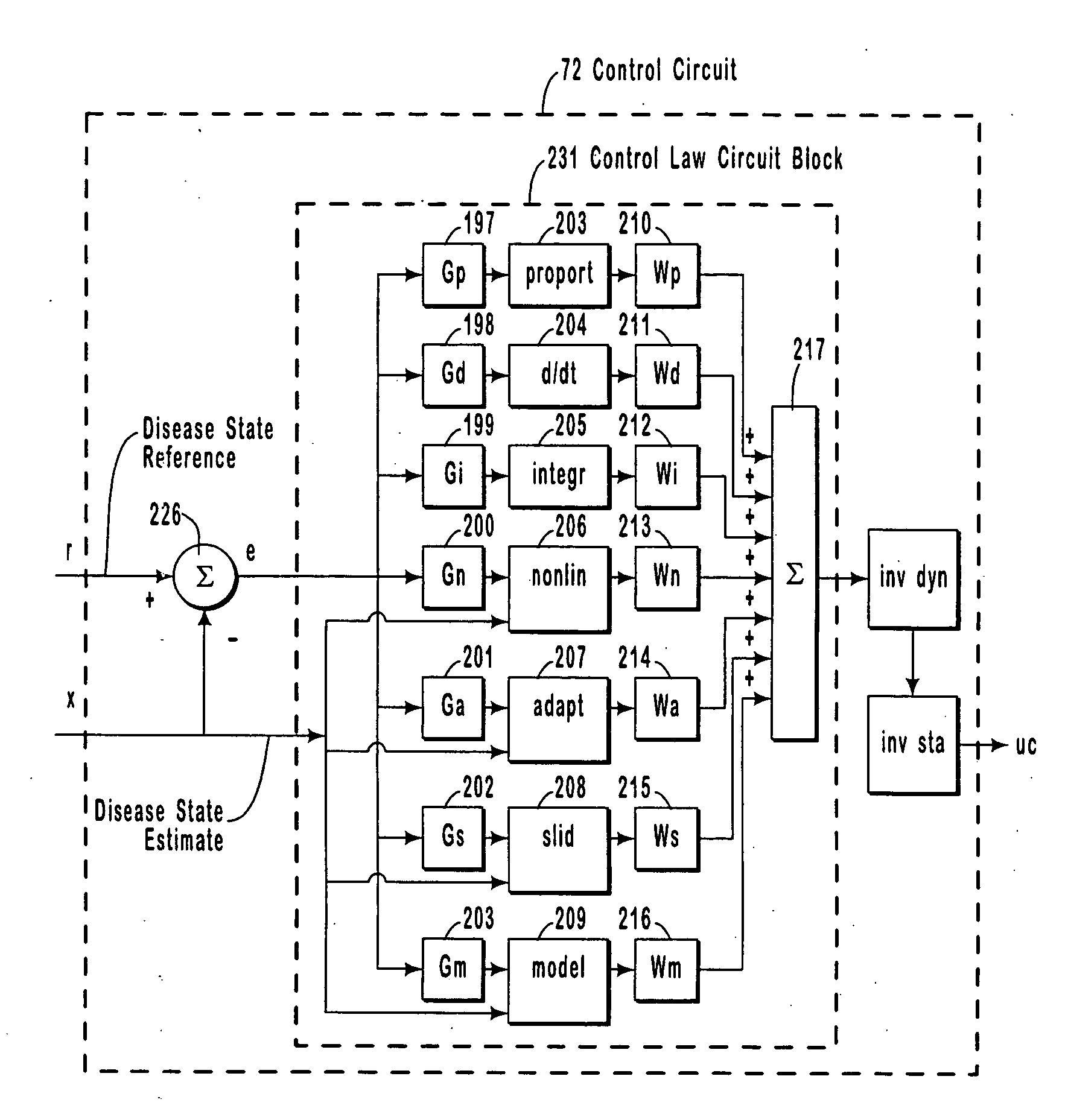
Systems and methods for monitoring a patient's neurological disease state
InactiveUS7747325B2Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsDiseaseNervous system
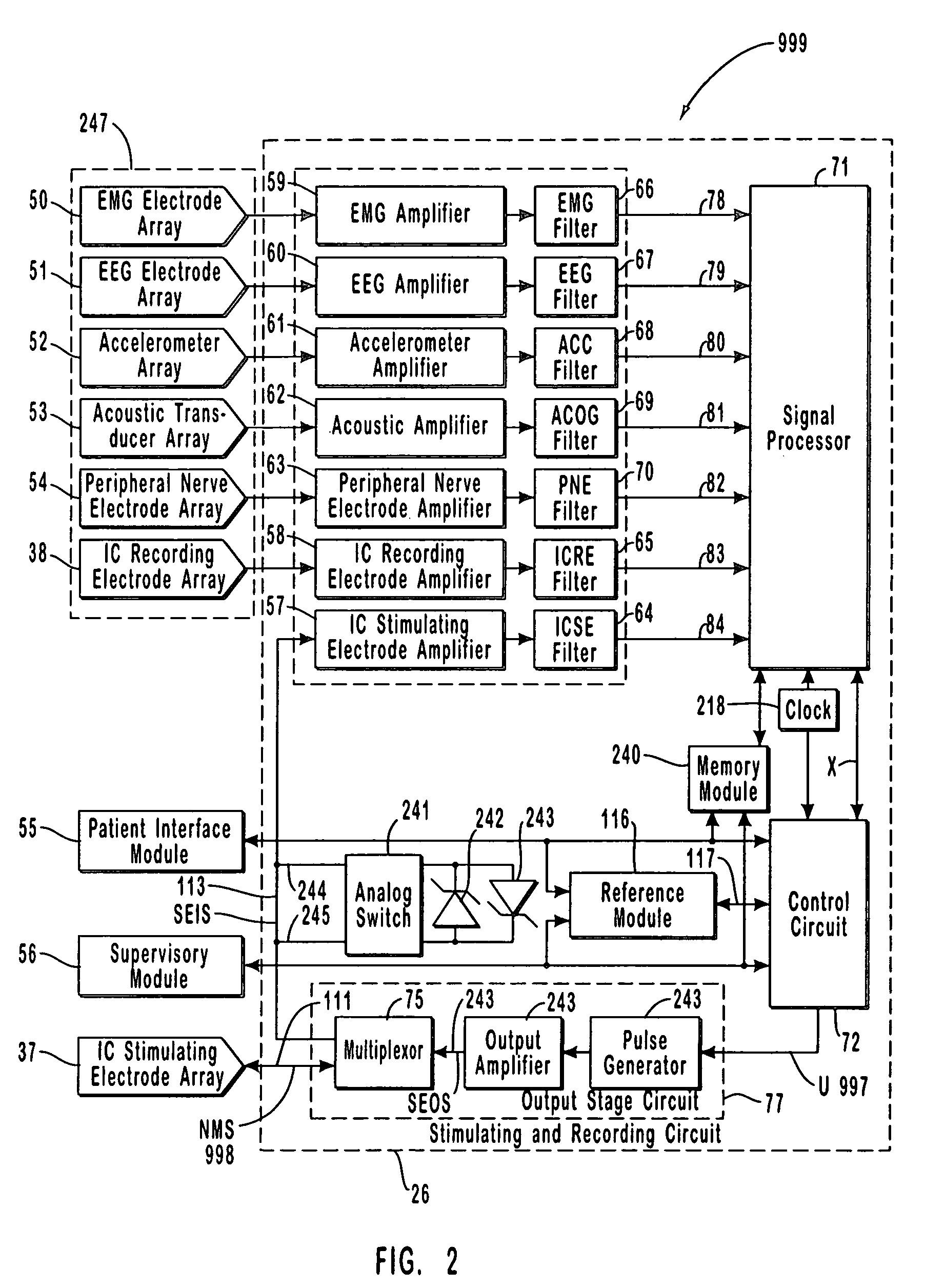
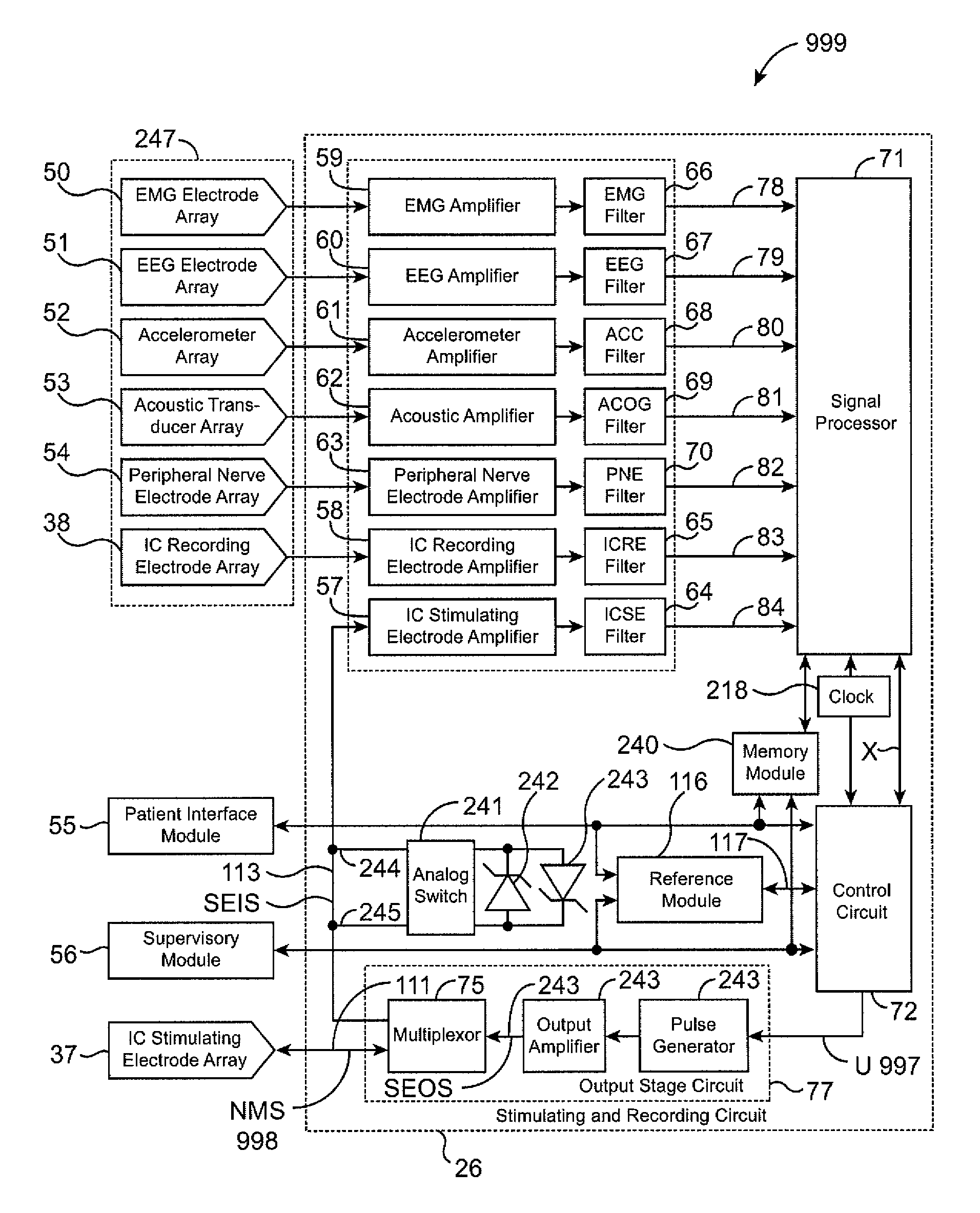
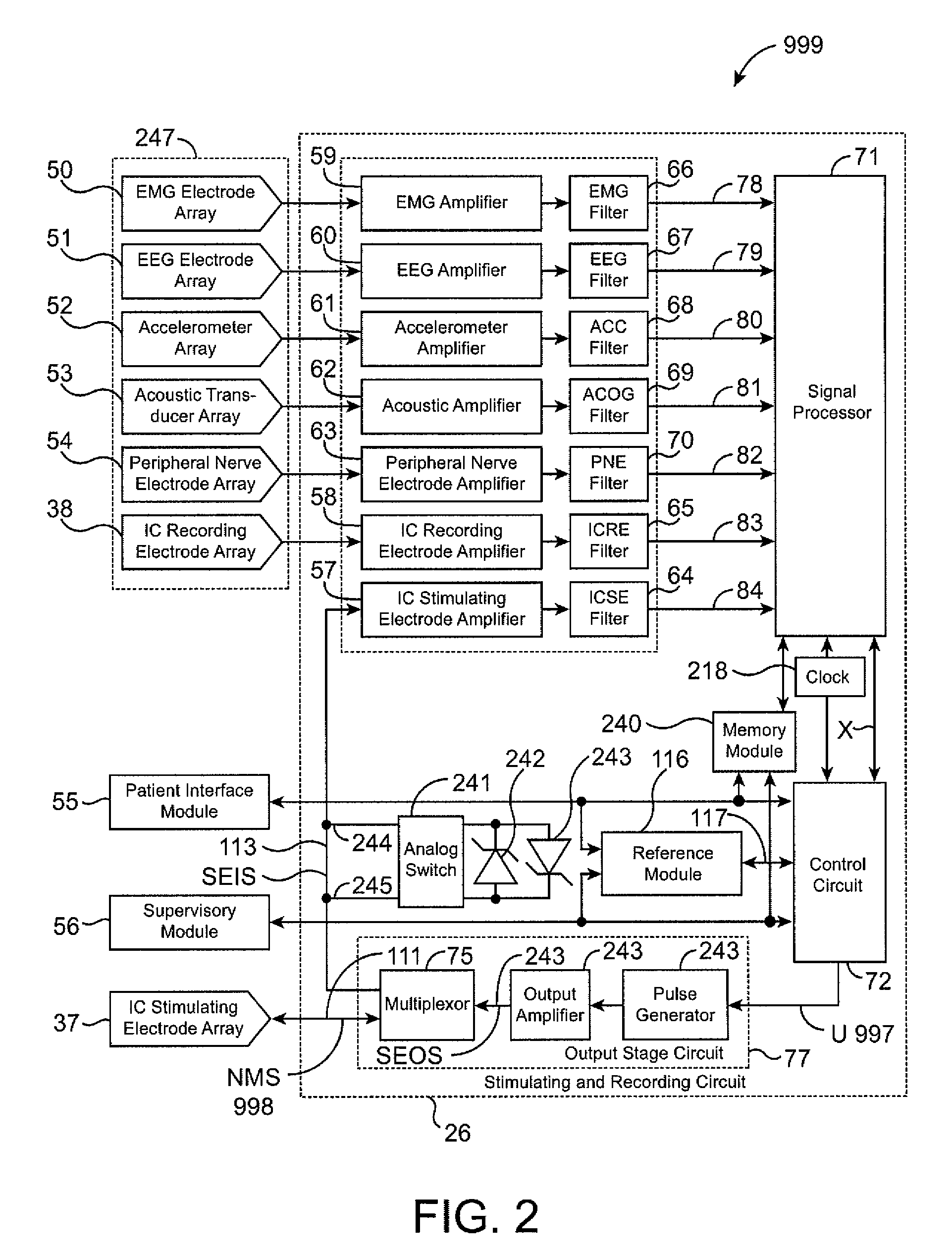
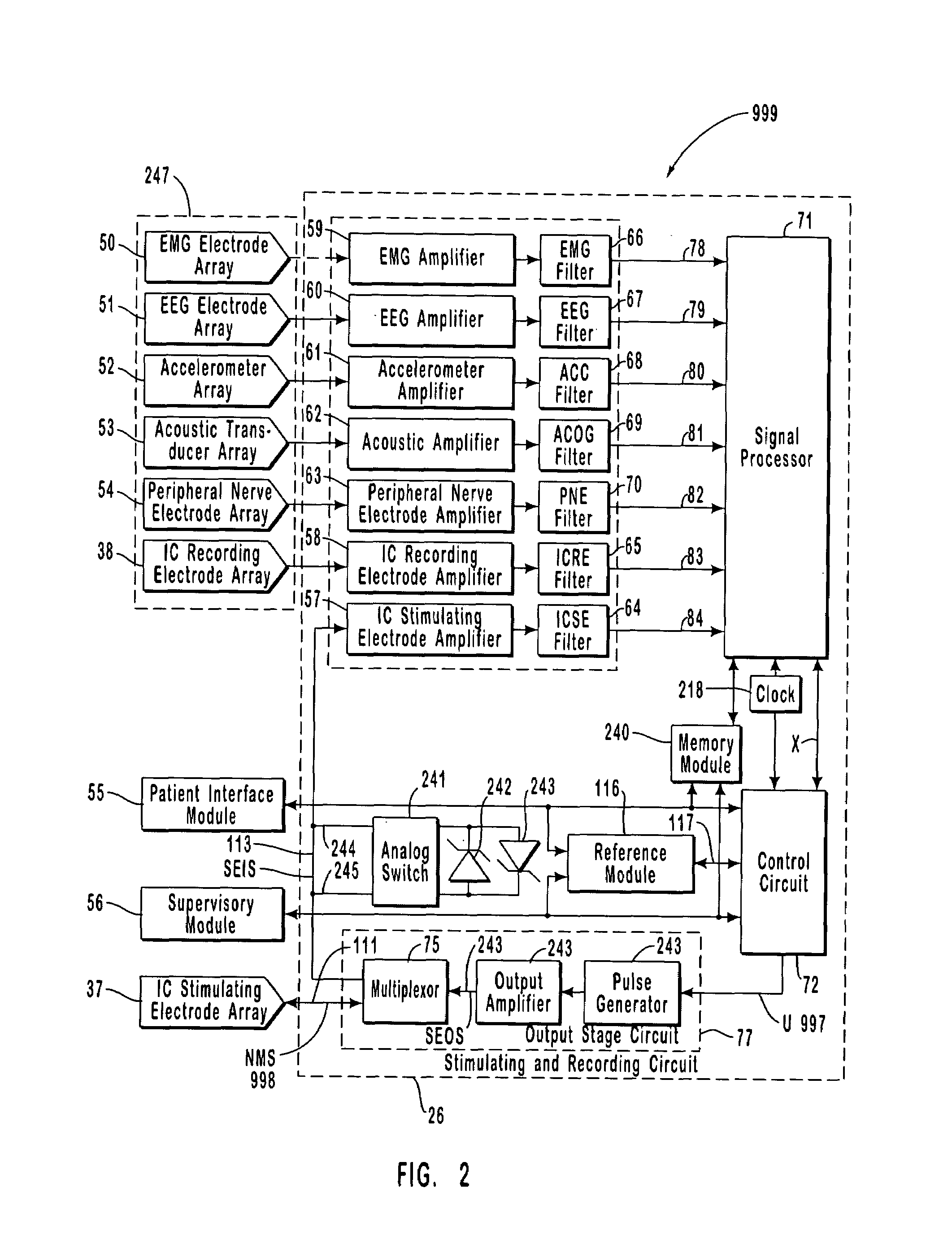
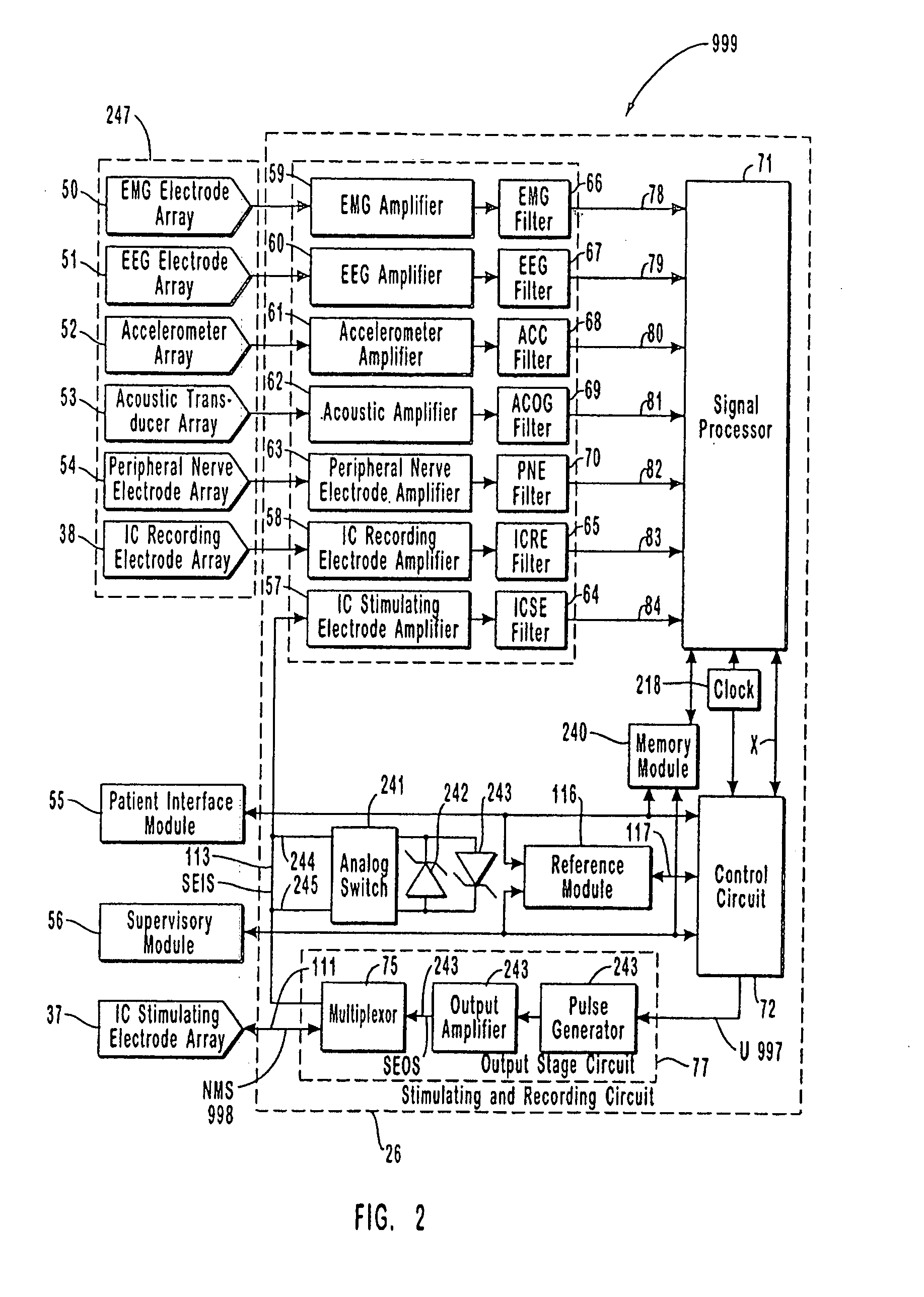
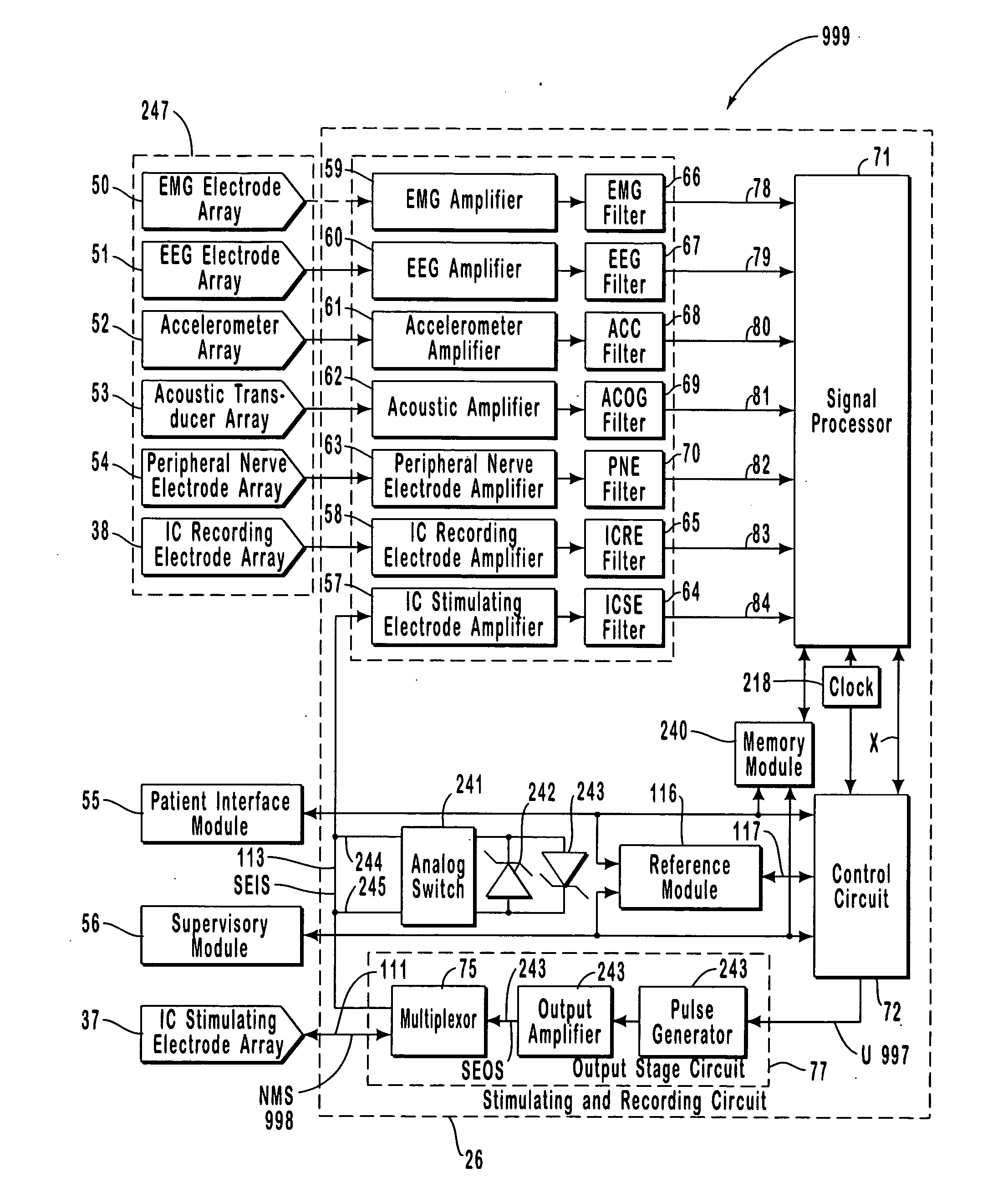
