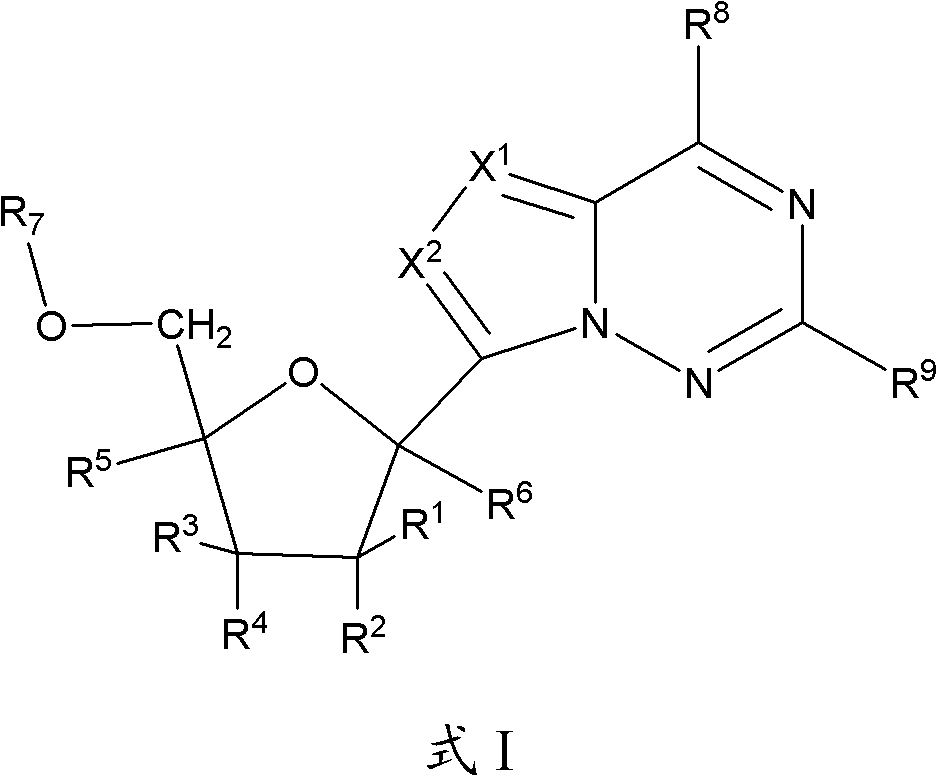
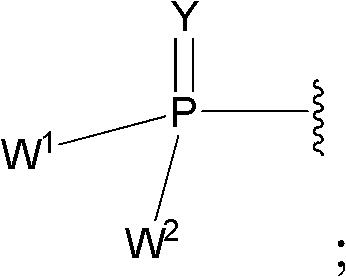
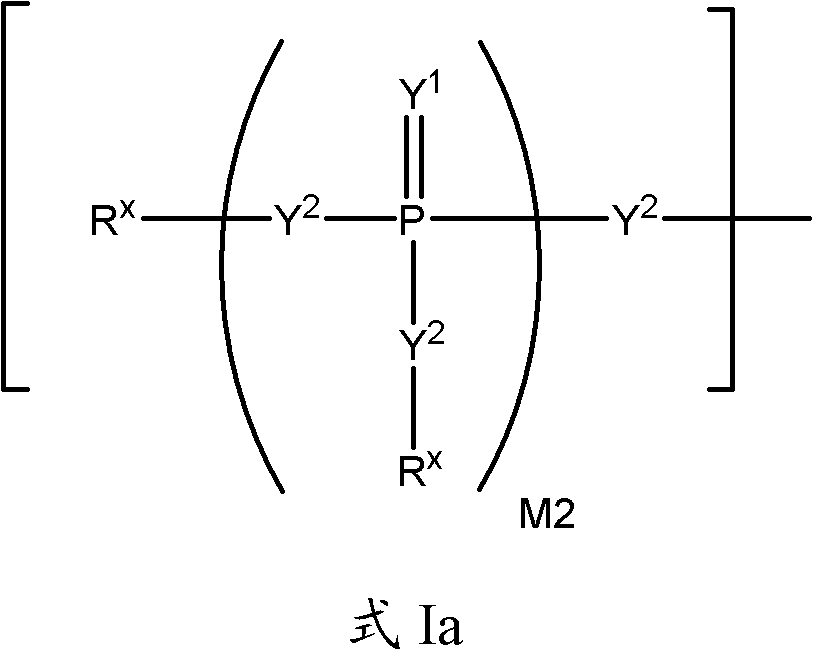
2' -fluoro substituted CARBA-nucleoside analogs for antiviral treatment
A C2-C8, C4-C8 technology, applied in the field of 2'-fluoro CARBA-nucleoside analogs for antiviral therapy, can solve problems such as unpublished
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0483] In describing experimental details, certain abbreviations and acronyms are used. Although most of them will be understood by those skilled in the art, Table 1 contains a listing of many such abbreviations and acronyms.
[0484] Table 1. List of abbreviations and acronyms
[0485]
[0486]
[0487]
[0488]
[0489] Compound preparation
[0490] Compound 1
[0491]
[0492] BuLi (1.6M in hexane, 1.61 mL, 2.41 mmol) was added dropwise to 7-bromo-2,4-bis-methylsulfanyl-imidazo[2,1-f] at -78°C [1,2,4] Triazine (prepared according to WO2008116064, 500 mg, 1.72 mmol) suspension in anhydrous THF (5 mL). After 5 minutes the suspension turned into a red-brown solution, then a mixture of 1a (prepared according to WO200631725, 675 mg, 1.81 mmol) and boron trifluoride etherate (2.40 mL, 1.89 mmol) in THF (5 mL) was added dropwise to the mixture. After stirring at -78°C for 2 hours, saturated NH 4 Cl to quench the reaction. The mixture was diluted with ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com