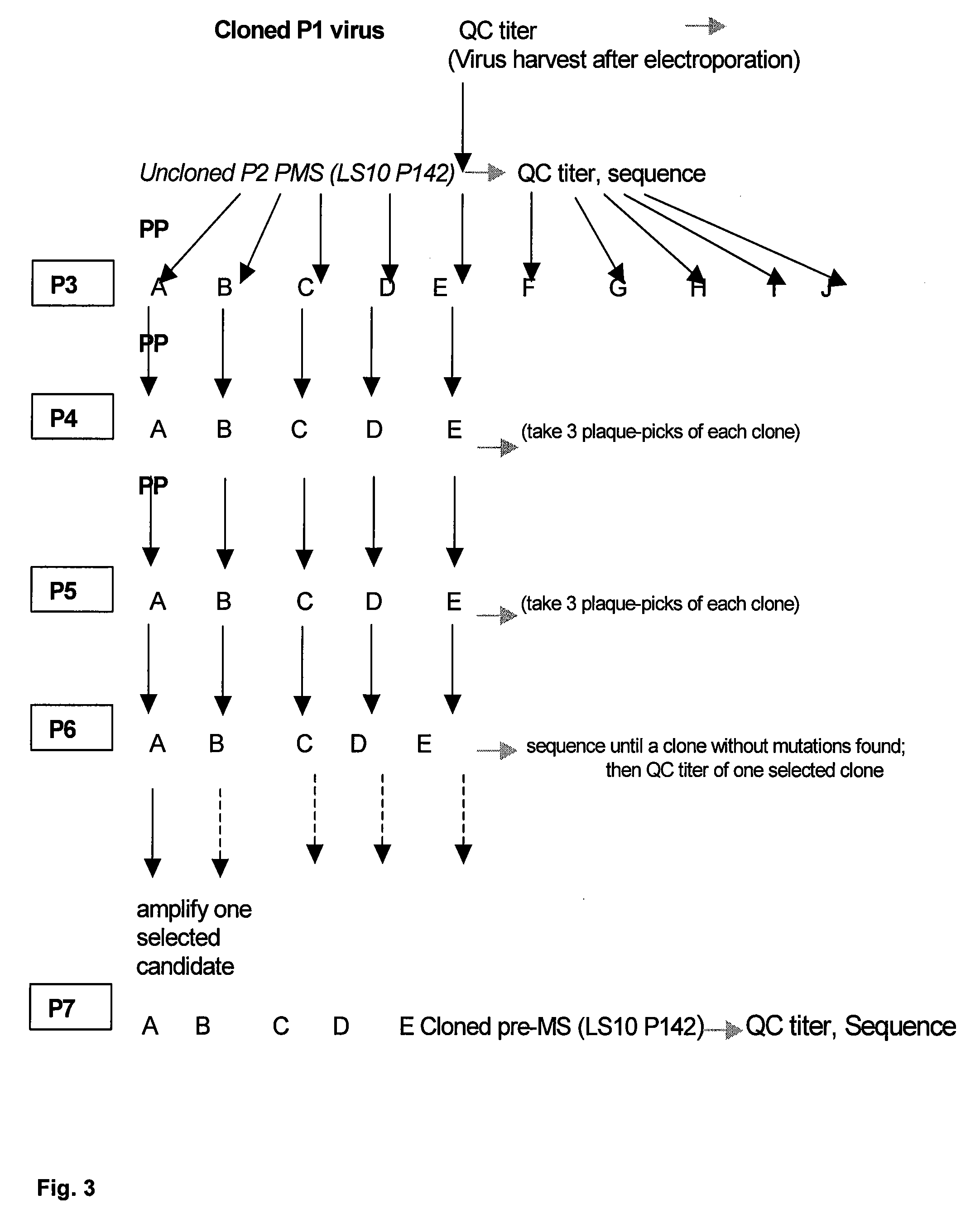
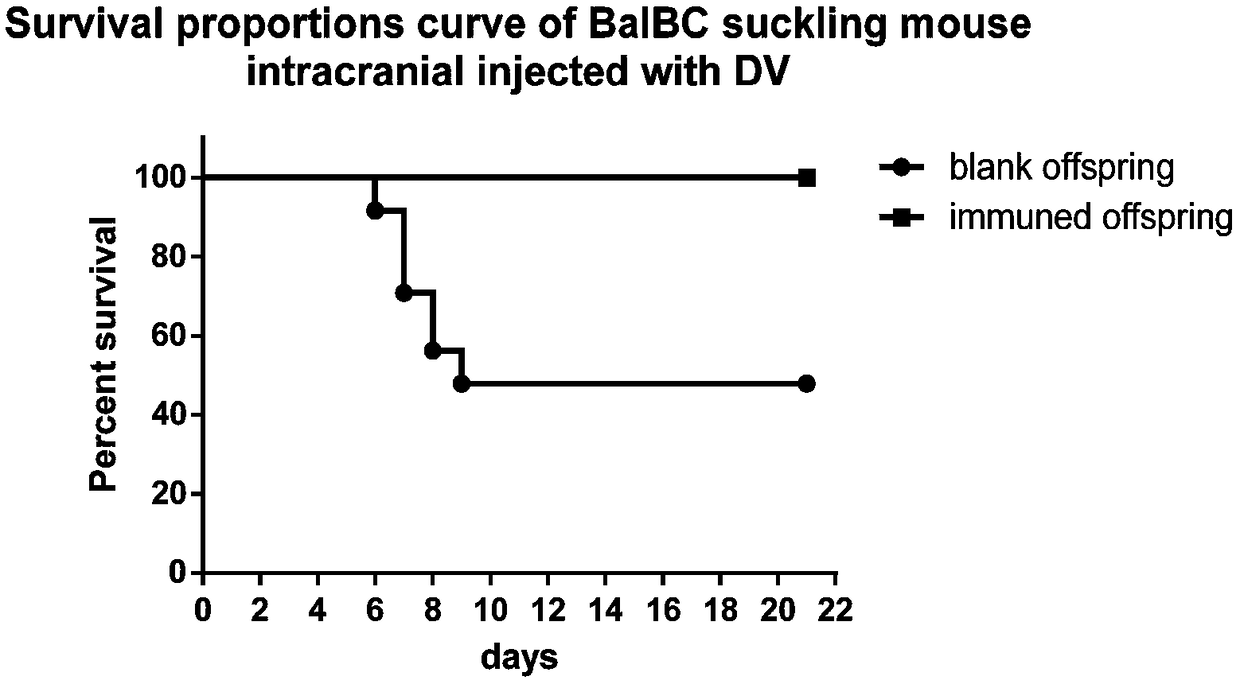
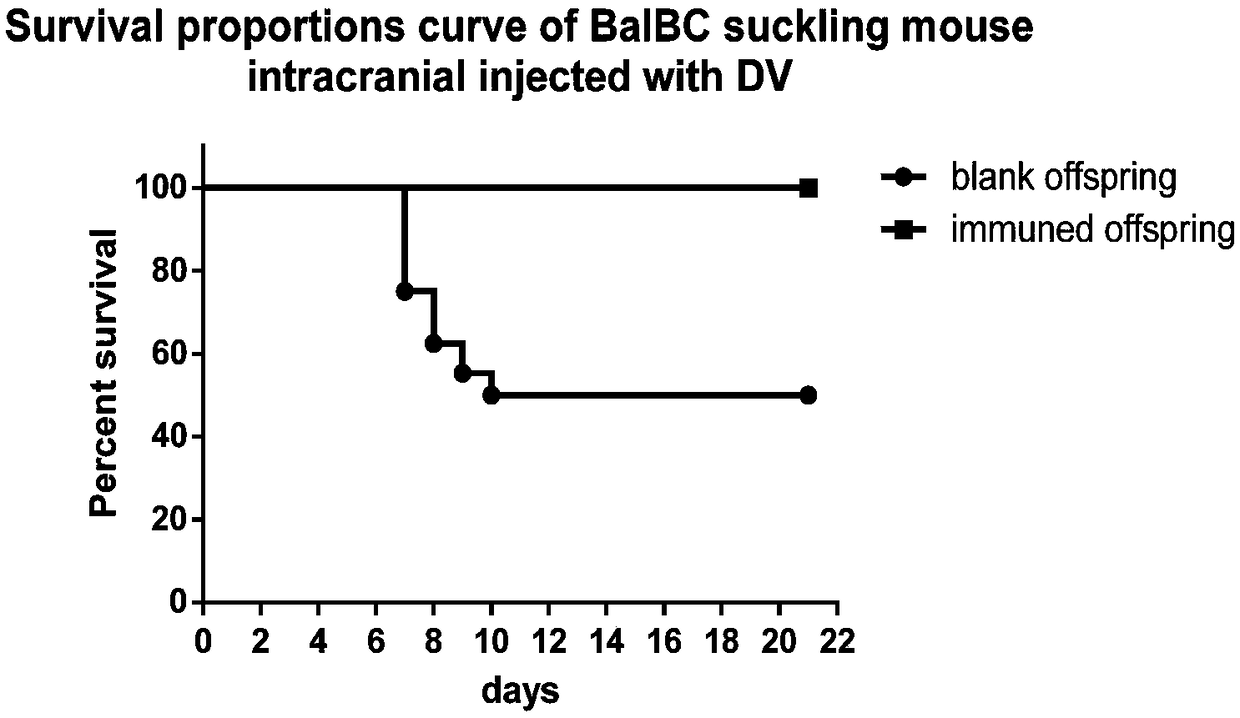
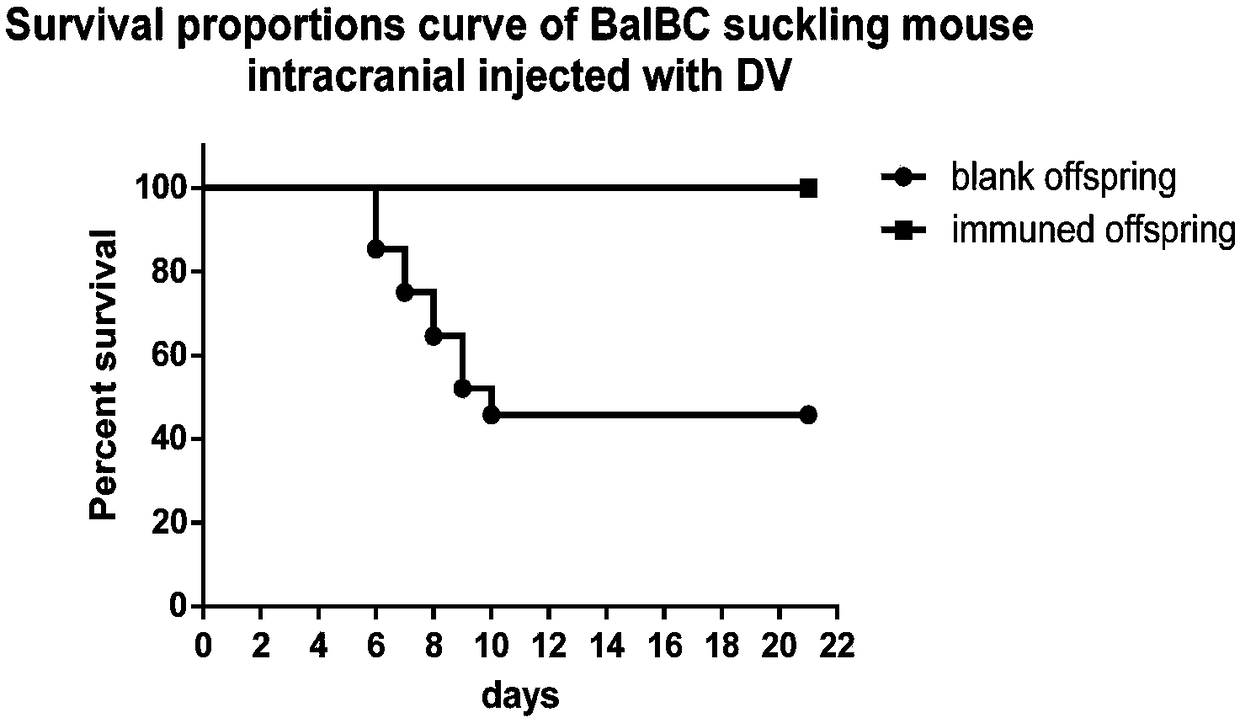
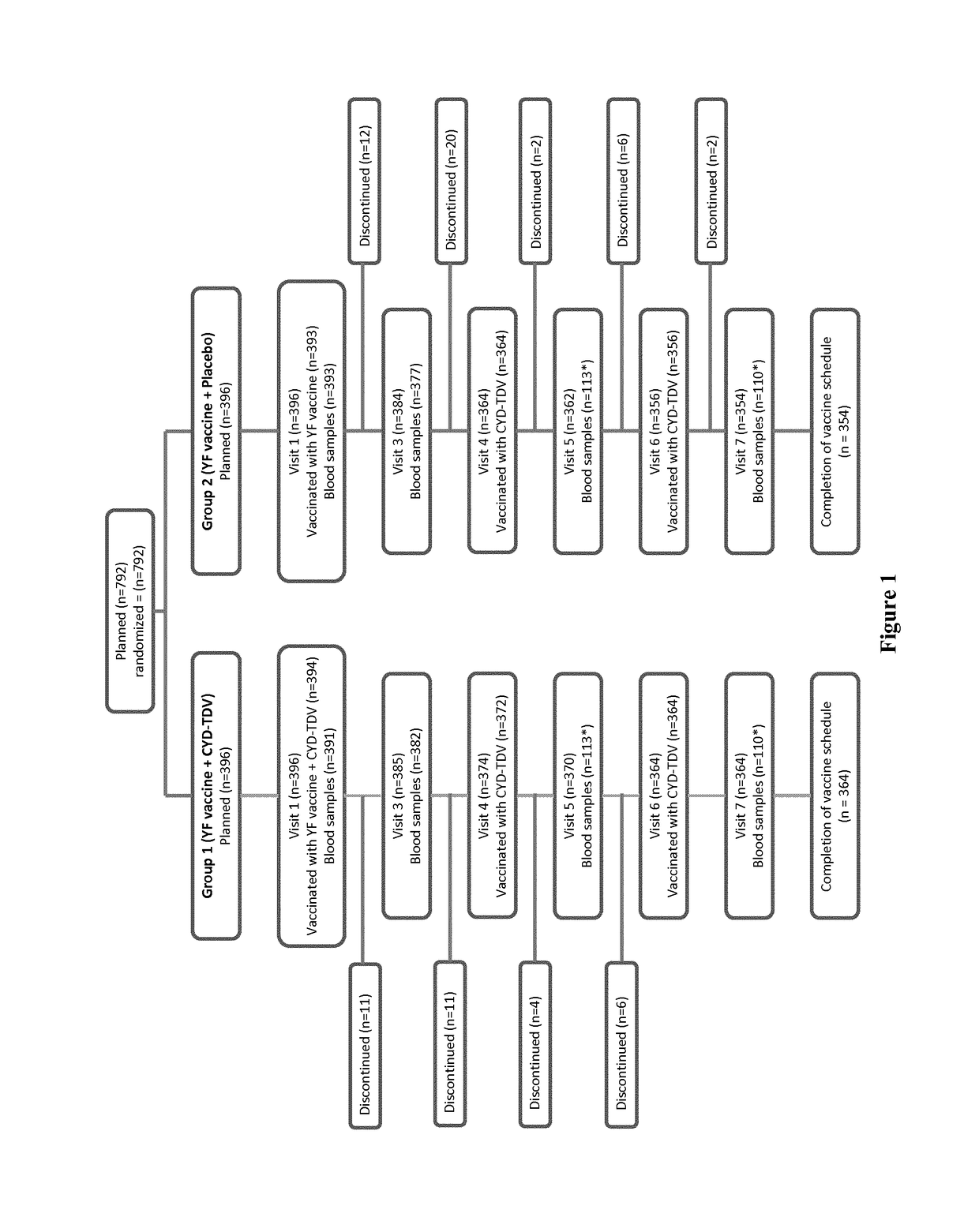
Patents
Literature
40 results about "Dengue vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dengue vaccine is a vaccine to prevent dengue fever in humans. The World Health Organization (WHO) only recommends the vaccine in those who have previously had dengue fever or populations in which most people have been previously infected. The value of this vaccine is limited by the fact that it may worsen outcomes in those who have not previously been infected.