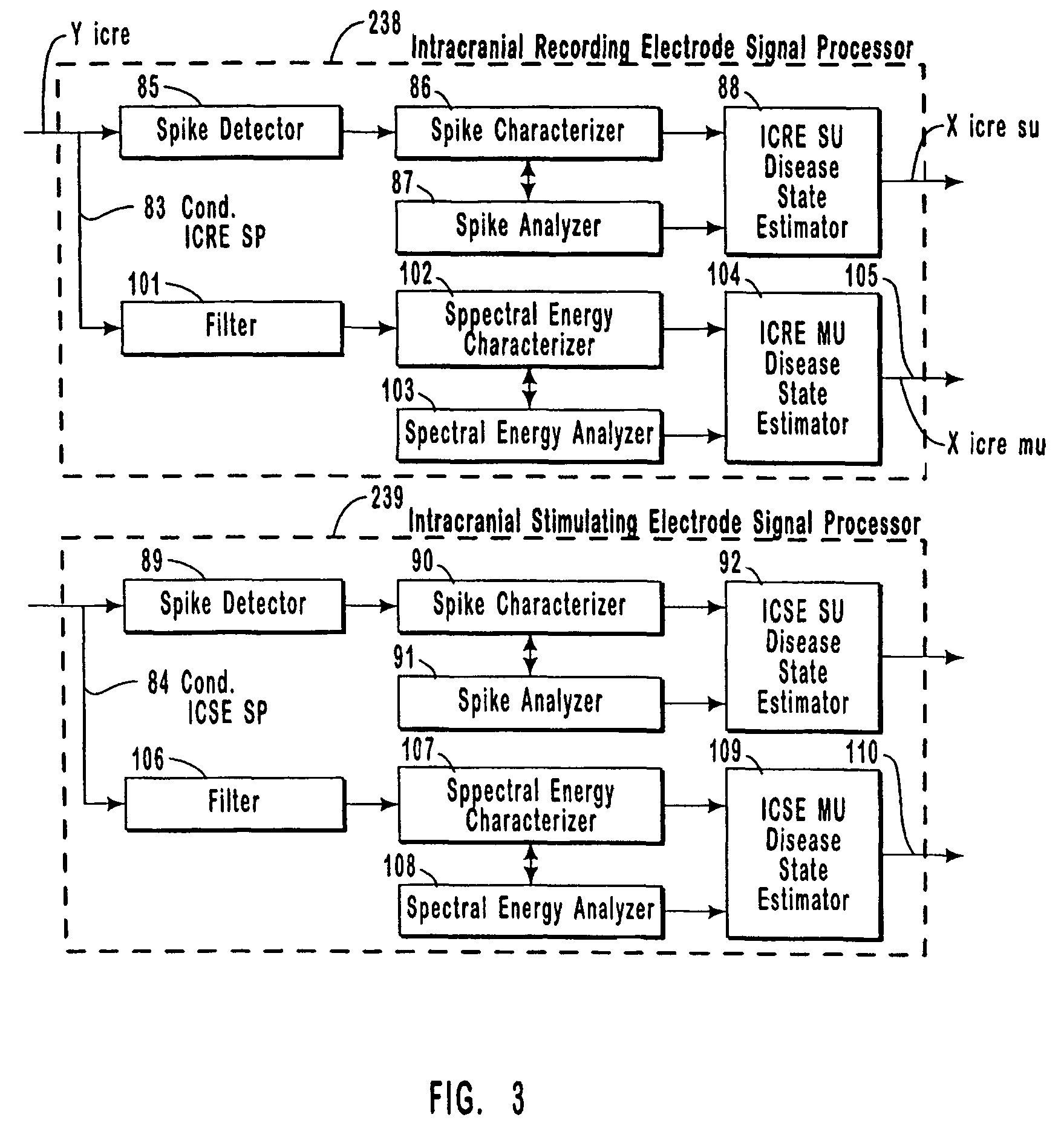
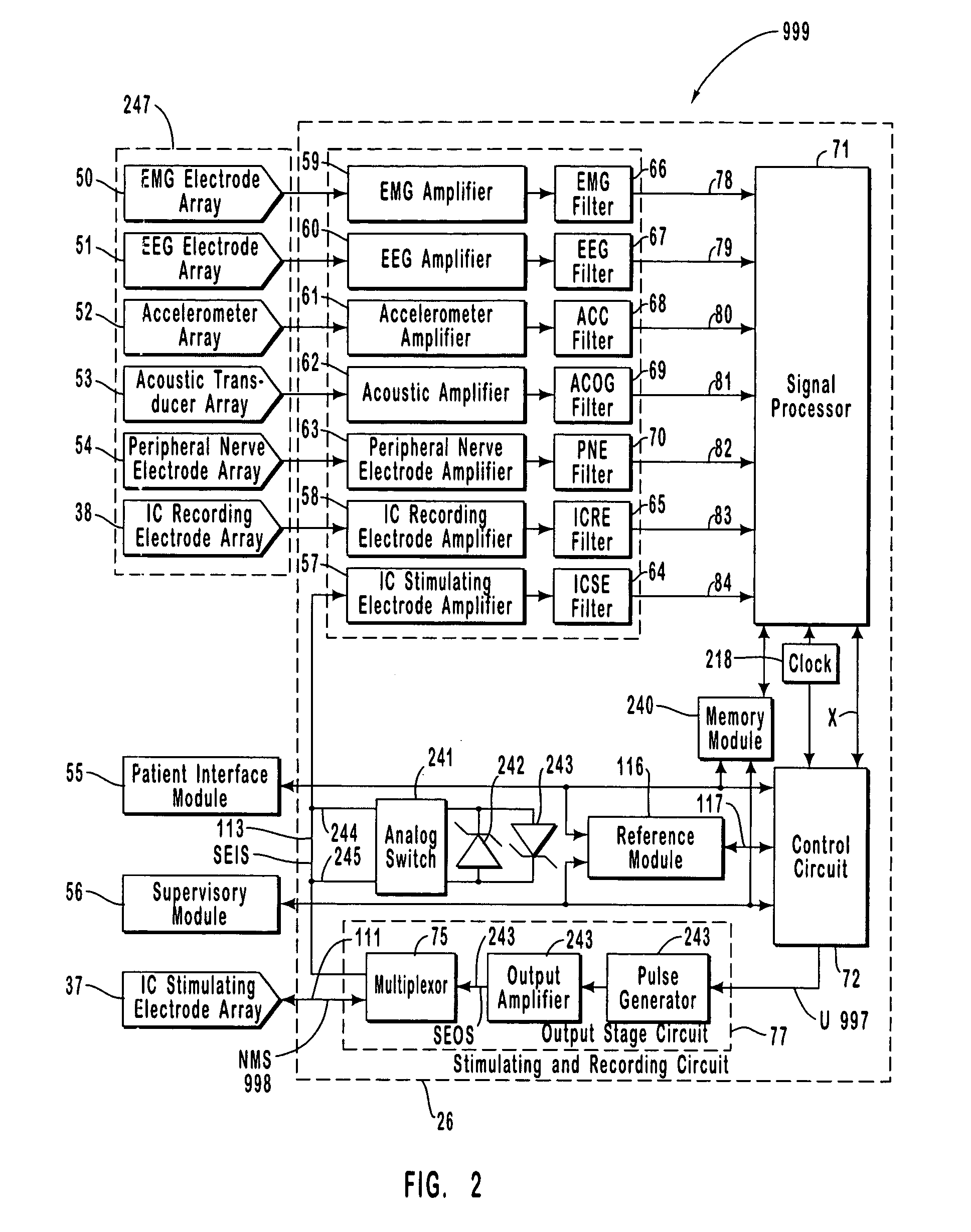
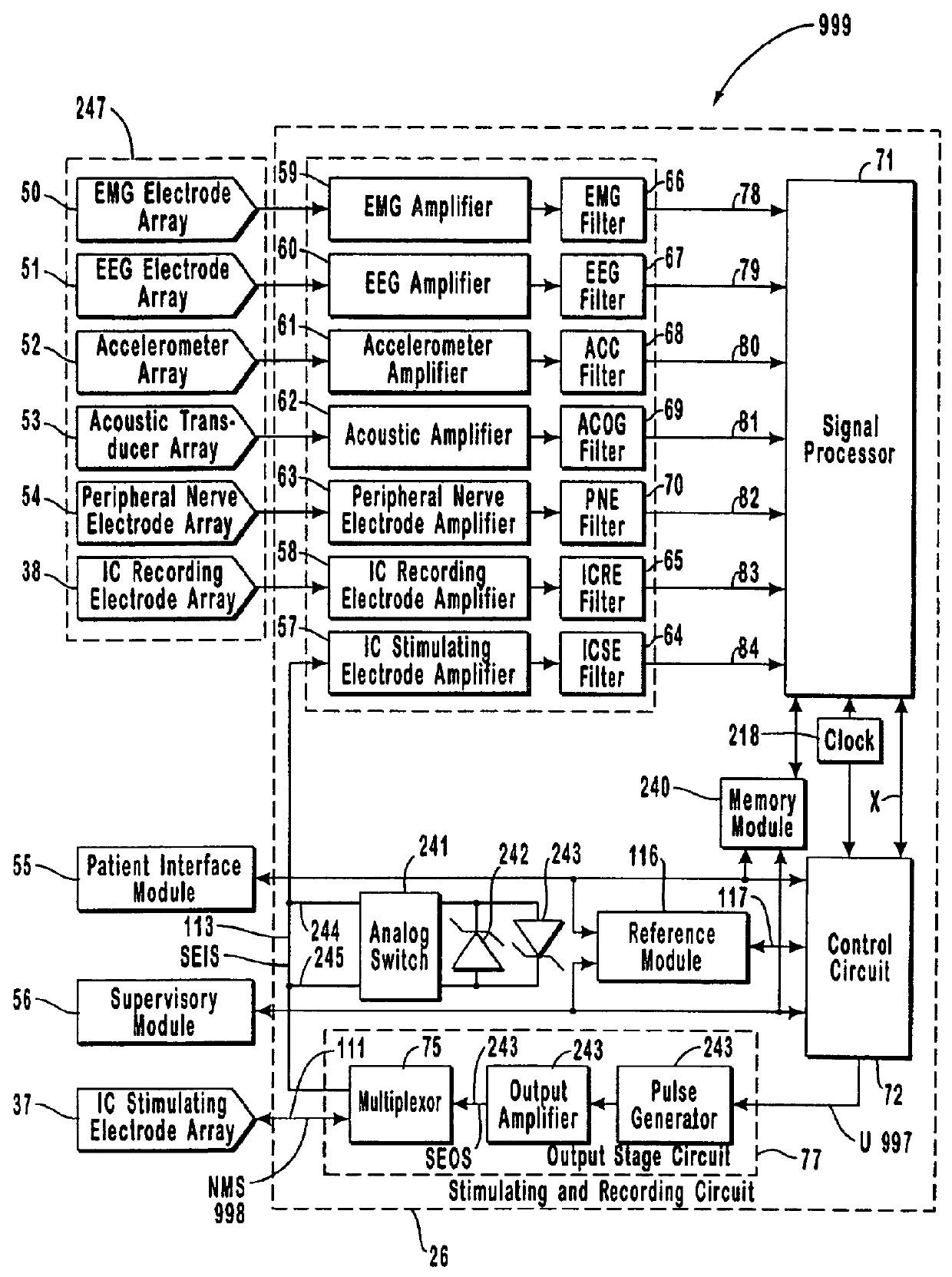
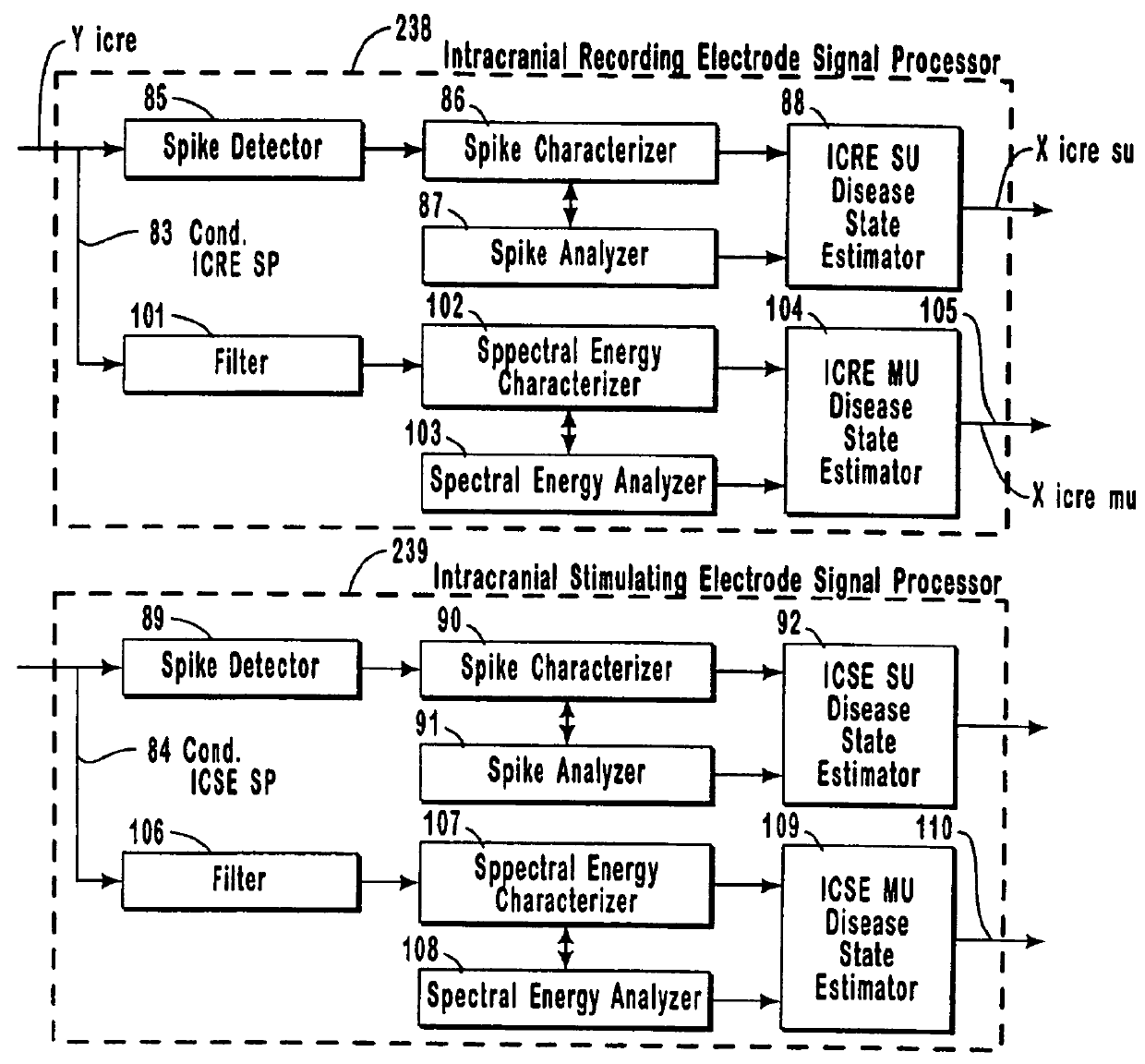
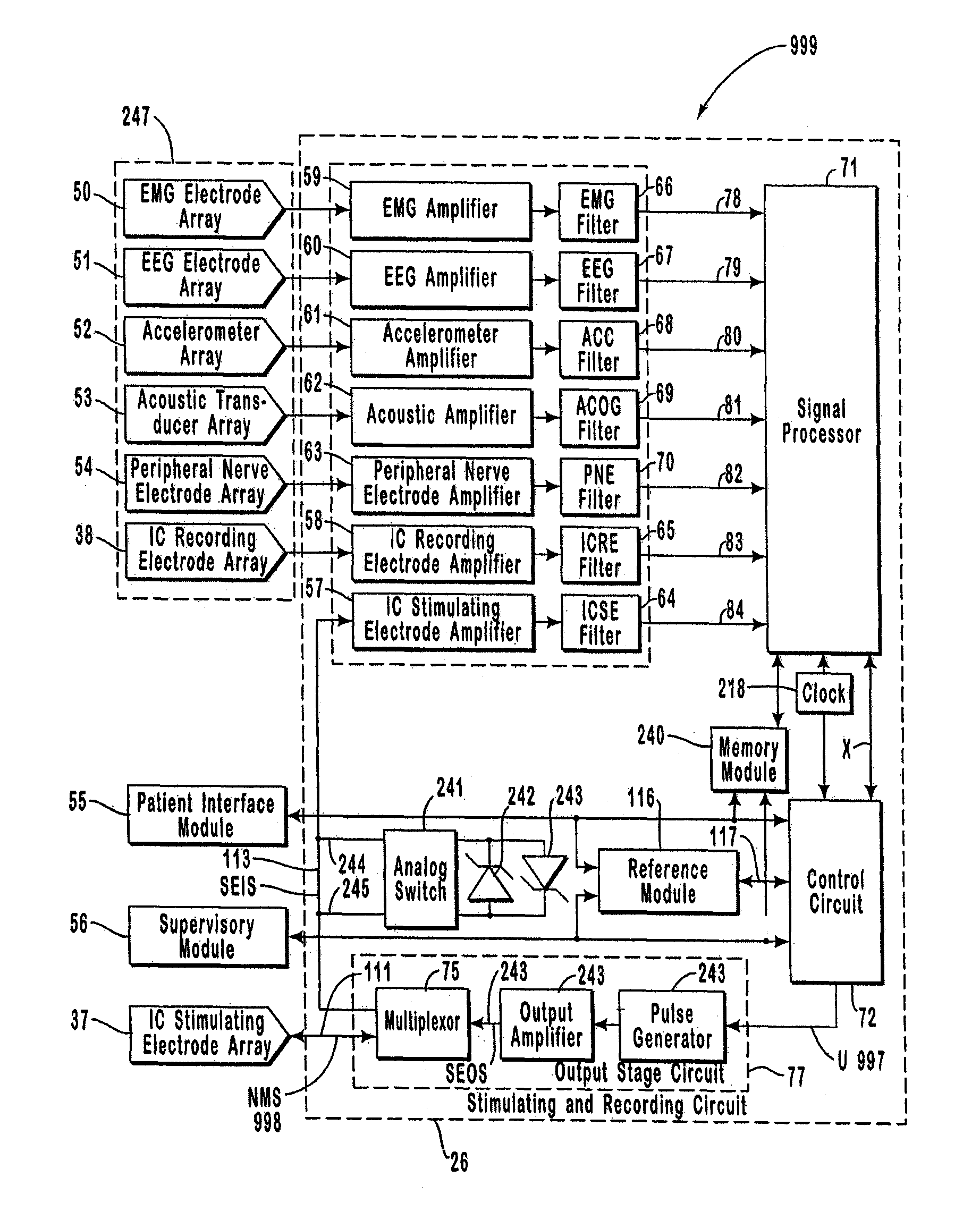
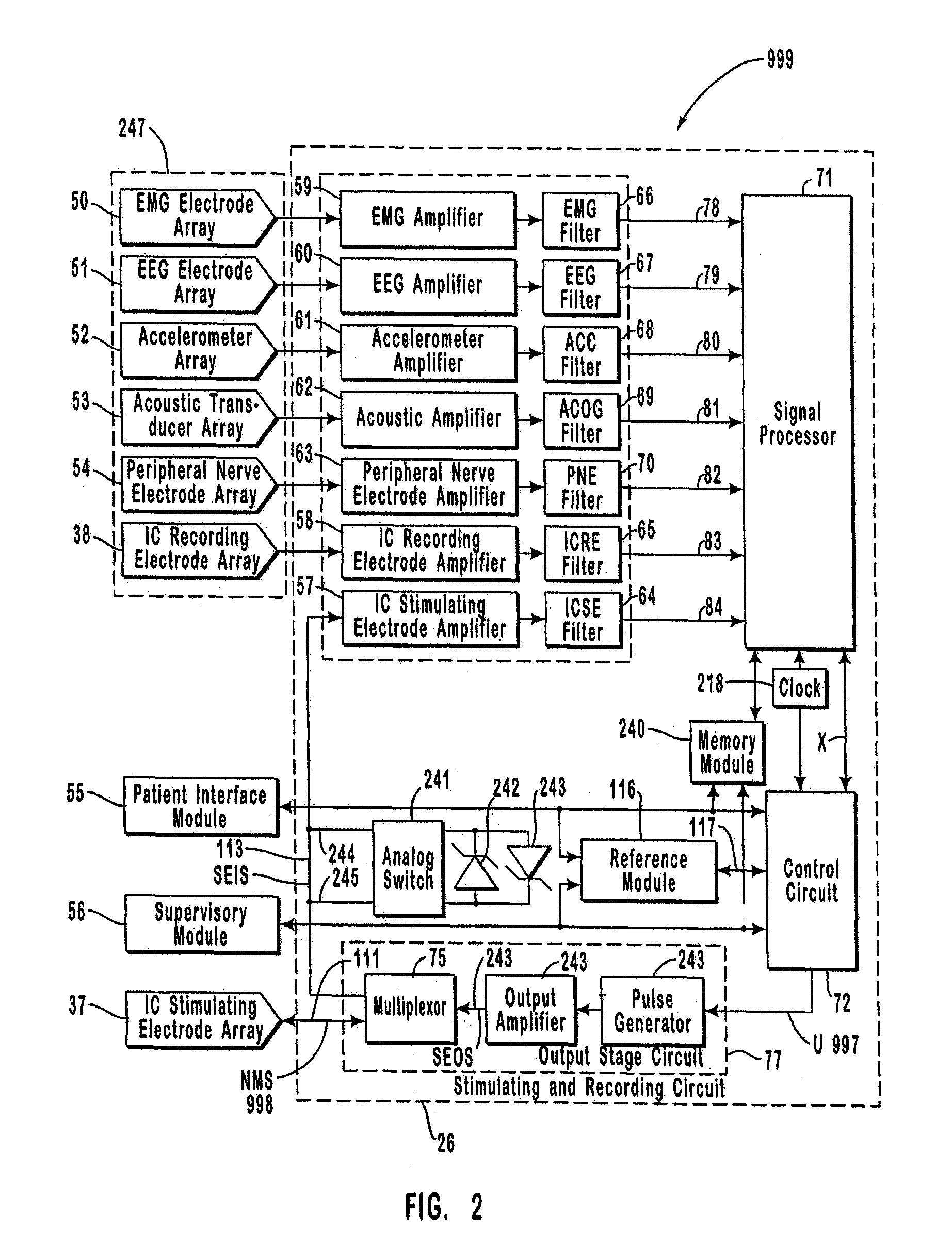
The present invention provides methods and systems for modulating a patient's neurological disease state. In one embodiment, the system comprises one or more sensors that sense at least one signal that comprise a characteristic that is indicative of a neurological disease state. A signal processing assembly is in communication with the one or more sensors and processes the at least one signal to estimate the neurological disease state and to generate a therapy to the patient that is based at least in part on the estimated neurological disease state. A treatment assembly is in communication with the signal processing assembly and delivers the therapy to a nervous system component of the patient.
