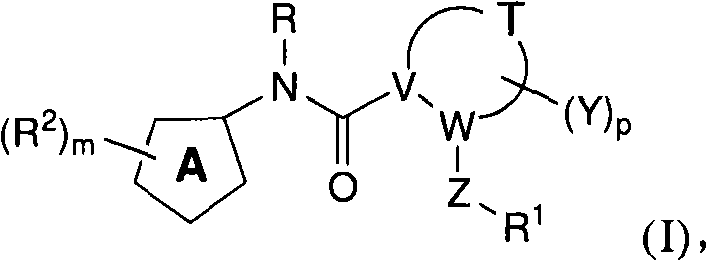
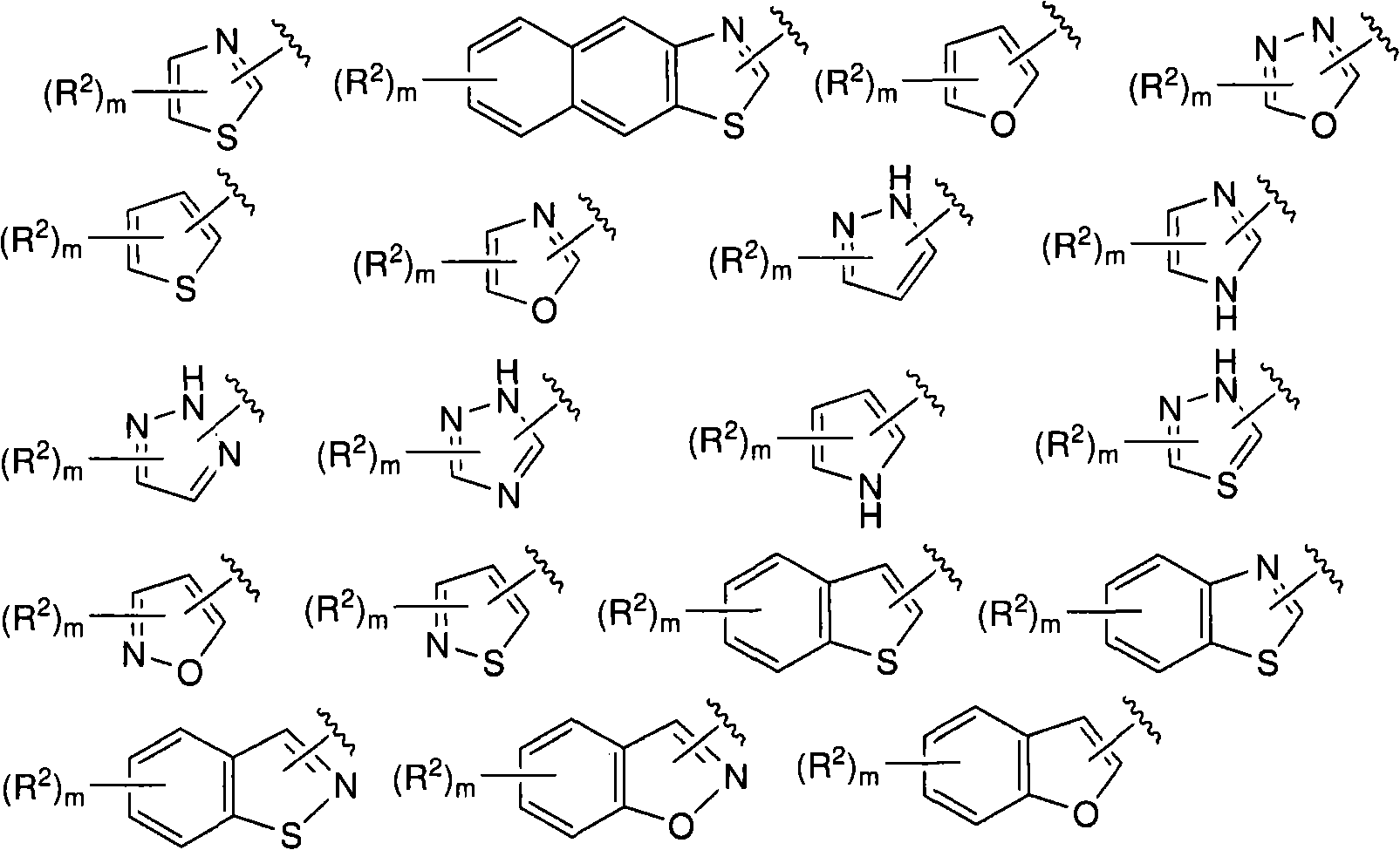
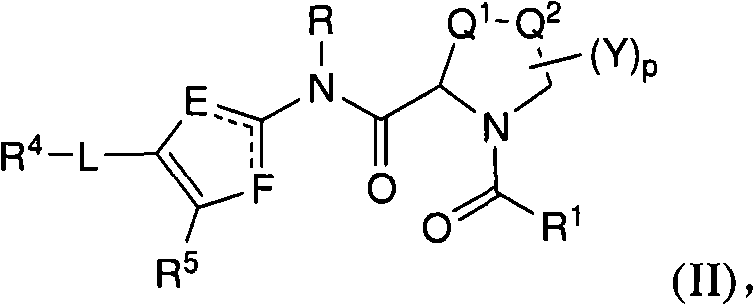
N-(5-membered aromatic ring)-amido anti-viral compounds
Technology of a compound, heteroaryl, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0296] In the following examples and throughout the application, the following abbreviations have the following meanings. If not defined, terms have their generally accepted meanings.
[0297] atm = atmospheric pressure
[0298] cm = centimeter
[0299] DIEA = Diisopropylethylamine
[0300] DMF = dimethylformamide
[0301] DMSO = dimethyl sulfoxide
[0302] eq. = equivalent
[0303] F.W. = formula weight
[0304] g = gram
[0305] HATU = N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethylene]-N-methylmethanoammonium N-hexafluorophosphate -Oxide
[0306] HPLC = High Pressure Liquid Chromatography
[0307] KOAc = potassium acetate
[0308] L = liter
[0309] MeCN = acetonitrile
[0310] mg = milligram
[0311] mL = milliliter
[0312] mmol = millimole
[0313] MS = mass spectrum
[0314] TEA = Triethylamine
[0315] TFA = trifluoroacetic acid
[0316] THF = Tetrahydrofuran
[0317] TLC = Thin Layer Chromatography
[0318] v / v = volume / volume
[031...
example 1