Recombinant subunit dengue virus vaccine
ActiveUS20130216575A1Prevention and treatmentAcceptable safety profileSsRNA viruses positive-senseViral antigen ingredientsAdjuvantImmune senescence
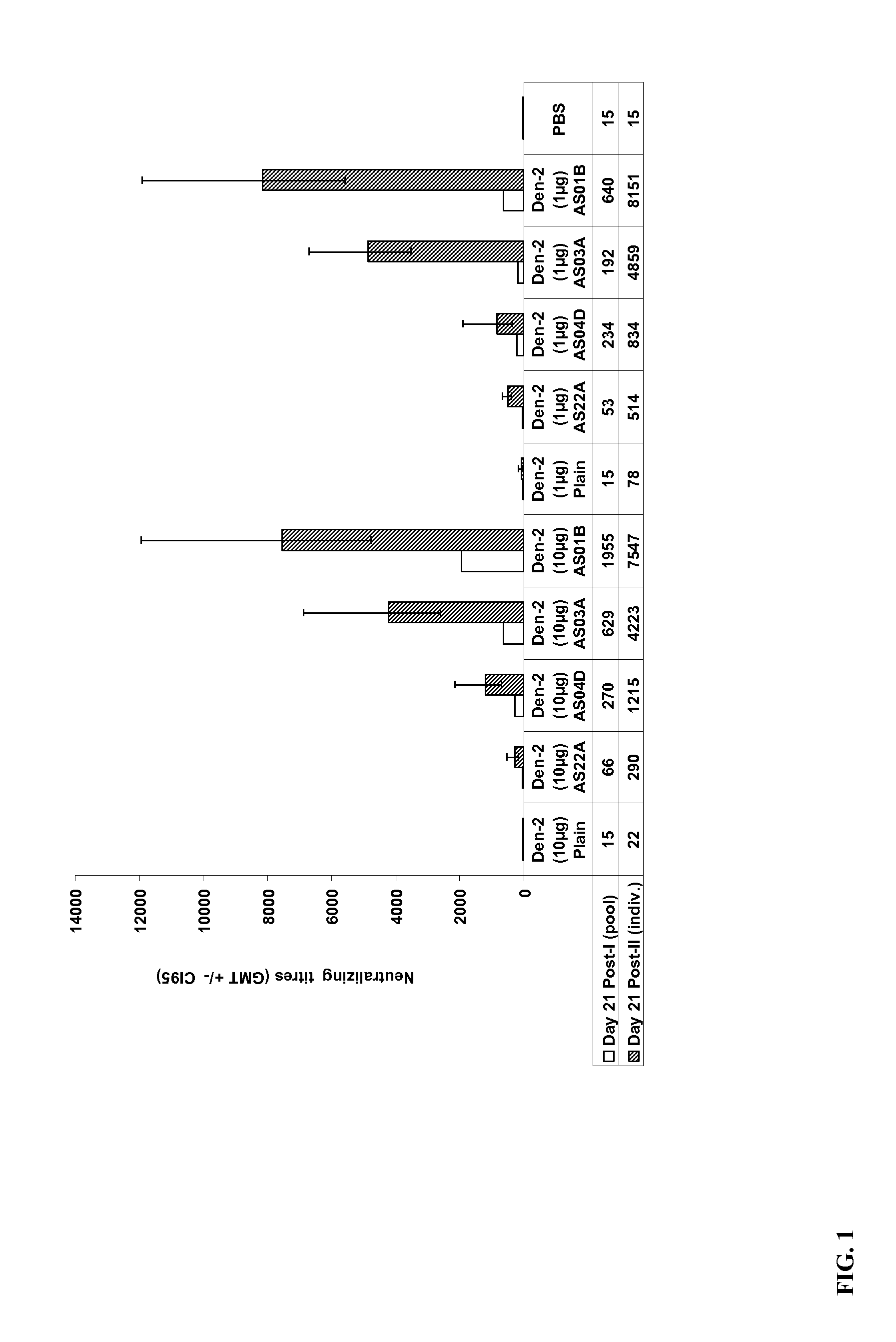
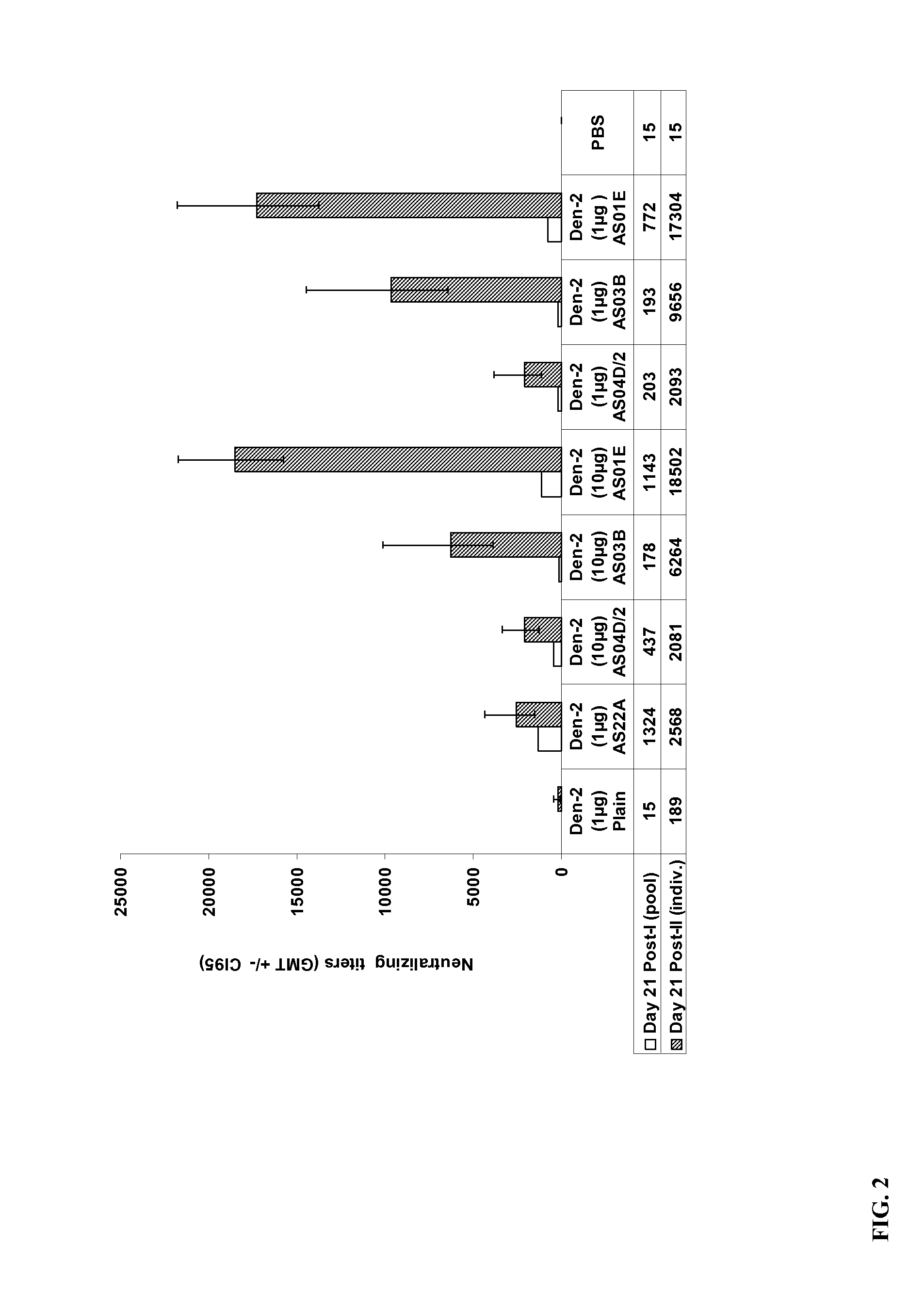
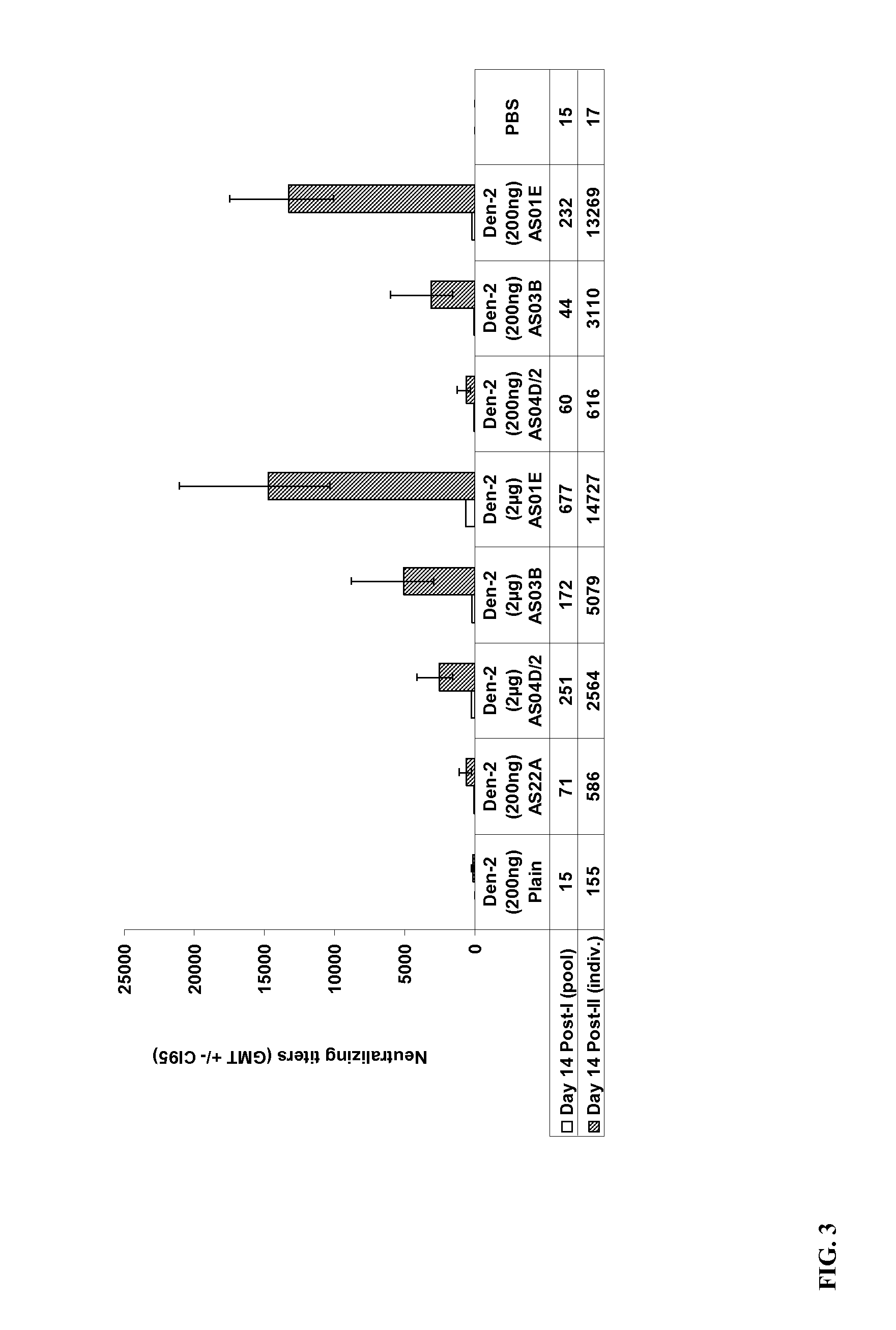
The present invention provides dengue virus vaccines and immunogenic compositions for administration to human subjects. The vaccine compositions of the present invention comprise recombinantly produced monomeric and / or dimeric forms of truncated dengue virus envelope glycoprotein that, when formulated together with an adjuvant and a pharmaceutically acceptable carrier, induce balanced tetravalent immune responses. In preferred embodiments of the compositions described herein, the DEN4 protein component is a dimeric form of DEN4. The compositions are designed to be acceptable for use in the general population, including immunosuppressed, immunocompromised, and immunosenescent individuals. Also provided herein are methods of inducing a protective immune response in a human patient population by administering the compositions described herein to the patients.
Owner:MERCK SHARP & DOHME LLC
Tetravalent Dengue Vaccines
InactiveUS20100158938A1Immune responseLong durationSsRNA viruses positive-senseViral antigen ingredientsDengue vaccineDengue fever
Owner:GUIRAKHOO FARSHAD
Japanese encephalitis/dengue chimeric virus and application thereof
InactiveCN101560520AStable passageViral antigen ingredientsAntiviralsJapanese encephalitisWestern blot