Owner:CYBERONICS INC
Multi-functional portable electro-medical device
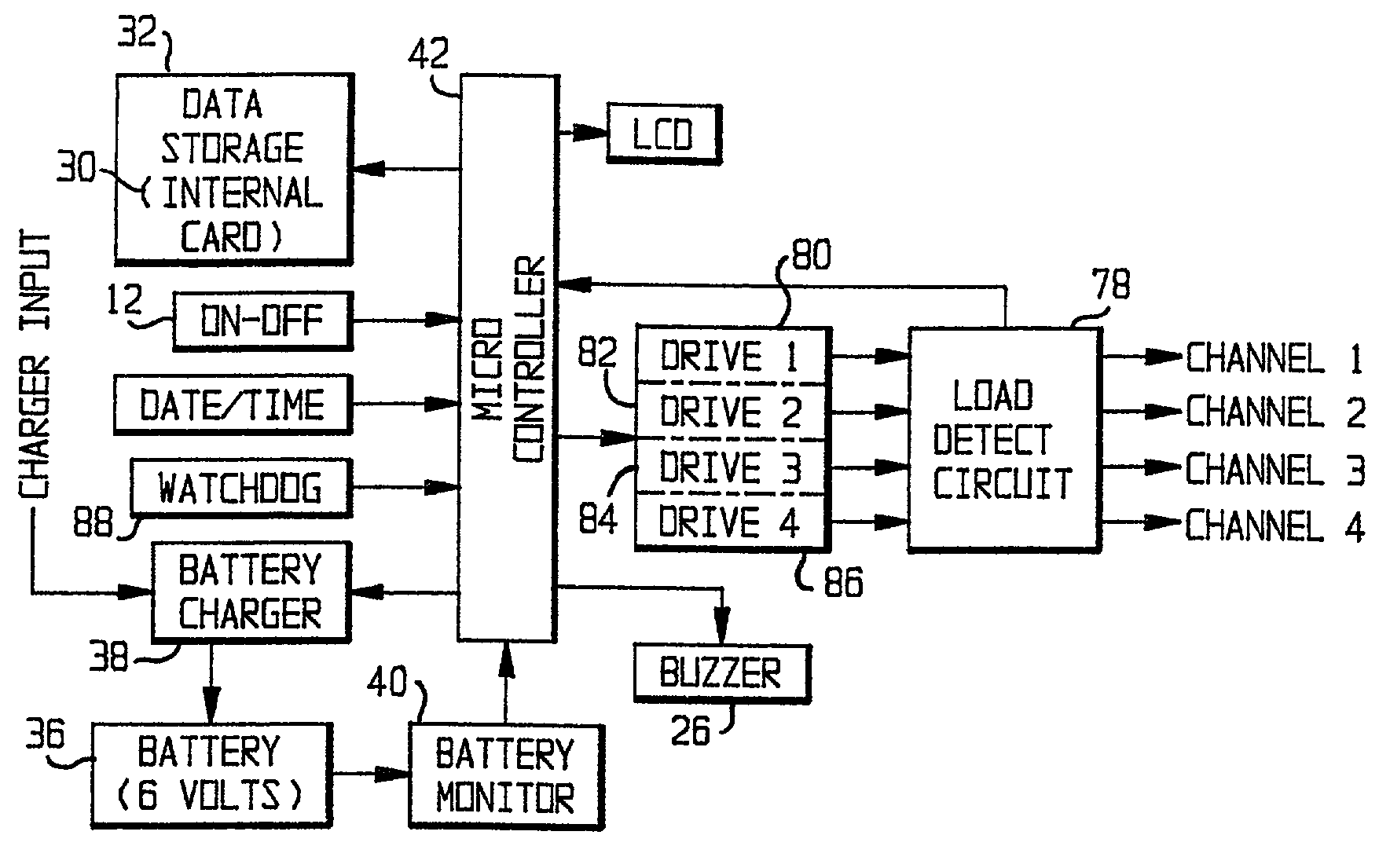
A multi-functional portable electro-medical device that is capable of providing muscle stimulation and interferential current stimulation. The multi-functional portable electro-medical device includes a multitude of safety features which are designed to prevent injury to the user while at the same time to ensure that the portable power electro-medical device is easy to use. The portable electro-medical device can be programmed to impart any combination of wave therapy to the patient.
Owner:INT REHABILITATIVE SCI
Closed-loop feedback-driven neuromodulation
InactiveUS8762065B2Reducing time and number of interactionSatisfactory treatmentHead electrodesAngle modulation detailsNervous systemClosed loop feedback
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators, comprising intracranial (IC) stimulating electrodes and other actuators, in accordance with treatment parameters. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:LIVANOVA USA INC
Closed-loop vagus nerve stimulation
InactiveUS9042988B2Avoid seizuresShorten the construction periodHead electrodesImplantable neurostimulatorsDiseaseMedicine
The present invention provides a closed-loop system for treating neurological disorders, such as epilepsy. In one embodiment the system comprises an input assembly that is adapted to receive one or more signals from a patient that are indicative of a patient's neurological state. The input assembly processes the one or more signals to generate one or more control input signals. An output assembly receives the one or more control input signals from the input assembly and generate a neuromodulation signal that is a function of the patient's neurological state. An electrode array is configured to deliver the neuromodulation signal to a patient's peripheral nerve, such as the vagus nerve.
Owner:LIVANOVA USA INC
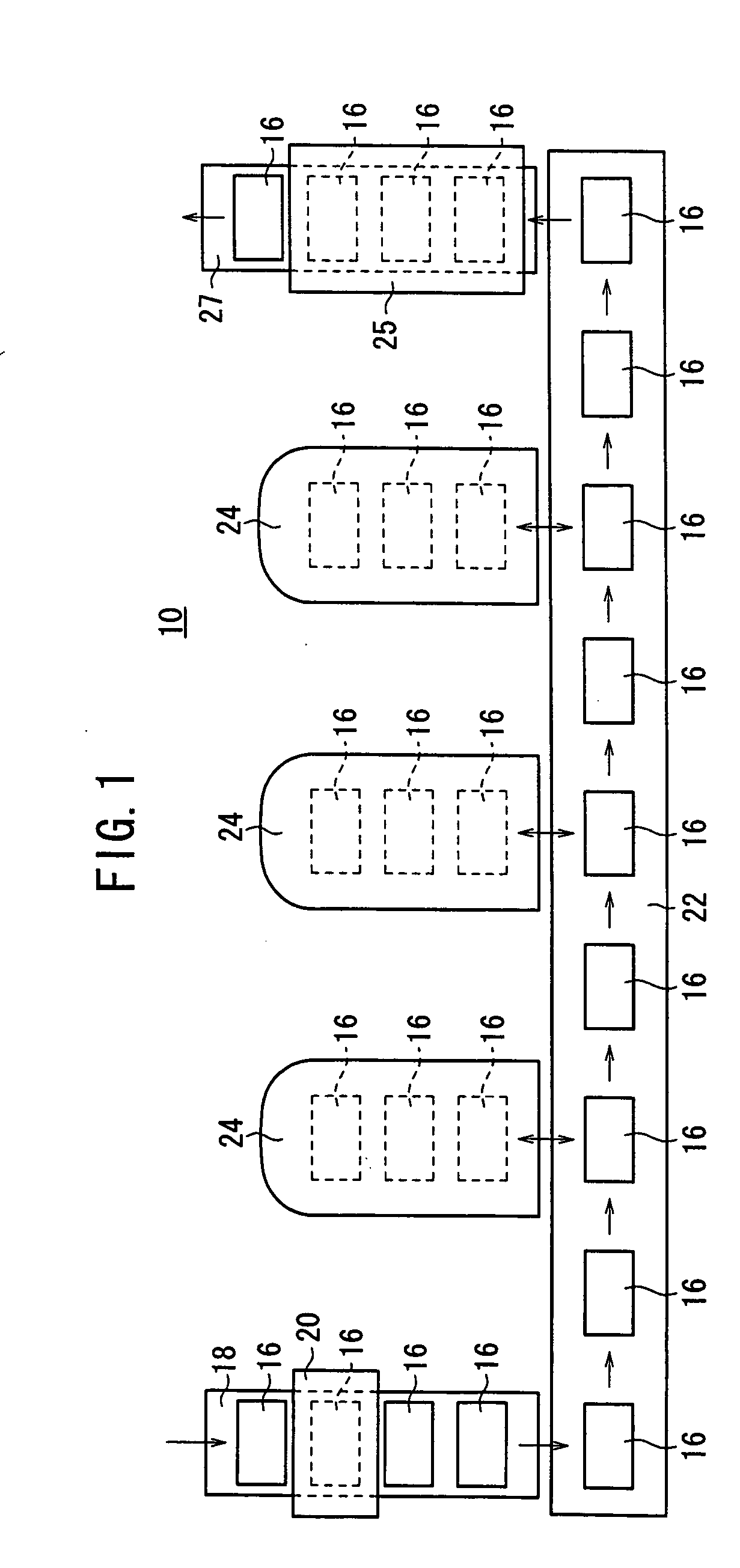
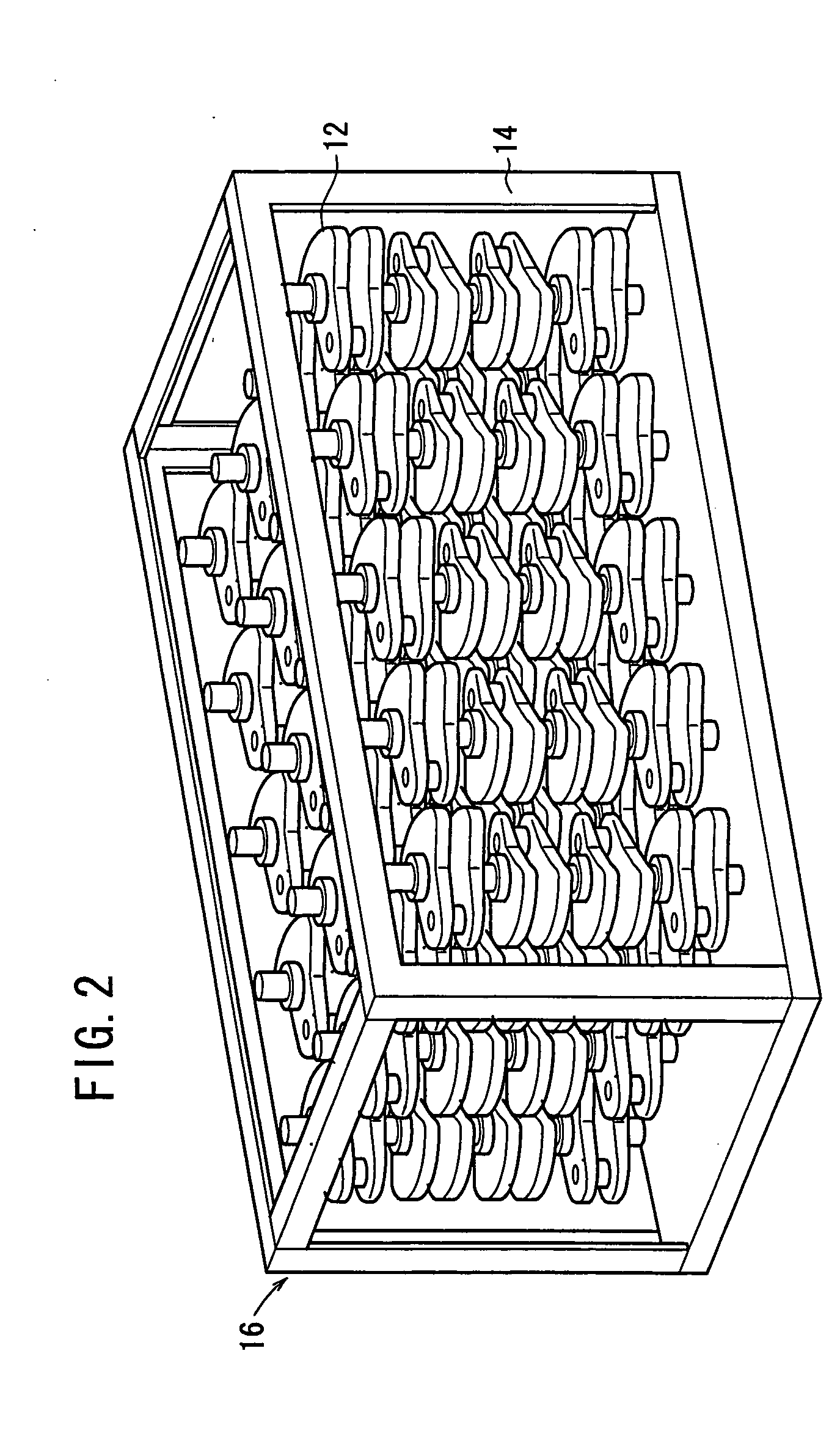
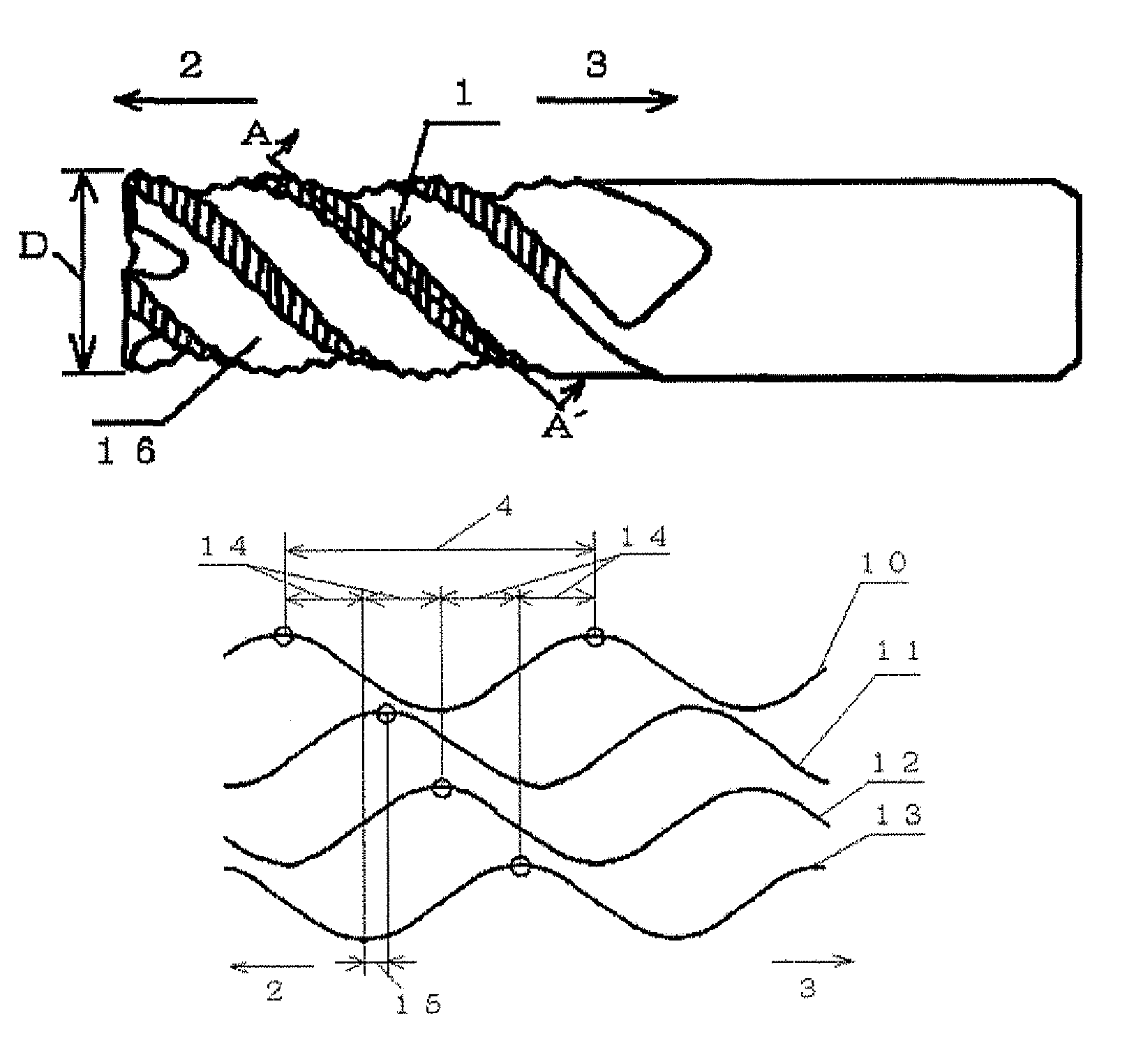
Carbide end mill and cutting method using the end mill
ActiveUS20120020749A1Improve featuresExtended service lifeMilling cuttersAdverse effect compensationCarbideEnd mill
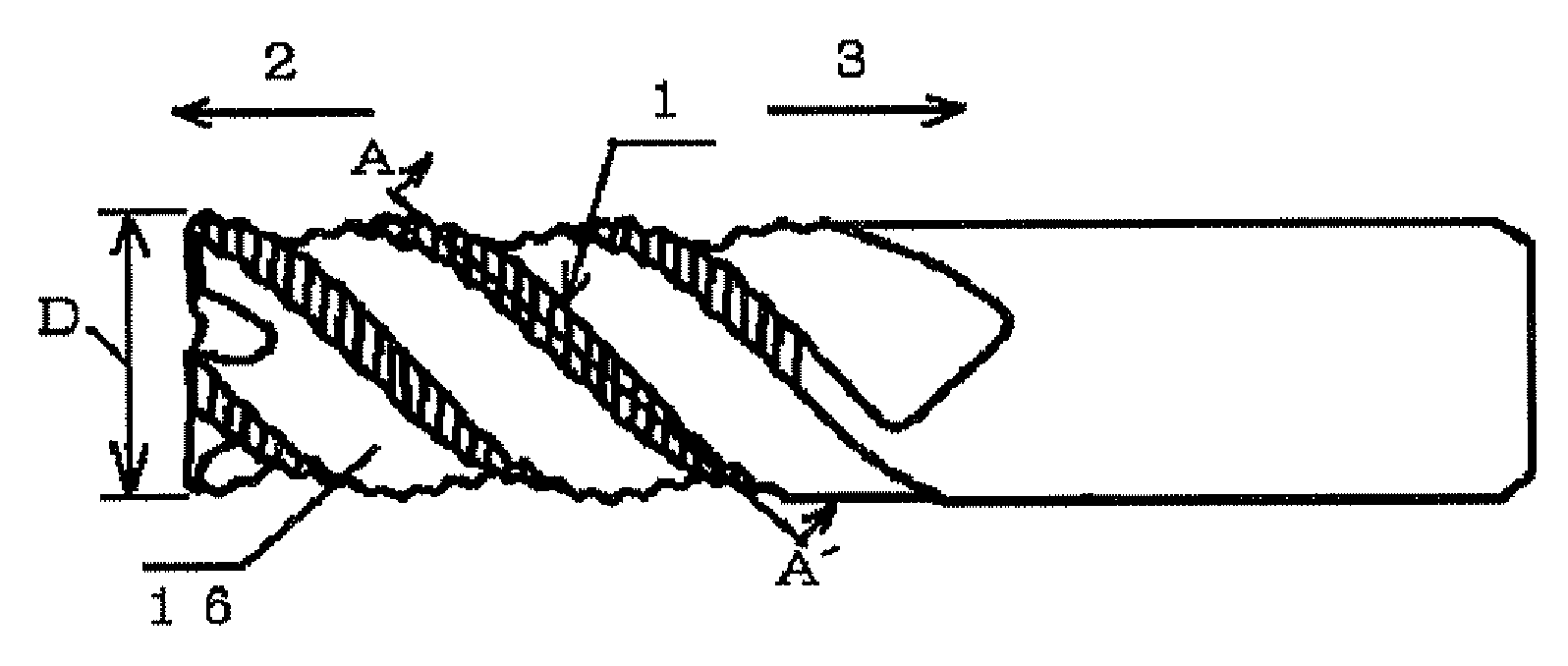
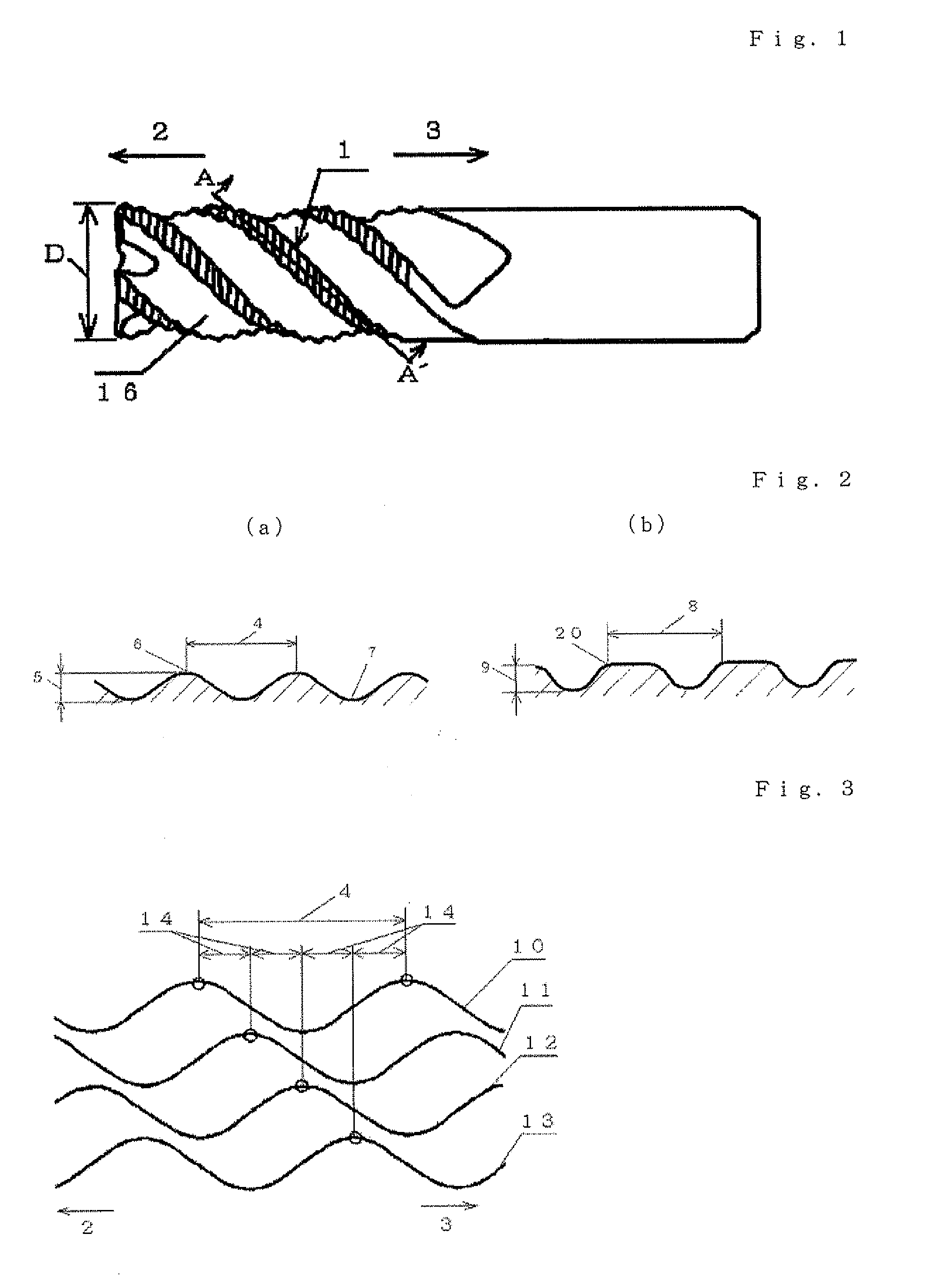
Provided is a long life carbide end mill which can perform stable cutting in high-efficiency machining such as die machining and parts machining. A cutting method using such an end mill is also provided. When a certain wavy or nicked peripheral cutting edge is considered a reference peripheral cutting edge with reference phases in a pitch of the reference peripheral cutting edge, wherein the distance of each reference phase is an amount corresponding to a value obtained by dividing the pitch of the nicks or waveform of each peripheral cutting edge by the number of the cutting edges; and the phase of at least one of the remaining peripheral cutting edges is deviated in the direction of the tool axis from the corresponding reference phase by an amount corresponding to 5% or less (excluding 0%) of the pitch.