[0369] 2-[4-(3-Amino-phenyl)-thiazol-2-ylcarbamoyl]-pyrrolidine-1-carboxylic acid benzyl ester (compound 5001)
[0370] Thiourea (0.31 g, 4.1 mmol) and sodium acetate (0.44 g, 5.3 mmol) were combined in 30 mL of absolute ethanol. To this suspension was added 2-bromo-1-(3-nitro-phenyl)-ethanone (1.0 g, 4.1 mmol). The reaction mixture was stirred overnight at room temperature. The reaction mixture was evaporated to dryness to yield 4-(3-nitro-phenyl)-thiazol-2-ylamine which was used in the next step without any further purification. MS: 222.0 (M+H + ).
[0371] Z-Pro-OH (1.5 g, 6.15 mmol) and HATU (2.3 g, 6.15 mmol) were combined in 30 mL of anhydrous DMF. To this solution was added DIEA (1.5 mL, 8.8 mmol). This solution was stirred at room temperature for 1 hour. To this solution was added 4-(3-nitro-phenyl)-thiazol-2-ylamine (0.9, 4.1 mmol). The reaction mixture was stirred overnight at room temperature. The crude material was purified using reverse phase HPLC to yield...
example 2
[0374] 2-(4-Benzoyl-5-yl-thiazol-2-ylcarbamoyl)-pyrrolidine-1-carboxylic acid benzyl ester (compound 5002)
[0375] From 10 mg of 4-benzo[1,2]dioxol-5-yl-thiazol-2-ylamine in 1 mL solution using general procedure G. MS: 452.0 (M+H + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com