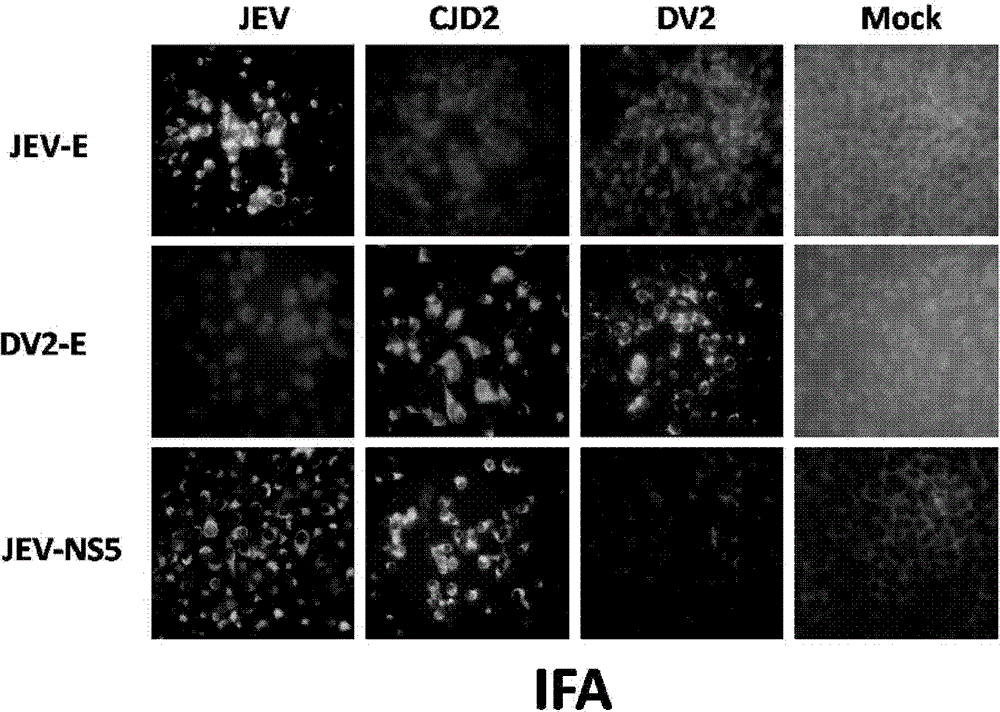
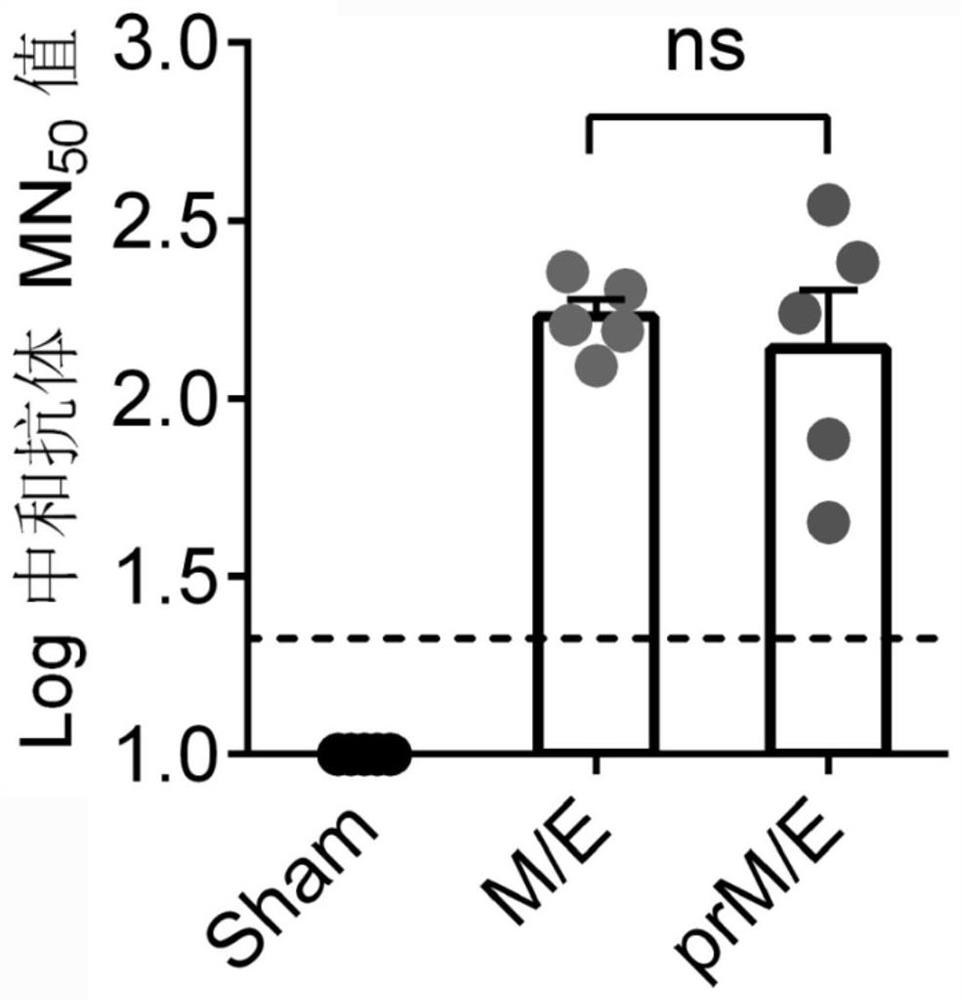
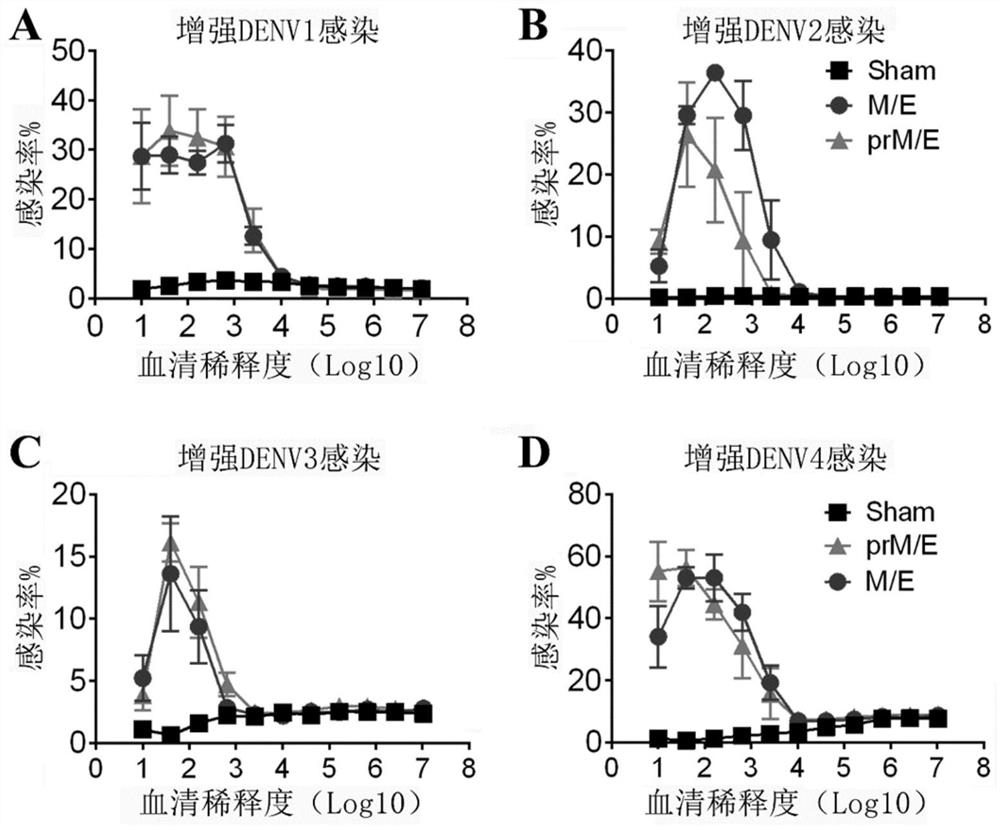
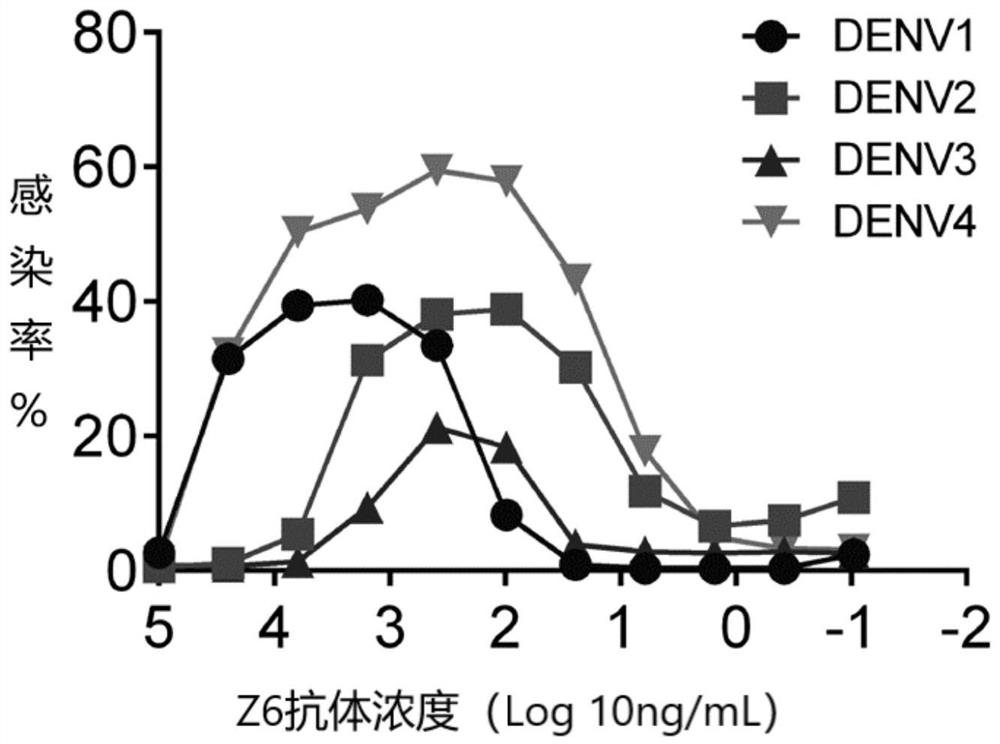
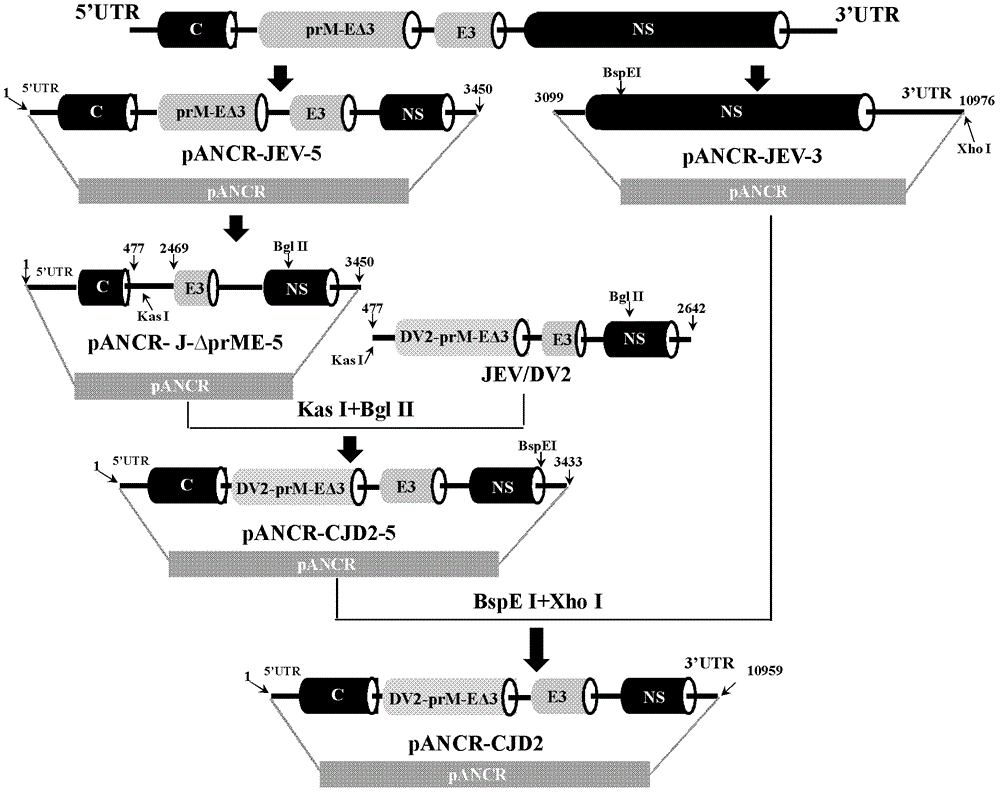
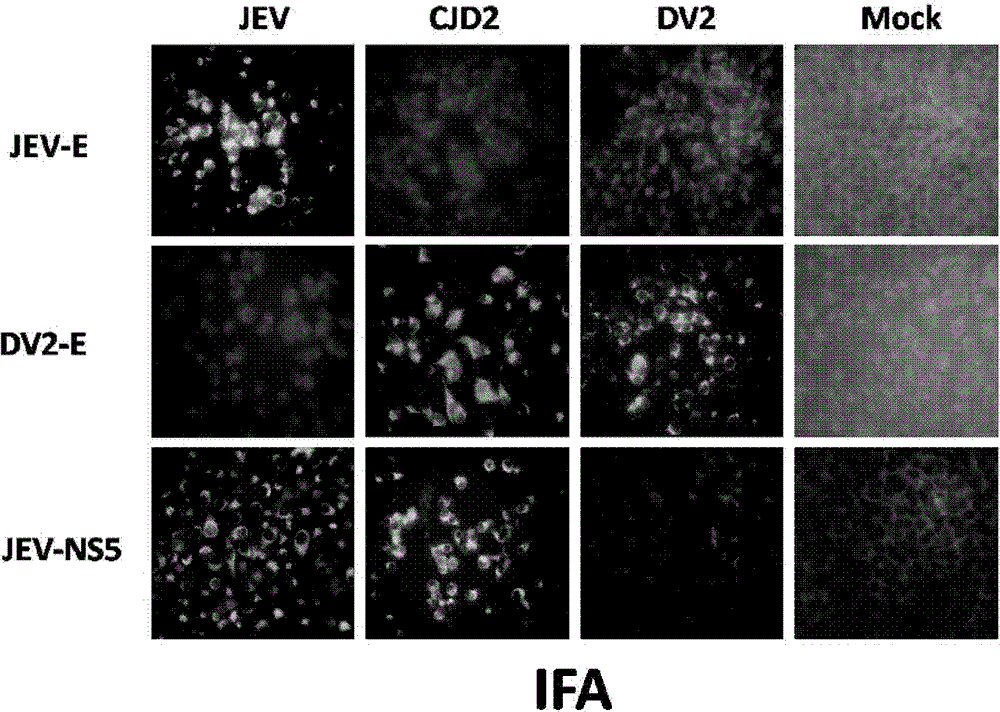
The invention provides a Japanese encephalitis / dengue chimeric virus, which is obtained by replacing a prM / E gene in Japanese encephalitis cDNA clone with the prM / E gene of dengue virus type II. IFA and Western blot confirm that the chimeric virus can express E protein of a dengue virus and can stabilize passage in C6 / 36 cells. The invention lays a foundation for developing a dengue virus vaccine. The chimeric virus can be taken as a vaccine to immunize animals so as to enable the animals to obtain immune protection against the dengue virus.
Owner:中国疾病预防控制中心病毒病预防控制所
Tetravalent Dengue Vaccines
InactiveUS20130095136A1Immune responseLong durationSsRNA viruses positive-senseViral antigen ingredientsMedicineDengue vaccine
Owner:SANOFI PASTEUR BIOLOGICS CO
Epidemic encephalitis B/dengue chimeric virus with epidemic encephalitis B virus attenuated strain as gene framework and application of epidemic encephalitis B/dengue chimeric virus
ActiveCN102796749AImprove securityStable attenuation characteristicsFungiBacteriaDengue vaccineTGE VACCINE