Owner:HITACHI TOOL ENG LTD
Closed-loop vagus nerve stimulation
InactiveUS20140288620A1Avoid seizuresShorten the construction periodHead electrodesImplantable neurostimulatorsDiseaseMedicine
The present invention provides a closed-loop system for treating neurological disorders, such as epilepsy. In one embodiment the system comprises an input assembly that is adapted to receive one or more signals from a patient that are indicative of a patient's neurological state. The input assembly processes the one or more signals to generate one or more control input signals. An output assembly receives the one or more control input signals from the input assembly and generate a neuromodulation signal that is a function of the patient's neurological state. An electrode array is configured to deliver the neuromodulation signal to a patient's peripheral nerve, such as the vagus nerve.
Owner:LIVANOVA USA INC
Systems and methods for monitoring a patient's neurological disease state
InactiveUS9375573B2Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsMedicineDisease status
The present invention provides a system for providing neurological disease state information to a patient. The system comprises one or more sensors that sense at least one signal that comprise feature(s) that are indicative of a neurological disease state. A signal processing assembly is in communication with the one or more sensors and processes the at least one signal to estimate the neurological disease state. A patient interface module is in communication with the signal processing assembly and communicates with a patient an output that is indicative of the patient's estimated neurological disease state.
Owner:CYBERONICS INC
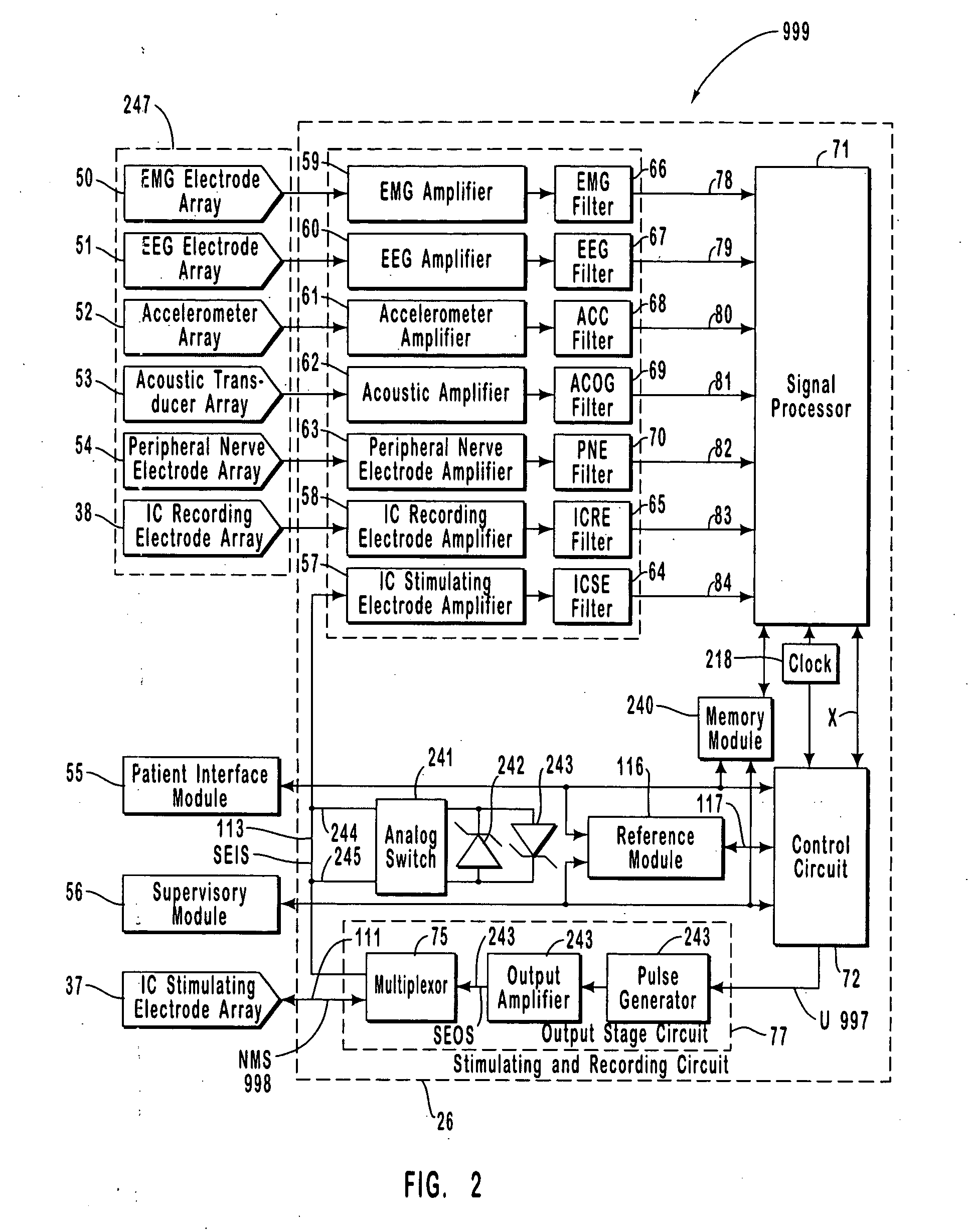
Monitoring an epilepsy disease state with a supervisory module
InactiveUS9415222B2Reducing time and number of interactionSatisfactory treatmentHead electrodesAngle modulation detailsDiseaseImplantable Electrodes
Systems and methods for monitoring an epilepsy disease state in a human patient are described. The system includes patient components and a supervisory module. The patient components include: an implantable electrode array for collecting neurological signals, an implantable recording electrode signal processor for processing the neurological signals collected by the electrode array, and a disease state estimator for estimating the patient's disease state. The supervisory module receives the patient's disease state from the patient components and displays a time history of the patient's disease state.
