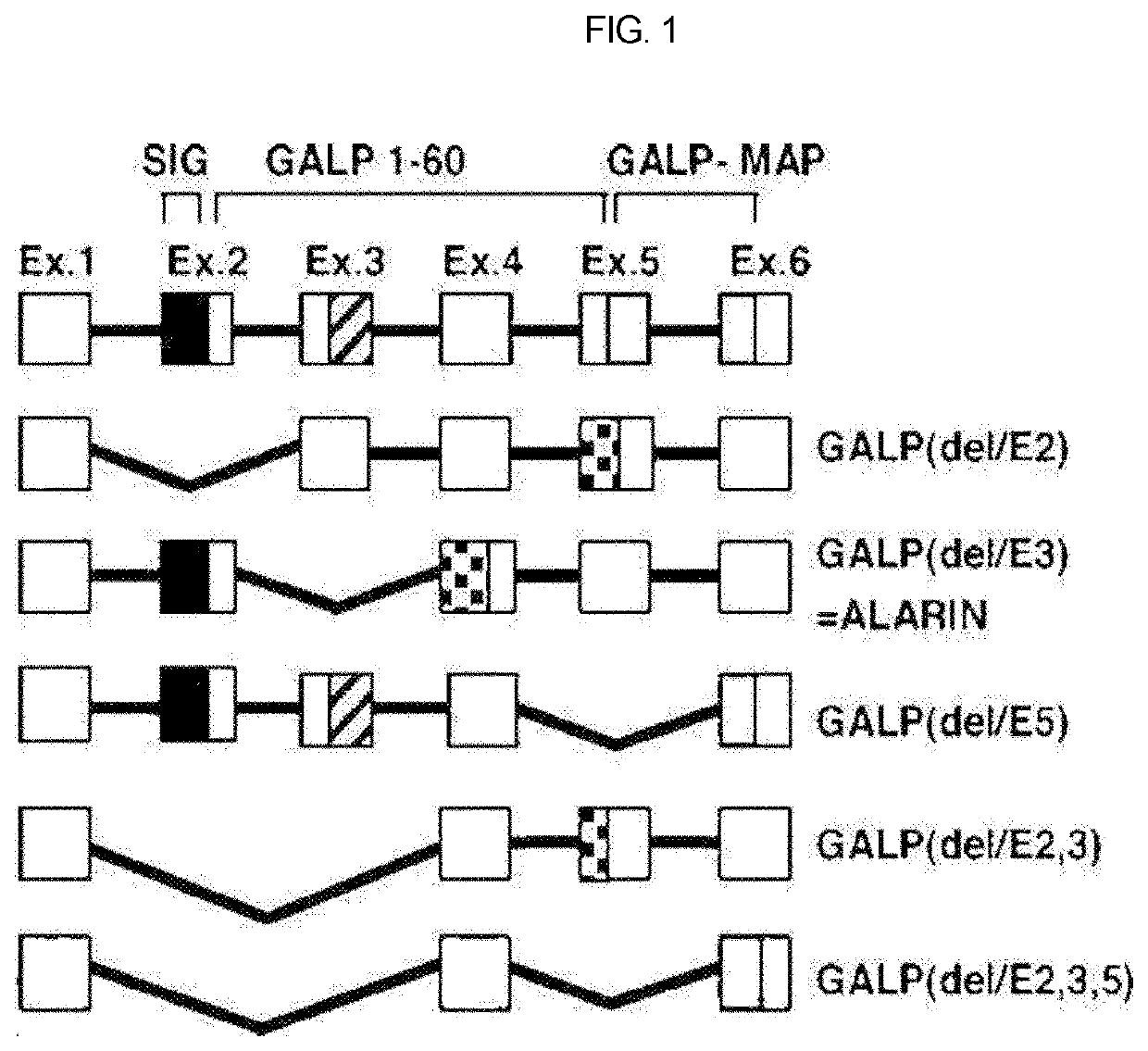
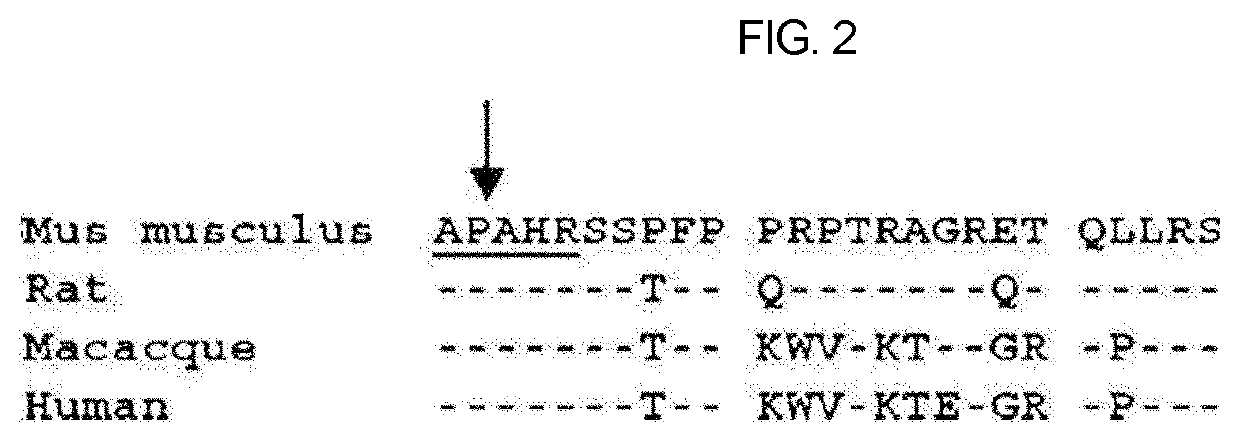
Therapeutic agent of uremia containing alarin as the main ingredient
a technology of uremia and therapeutic agent, which is applied in the direction of pharmaceutical active ingredients, pharmaceutical delivery mechanisms, peptide/protein ingredients, etc., can solve the problems of uremia and the difficulty of treating uremia with medicine, and the death of the person with uremia,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
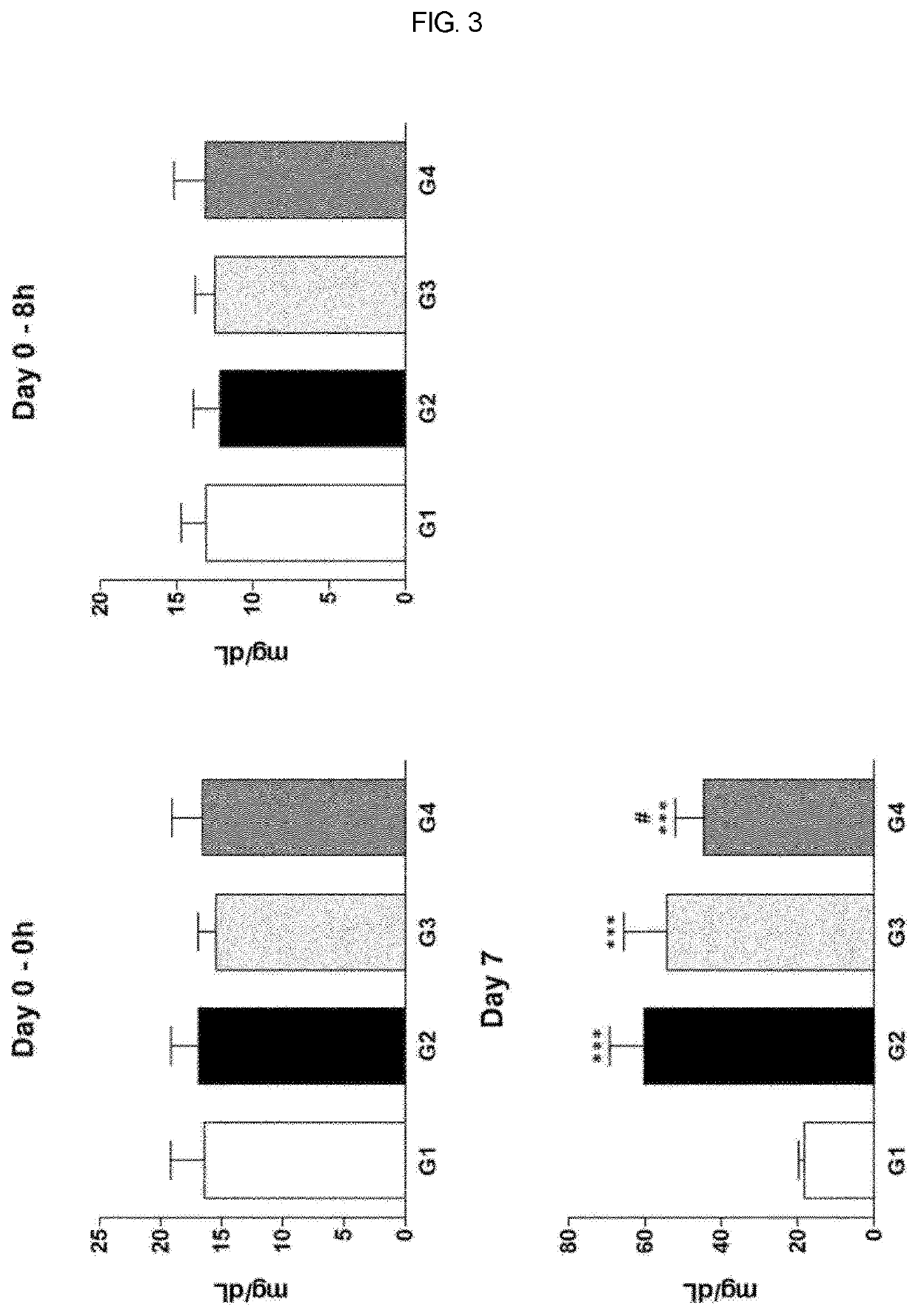
[0046]Cisplatin-induced uremia was evaluated for the effect of administration of test substance on an improvement of uremia in the Sprague-Dawley rat model. Here, G1 is normal control group which is administered with an excipient, G2 is induced control group which is administered with an excipient, G3 is group which test substance is administered with 10 μg / 1 mL / head, and G4 is group which test substance is administered with 20 μg / mL / head. Here, test substance is alarin (human) trifluoroacetate salt of the present invention. Cisplatin was intraperitoneally administered (IP) and test substance was intravenously administered (IV).
[0047]In addition, the test substance (G3-G4) was administered once at 8 hours (Day 0) after cisplatin administration, and in the case of cisplatin (G2-G4), dose of 5 mg / 10 mL / kg was administered once at 8 hours (Day 0) before test substance administration.
[0048]The intravenous administration was done by fixing an animal into a correction frame, and slowly in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com