The invention discloses a pidemic encephalitis B / dengue chimeric virus with an epidemic encephalitis B virus attenuated strain as a gene framework and application of the epidemic encephalitis B / dengue chimeric virus. The invention provides a deoxyribonucleic acid (DNA) molecule, and the DNA molecule is recombinant DNA which is obtained by replacing an encoding gene of protein A in complementary DNA (cDNA) corresponding to genome ribonucleic acid (RNA) of an epidemic encephalitis B virus by using an encoding gene of protein B; the protein A consists of an amino acid sequence shown as a sequence 1 in a sequence table; and the protein B consists of an amino acid sequence shown as a sequence 2 in the sequence table. The pidemic encephalitis B / dengue chimeric virus with the epidemic encephalitis B virus attenuated strain as the gene framework is a recombinant virus containing the DNA molecule. The pidemic encephalitis B / dengue type II chimeric virus also has a good application prospect when taken as a dengue vaccine and used for preventing dengue virus infection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI +1
Inactivated dengue virus vaccine with aluminium-free adjuvant
InactiveUS20110318407A1Reduce capacityShort durationSsRNA viruses positive-senseViral antigen ingredientsDiseaseAdjuvant
The present disclosure provides immunogenic compositions for the prevention and / or treatment of disease caused by dengue virus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
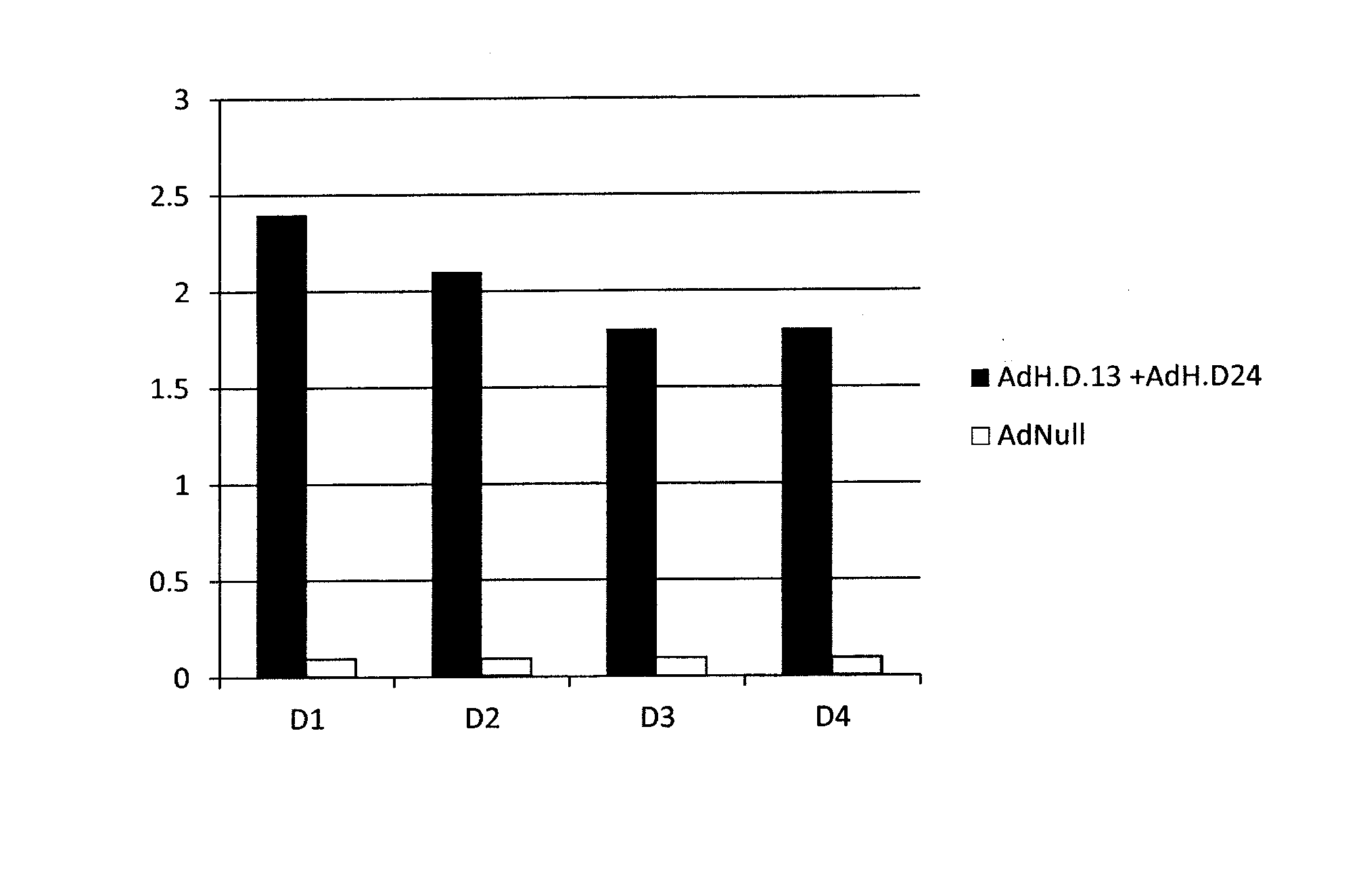
Adenoviral vector-based dengue fever vaccine