Owner:CYBERONICS INC
Automated vehicle wash system
InactiveUS20100206341A1Simple and efficientEasily adjustCleaning apparatus for vehicle exteriorsCleaning using liquidsAutomotive engineeringCompound (substance)
An automated vehicle wash system including vehicle wash equipment, a mechanical room and a control module, such as a control pod. The control pod is positioned outside the mechanical room and adjacent the vehicle was equipment so that an operator of the control pod can directly view the wash equipment as the operator adjusts controls of the control pod to precisely regulate materials, such as chemicals and other treatment liquids, delivered by the wash equipment to a vehicle as the vehicle passes along a path.
Owner:V Q
Closed-loop feedback-driven sympathetic neuromodulation for affect control
ActiveUS9345880B1Reducing time and number of interactionSatisfactory treatmentHead electrodesCatheterNervous systemClosed loop feedback
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators, comprising intracranial (IC) and peripheral stimulating electrodes and other actuators, in accordance with treatment parameters. The neurological control system may directly or indirectly control a portion of the autonomic nervous system, including the sympathetic and parasympathetic nervous system. Such treatment parameters may be derived from a neural or physiological response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:DILORENZO DANIEL J
Ion implantation apparatus and ion implantation method
InactiveUS20100006779A1Satisfactory treatmentPrevention of wafer droppageElectric discharge tubesRadiation therapyAngle of incidenceBiomedical engineering
This ion implantation apparatus is provided with a holding devise which holds the wafer, and which turns it along its circumference. In addition to holding the wafer at a prescribed position, the ion implantation apparatus subjects the wafer to ion implantation in regions where there is partial overlap of its circumference. The holding devise turns and inclines the wafer, and also holds the wafer by three or more holding pins. The side face of the holding pin has an inversely tapered shape, and the multiple holding pins include a first holding pin whose protrusion amount is relatively small, and a second holding pin whose protrusion amount is relatively large. The holding pin which is on the upper side from the center of the wafer in the planar direction of the inclined wafer is the second holding pin, and the angle of inclination of the side face of the second holding pin at a position where ions are implanted into the wafer has an angular degree which is equal to or less than an angle of incidence of the ion beam relative to the wafer.
Owner:SUMCO CORP
Medicine for treating psoriasis
ActiveCN1954847ANo side effectsGood effectGranular deliveryDermatological disorderPsoriasis patientTraditional medicine
A Chinese medicine in the form of particles for treating psoriasis is prepared from 13 Chinese-medicinal materials including gypsum, rehmannia root, red peony root, scutellaria root, etc. Its preparing process is also disclosed.
Owner:TIANJIN TASLY PHARMA CO LTD
Nitriding method and device
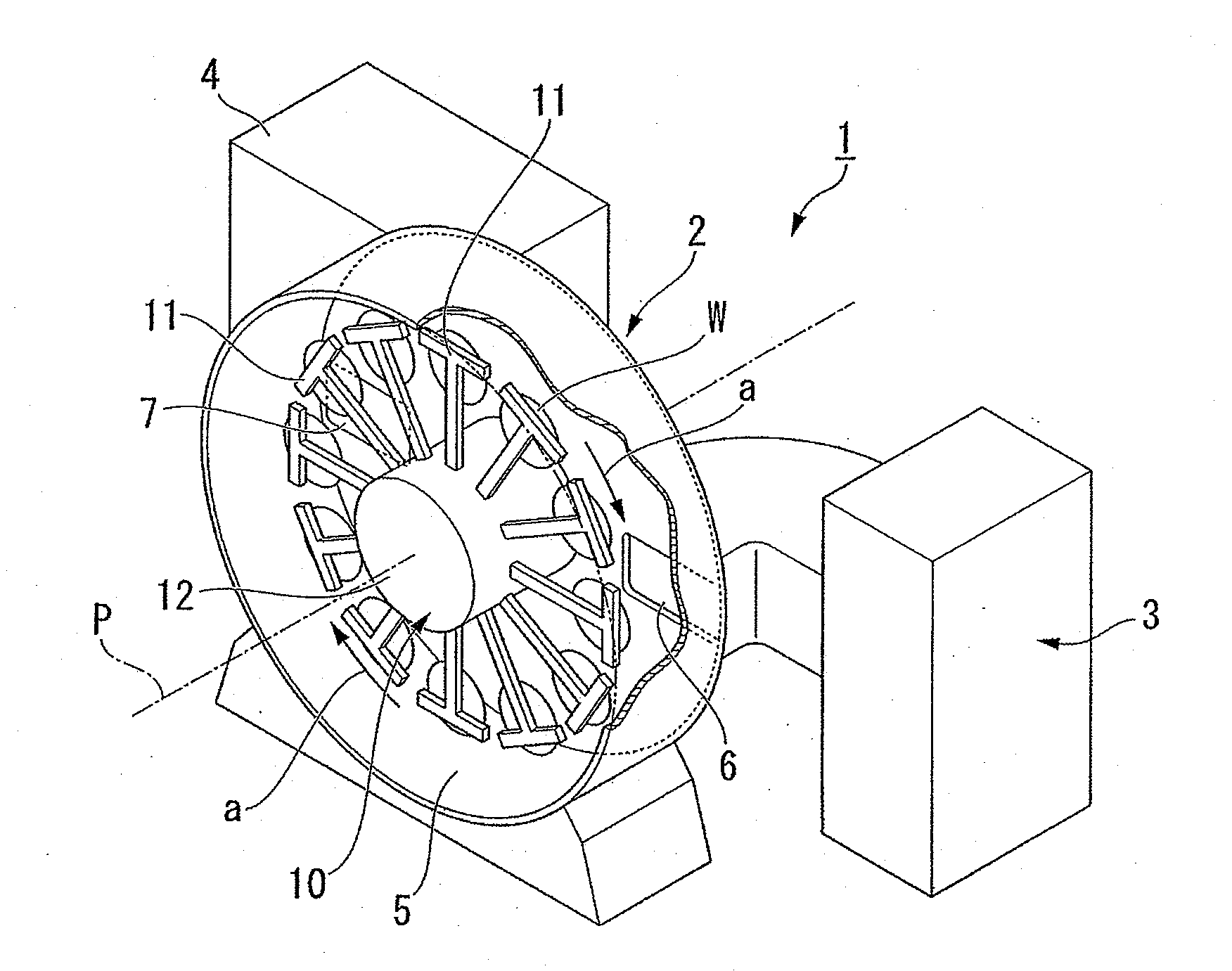
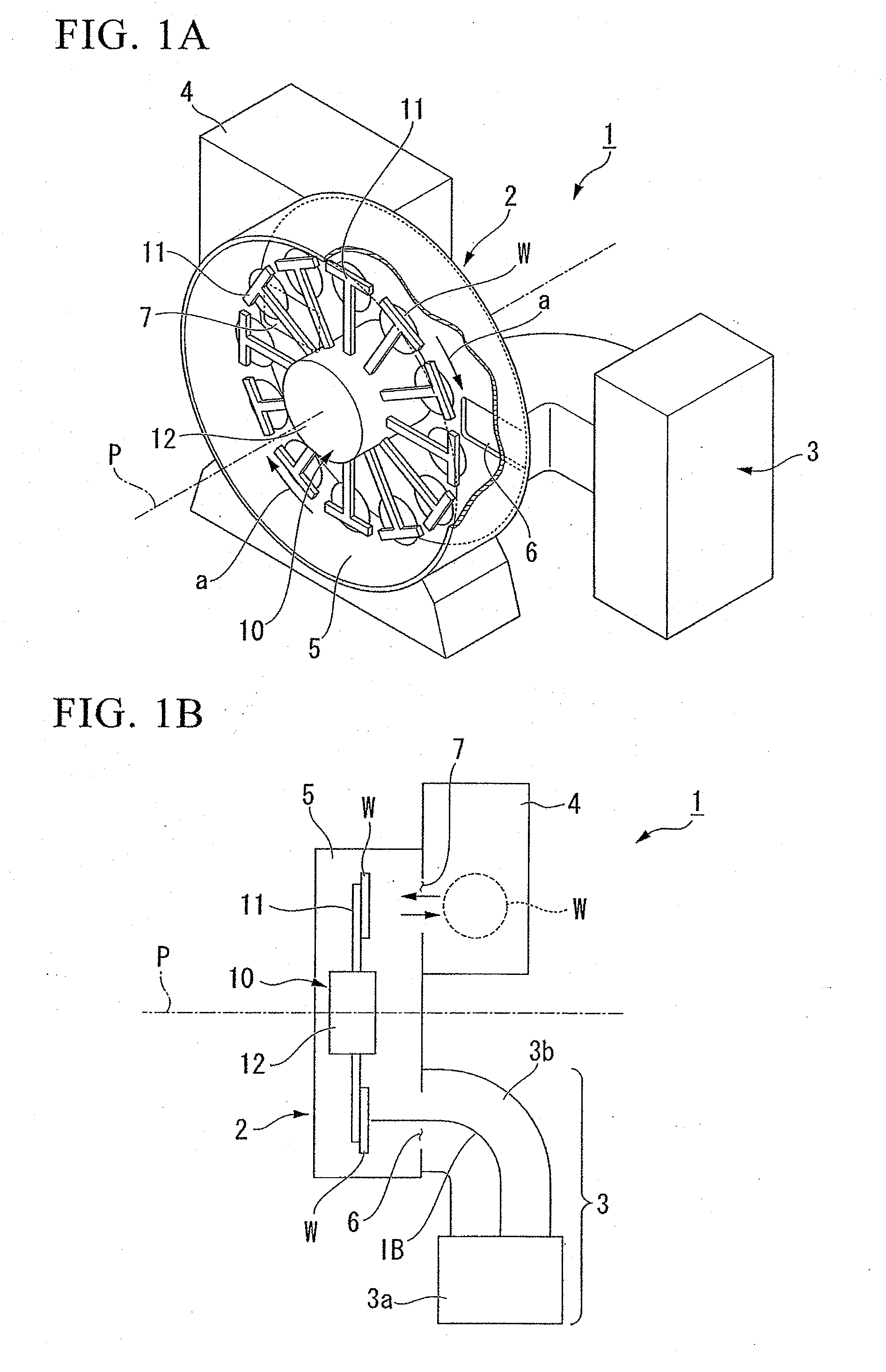
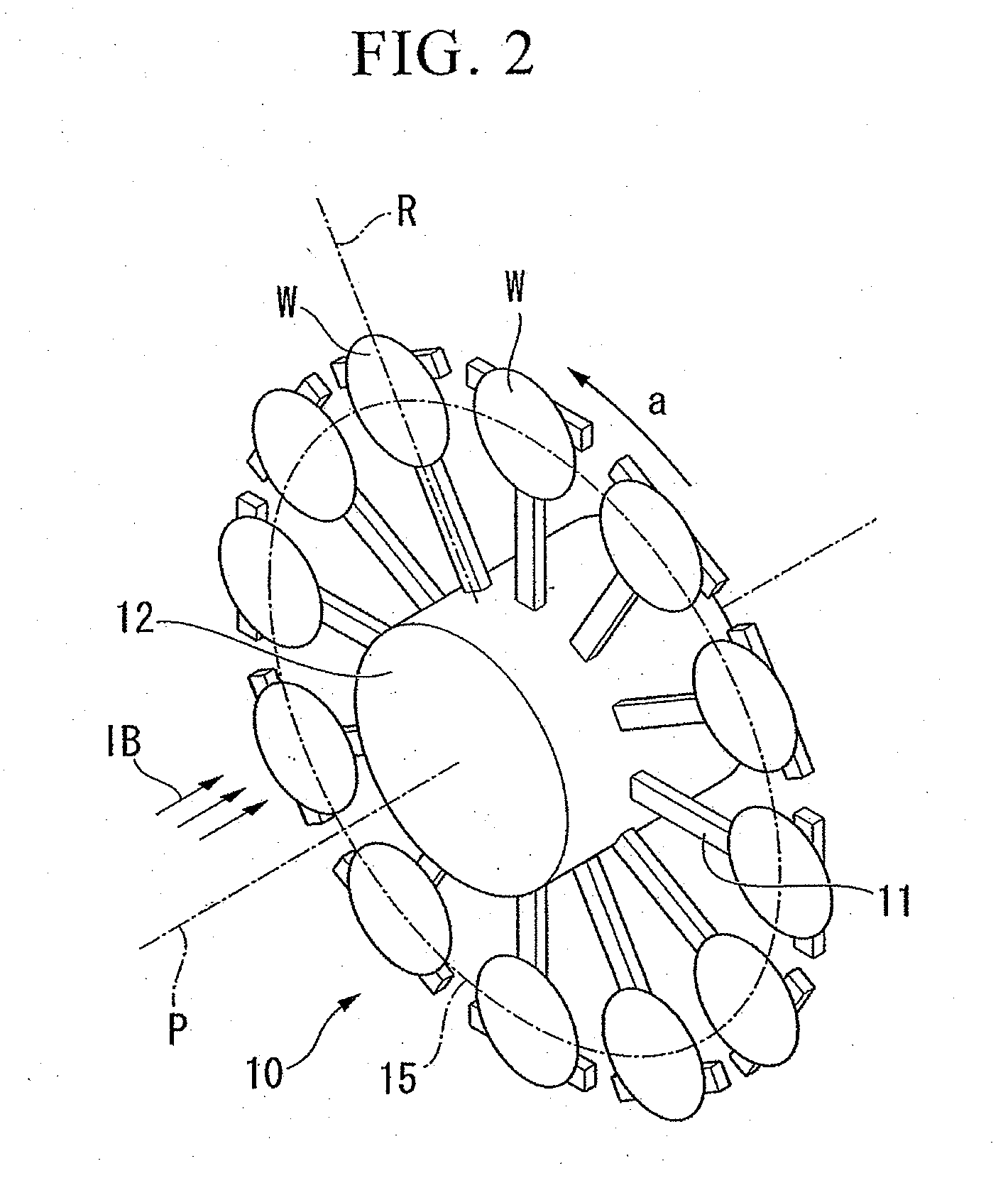
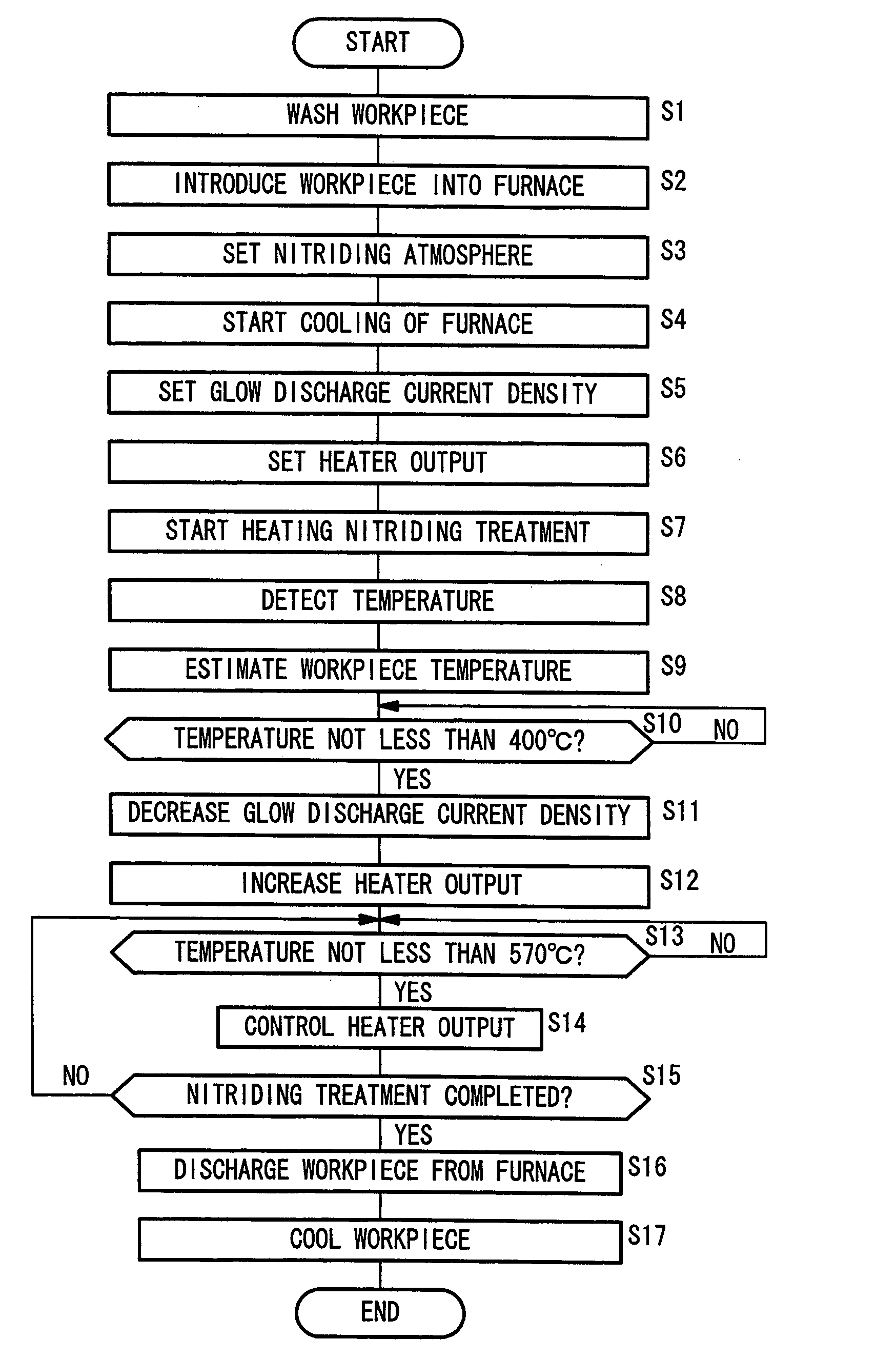
InactiveUS20060124202A1Heated quickly and uniformlyTemperature detectionVacuum evaporation coatingSputtering coatingVolumetric Mass DensityPulse voltage
A pulse voltage having a frequency of 15 kHz is applied from a discharging power supply unit (48) to between a crankshaft (12) and an electrode plate (45) at a current density of 2.5 mA / cm2 to generate a glow discharge and an electric heater (34) is driven at a 40% output (64 kW / kg) to heat the crankshaft (12) to up to 400° C., and then heating is continued with the current density of a glow discharge set at 0.5 mA / cm2 and the output of the electric heater (34) set at 90% (144 kW / kg), thereby effecting nitriding at a desired nitriding temperature.
Owner:HONDA MOTOR CO LTD
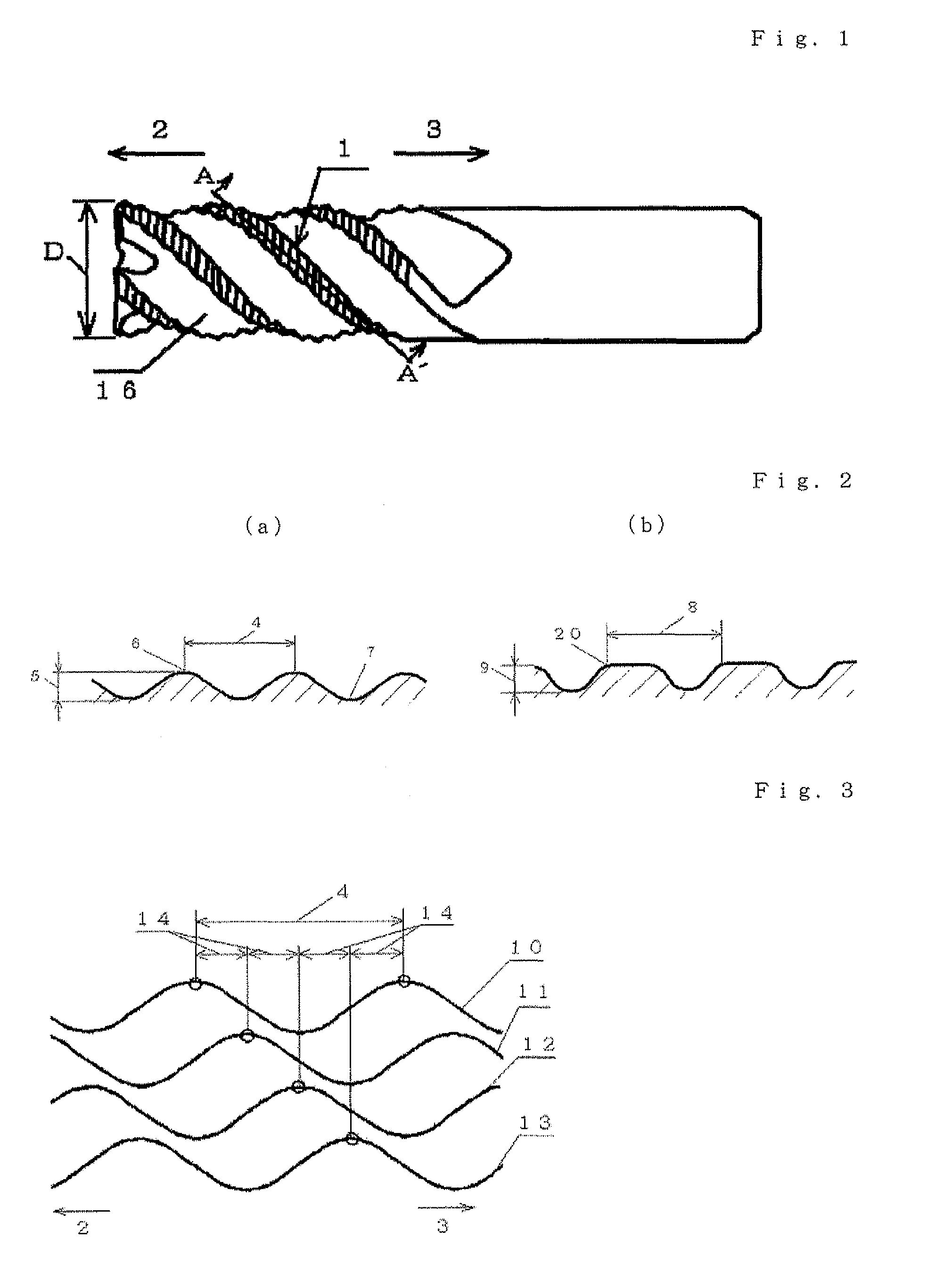
Carbide end mill and cutting method using the end mill
ActiveUS8827600B2Vibration minimizationSatisfactory treatmentMilling cuttersAdverse effect compensationCarbideEngineering
Provided is a long life carbide end mill which can perform stable cutting in high-efficiency machining such as die machining and parts machining. A cutting method using such an end mill is also provided. When a certain wavy or nicked peripheral cutting edge is considered a reference peripheral cutting edge with reference phases in a pitch of the reference peripheral cutting edge, wherein the distance of each reference phase is an amount corresponding to a value obtained by dividing the pitch of the nicks or waveform of each peripheral cutting edge by the number of the cutting edges; and the phase of at least one of the remaining peripheral cutting edges is deviated in the direction of the tool axis from the corresponding reference phase by an amount corresponding to 5% or less (excluding 0%) of the pitch.
Owner:HITACHI TOOL ENG LTD
Cyclic GMP dependent protein kinase as a chemotherapeutic target for antiprotozoal agents
InactiveUS7125700B2Facilitate processingSatisfactory treatmentSugar derivativesProtozoaEnzymeMolecular biology
Protozoal cyclic GMP dependent protein kinases have been isolated and cloned. These enzymes may be used in screening assays to identify potential antiprotozoal agents.
Owner:MERIAL LTD
Medicine for treating psoriasis
ActiveCN1954847BThe solution is not significantSatisfied with the curative effectGranular deliveryDermatological disorderPsoriasis patientTraditional medicine
Owner:TIANJIN TASLY PHARMA CO LTD
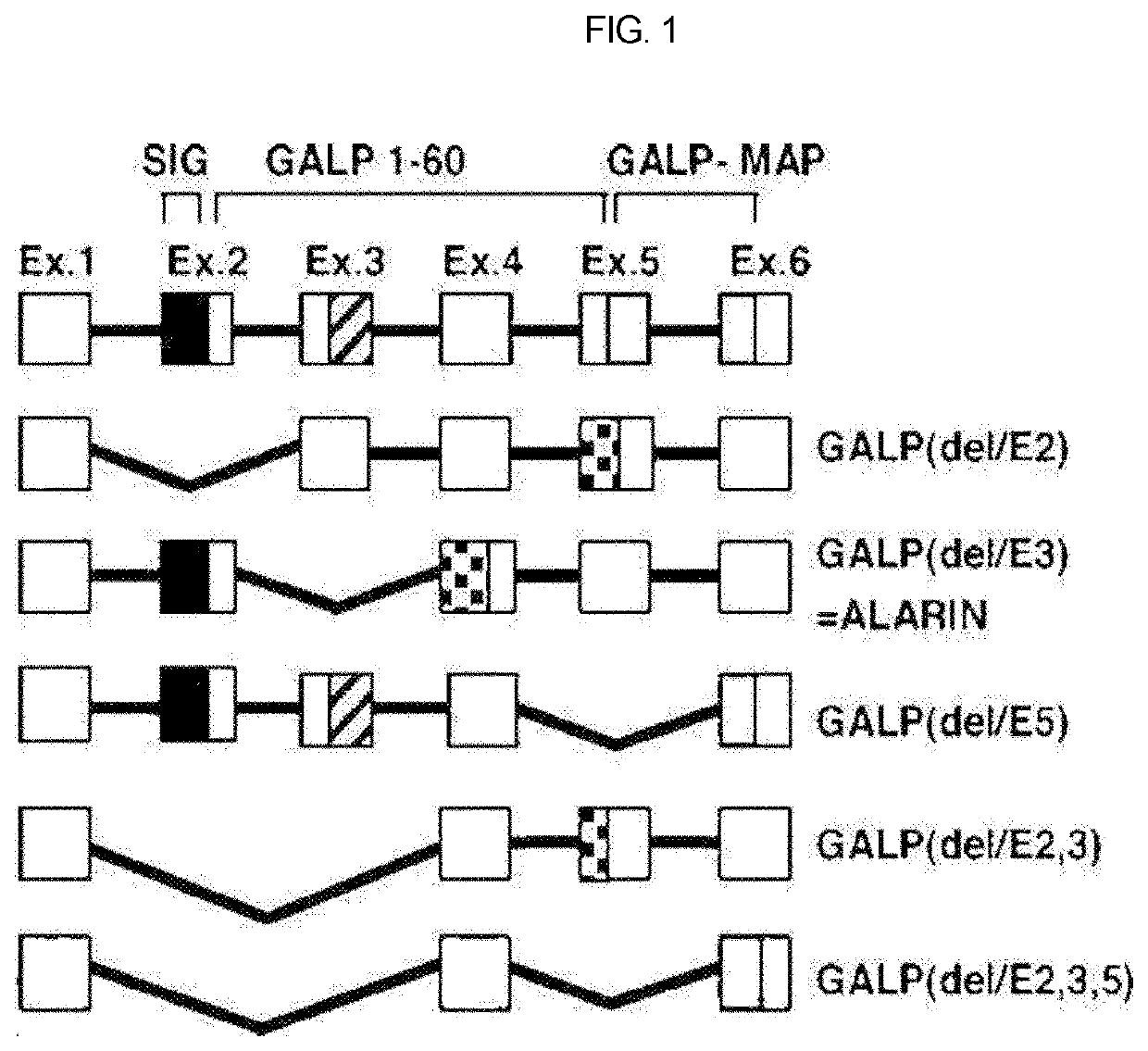
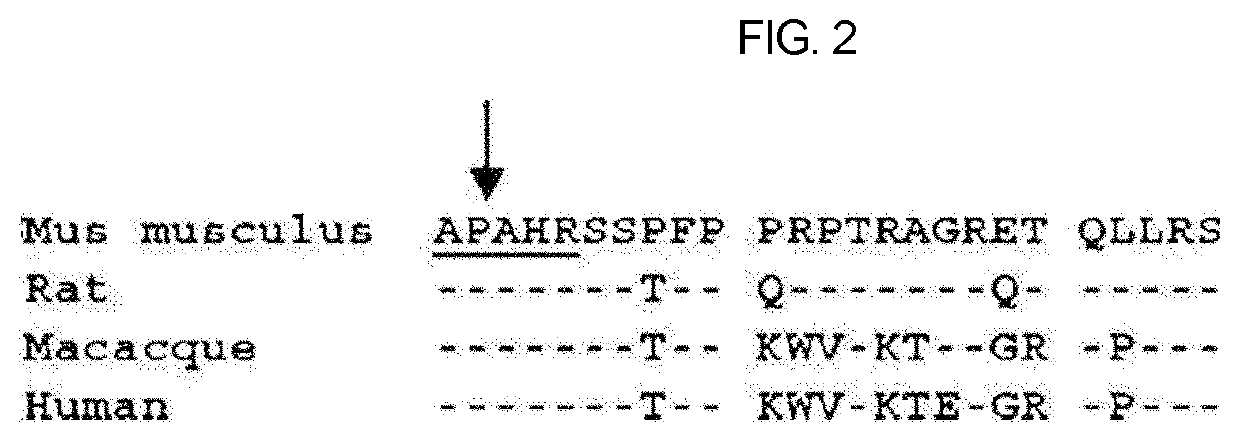
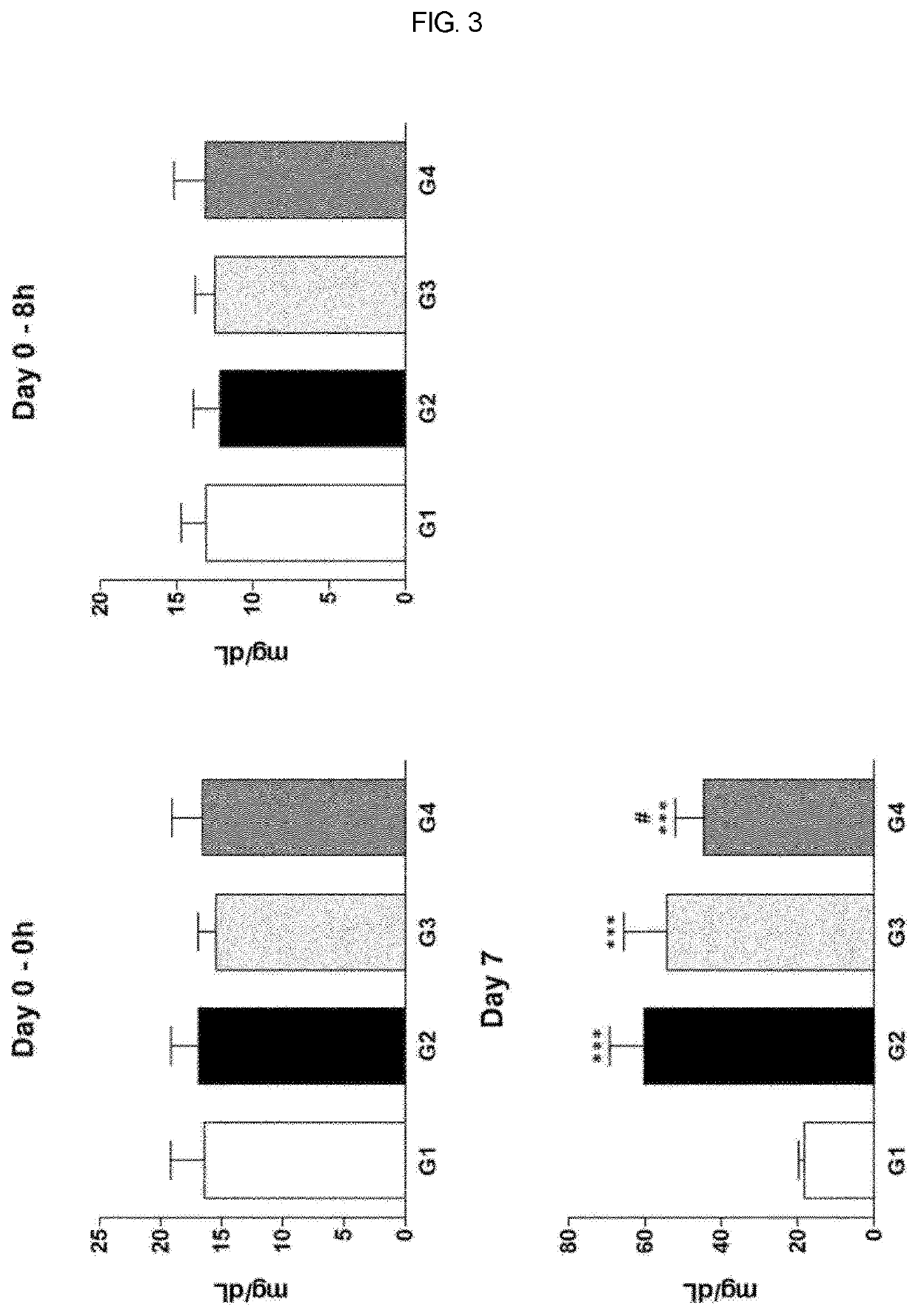
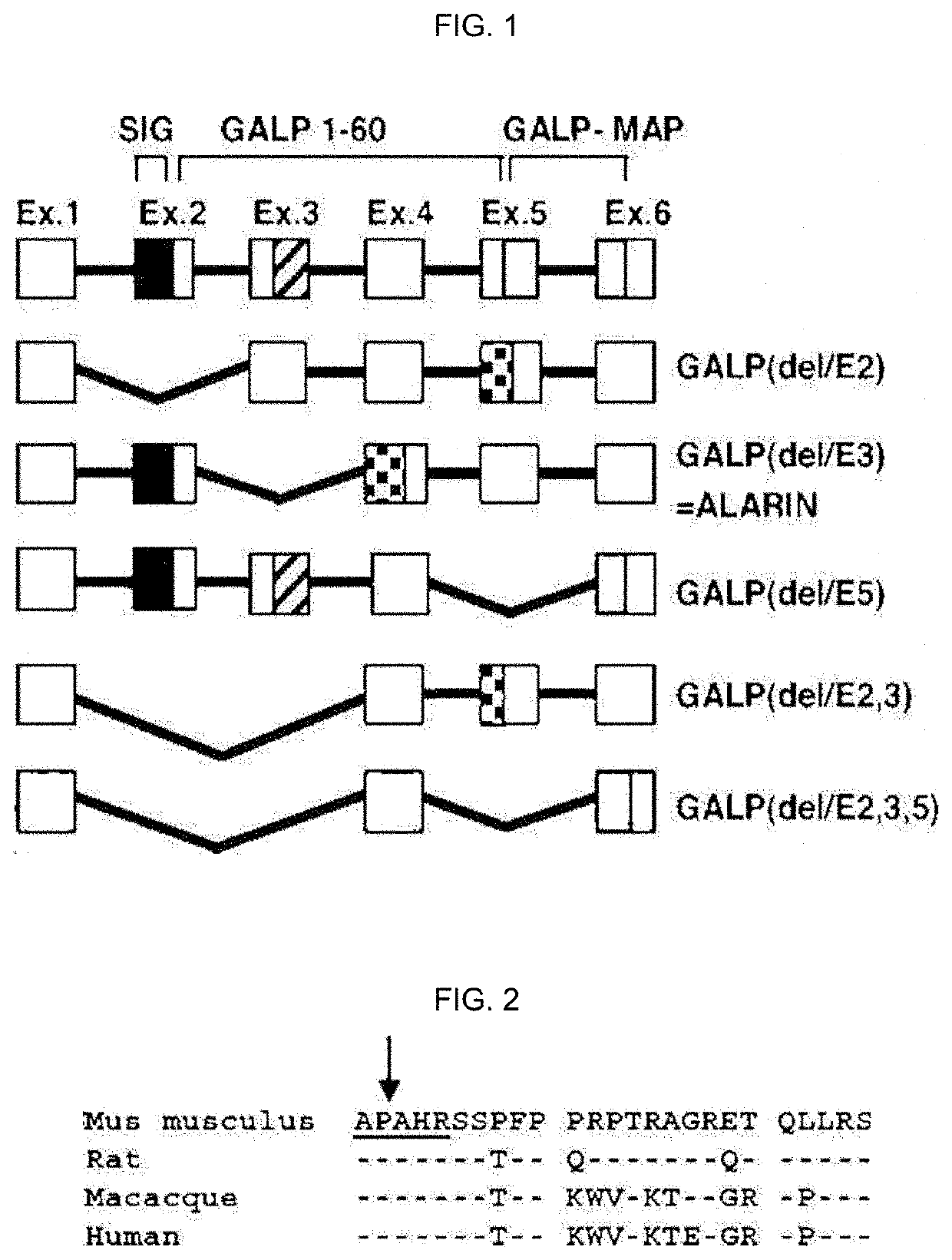
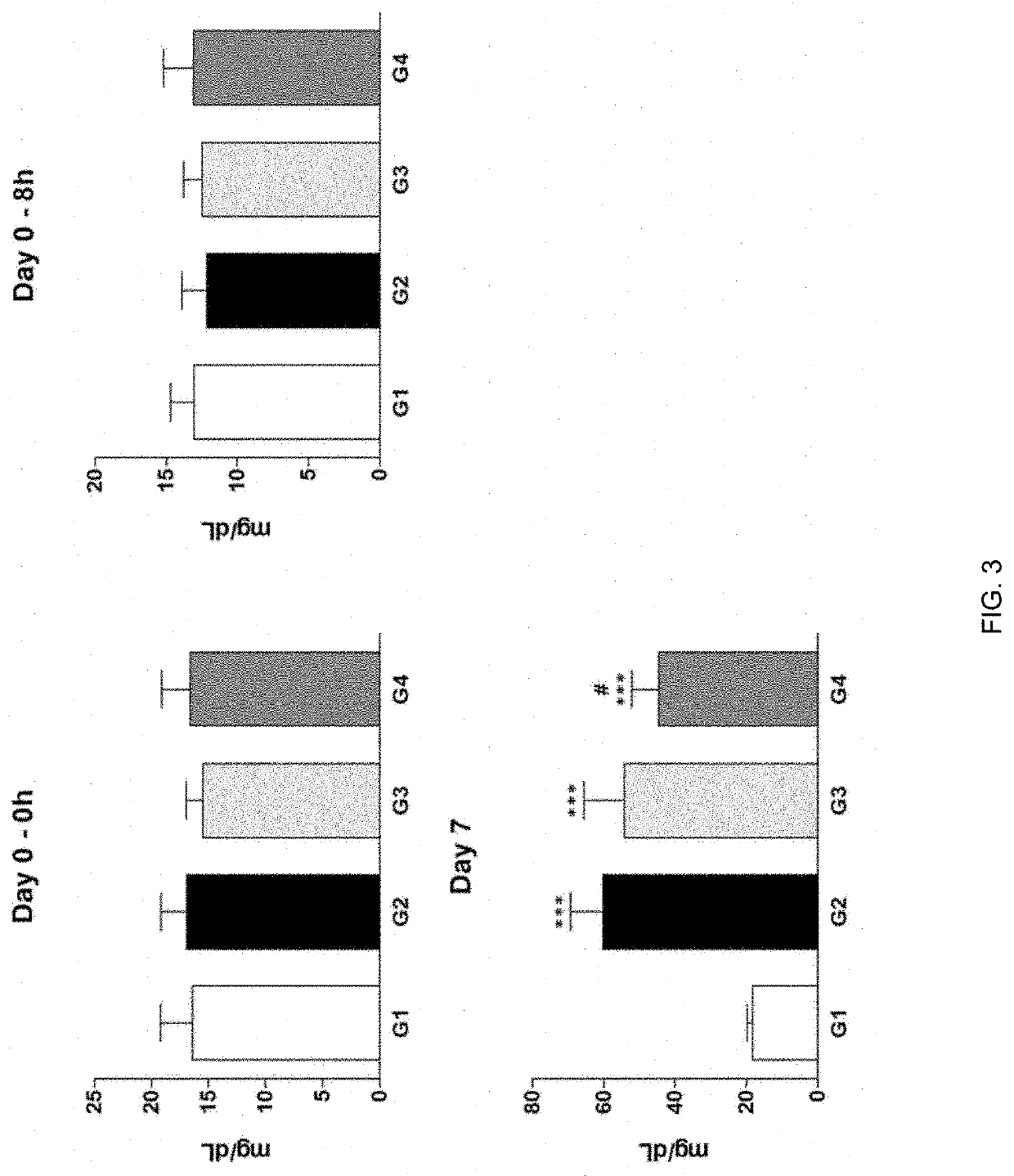
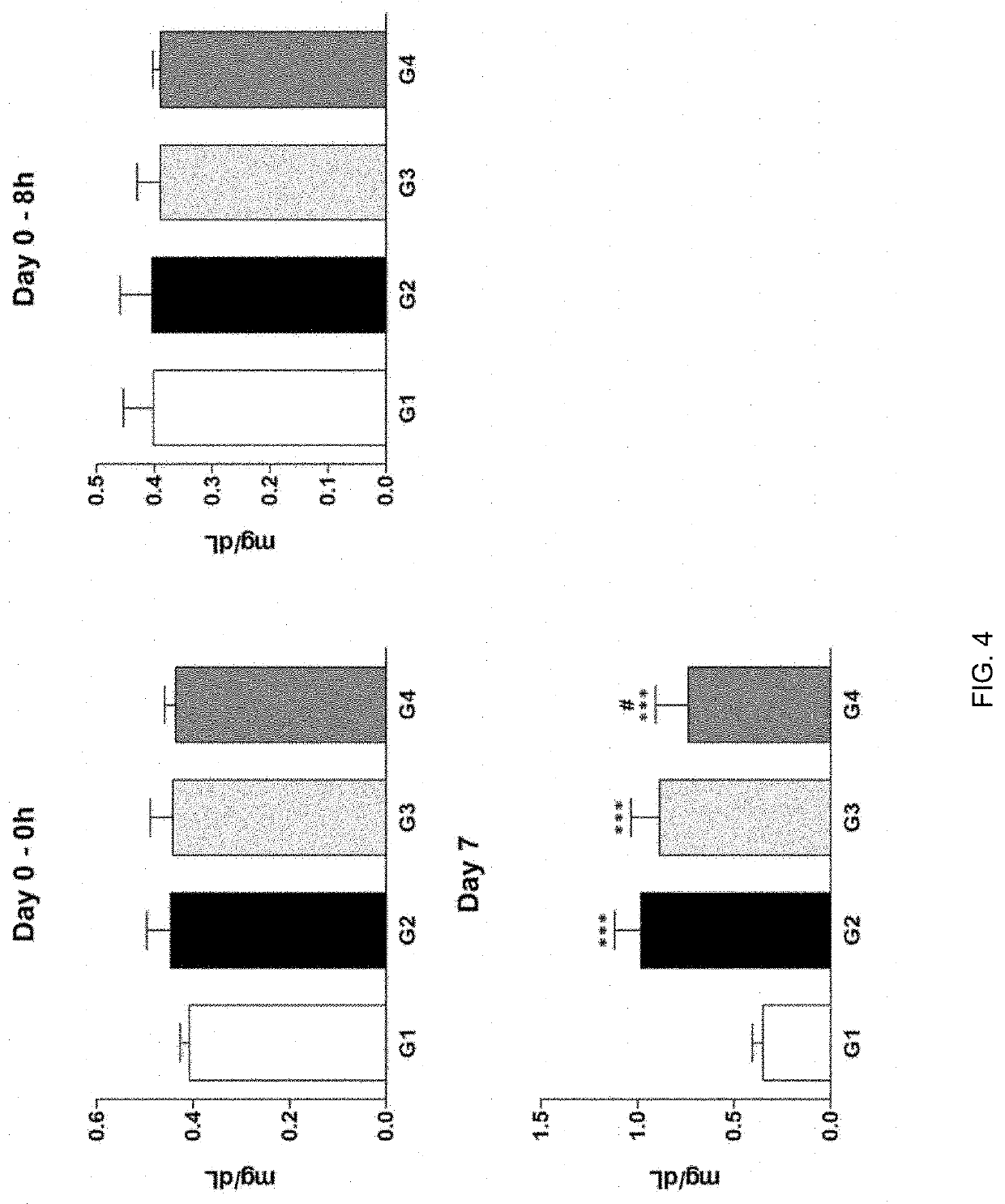
Therapeutic agent of uremia containing alarin as the main ingredient
InactiveUS20200254059A1High treatment costSatisfactory treatmentPeptide/protein ingredientsPharmaceutical delivery mechanismGalPUremia
The present invention relates to a novel use of alarin derived from a splice variant of galanin-like peptide (GALP) RNA and thus having an amino acid sequence similar to that of galanin, with a therapeutic agent of uremia, and the therapeutic agents of uremia according to the present invention include alarin as the main ingredient.