The invention relates to a replication-deficient adenoviral vector comprising two or more nucleic acid sequences encoding Dengue virus antigens and a chimeric hexon protein. The chimeric hexon protein comprises a first portion and a second portion. The first portion comprises at least 10 contiguous amino acid residues from a first adenovirus serotype (e.g., serotype 5 adenovirus hexon protein), optionally with one amino acid substitution. The second portion comprises (a) at least one hypervariable region (HVR) of a hexon protein of an adenovirus of a second adenovirus serotype, or (b) at least one synthetic hypervariable region (HVR) that is not present in the hexon protein of the wild-type adenovirus of the first adenovirus serotype.
Owner:GEN VEC INC
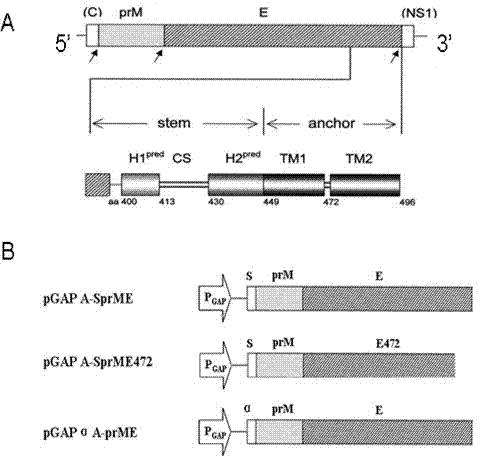
Dengue virus (DENV)-like particle as well as preparation method and application thereof
InactiveCN102363751AInnovativeSuitable for large-scale productionFungiViral antigen ingredientsYeastVirus-like particle
The invention discloses a dengue virus (DENV)-like particle as well as a preparation method and the application thereof. P. pastoris yeast strains transformed by recombinant carriers of virus-like particles (VLPs) of DENV1-4, which are respectively pGAPZ-Alpha-PrME-D1-X33, pGAPZ-sPrME472-D2-X33, pGAPZ-sPrME-D3-X33 and pGAPZ-sPrME-D4-X33, are respectively preserved at China center for type culture collection (CCTCC) at Wuhan University on March 14th, 2011 and have the serial numbers of CCTCCM2011063, CCTCCM2011064, CCTCCM2011065 and CCTCCM2011066. The VLPs of the DENV1-4 are obtained by transfecting