Owner:JU TIDE CO LTD
Linseed oil based external drug for infantile eczema and preparation method thereof
InactiveCN102973612ASimple recipeEasy and quick to makeDermatological disorderAluminium/calcium/magnesium active ingredientsDrugChemistry
The invention provides a linseed oil based external drug for infantile eczema. The linseed oil based external drug comprises a raw reagent A, a compatible reagent B and a raw reagent C, wherein the raw reagent A comprises the following component by weight: 1 part of fresh egg; the compatible reagent B comprises the following components: 8 to 13% of realgar, 8 to 12% of sulphur, 10 to 15% of dried alum, and 10 to 15% of plant soot; and the raw reagent C comprises the following component in percentage by mass based on the mass of the egg in the raw reagent A: 45 to 55% of linseed oil. The preparation method of the linseed oil based external drug comprises the following steps in sequence: grinding each component in compatible reagent B into powder; sequentially adding the powder to the raw reagent A; sealing and uniformly shaking; wrapping the egg through soil and sealing; burning the egg with the soil until carbonizing; cooling in the air; removing the soil housing; grinding the soil-removed material into fine powder; heating the raw reagent C; adding the heated raw reagent C to the fine powder under fire removing state; stirring uniformly; and cooling until reaching the room temperature, thus obtaining the linseed oil based external drug for infantile eczema. The linseed oil based external drug for infantile eczema is simple in prescription, simple and quick to manufacture, low in cost, convenient to use, free from toxic or side effect during external use, and short in cure cycle.
Owner:周廷智
Therapeutic agent of uremia containing alarin as the main ingredient
ActiveUS20220031804A1High treatment costSatisfactory treatmentPeptide/protein ingredientsPharmaceutical delivery mechanismGalPUremia
Provided is a novel use of alarin derived from a splice variant of galanin-like peptide (GALP) RNA and thus having an amino acid sequence similar to that of galanin, with a therapeutic agent of uremia, and the therapeutic agents of uremia according to the presently claimed subject matter include alarin as the main ingredient.
Owner:JU TIDE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com