the recombinant carriers stated in claim 1 with P. pastoris yeast cells X33 and enabling the recombinant carriers to secrete the VLPs. According to the preparation method, a P. pastoris yeast system not relied on carbinol is adopted to prepare the DENV-like particle, which is not reported at home and abroad yet, so the innovativeness is achieved. The DENV-like particle prepared from the system can be used for preparing vaccines, and various defects in the research of dengue vaccines such as attenuated live vaccines, subunit vaccines, carrier vaccines and the like are overcome; and the DENV-like particle has the advantages of safety and high efficiency, and is suitable for large scale production.
Owner:SUN YAT SEN UNIV
Adenoviral vector-based dengue fever vaccine
The invention relates to a replication-deficient adenoviral vector comprising two or more nucleic acid sequences encoding Dengue virus antigens and a chimeric hexon protein. The chimeric hexon protein comprises a first portion and a second portion. The first portion comprises at least 10 contiguous amino acid residues from a first adenovirus serotype (e.g., serotype 5 adenovirus hexon protein), optionally with one amino acid substitution. The second portion comprises (a) at least one hypervariable region (HVR) of a hexon protein of an adenovirus of a second adenovirus serotype, or (b) at least one synthetic hypervariable region (HVR) that is not present in the hexon protein of the wild-type adenovirus of the first adenovirus serotype.
Owner:GENVEC INC
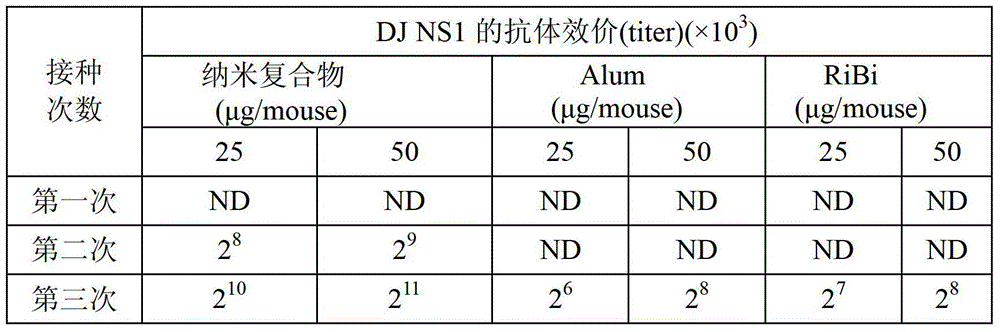
Dengue vaccine, pharmaceutical composition comprising the same, nucleotide sequence and antibody composition
InactiveUS20120070454A1Avoid potential risksImprove stabilitySsRNA viruses positive-senseSugar derivativesNucleotideAutoimmune responses
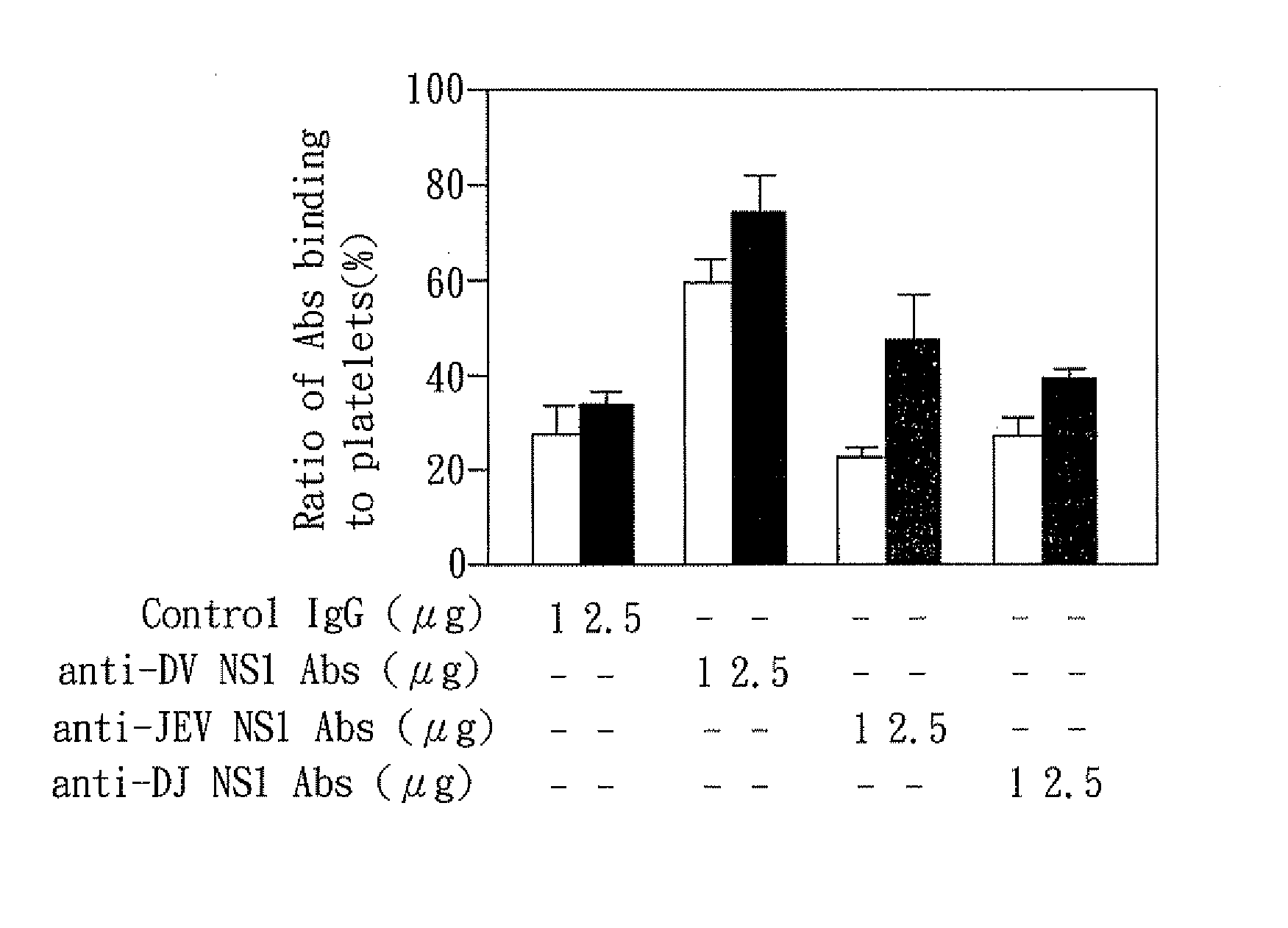
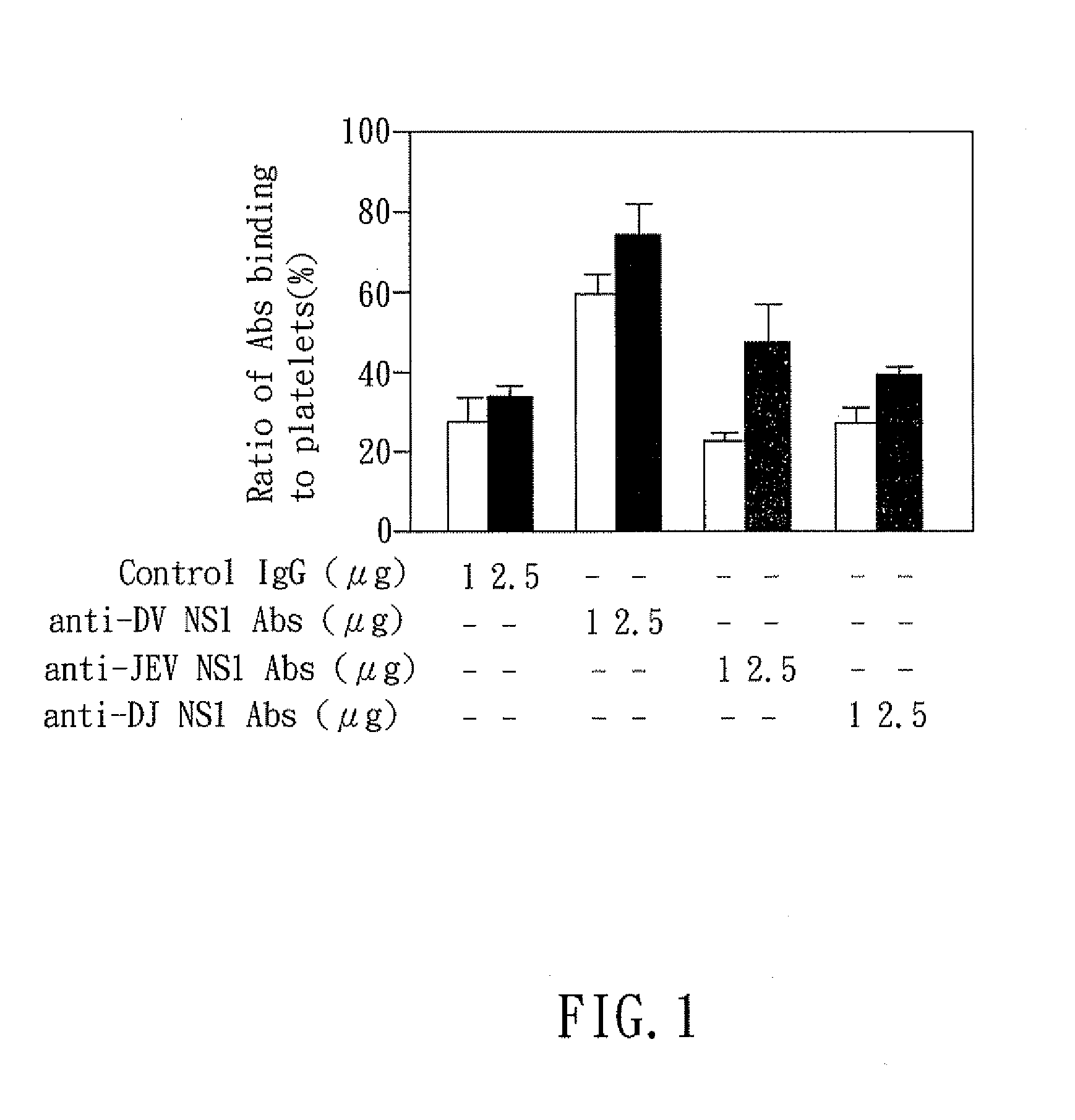
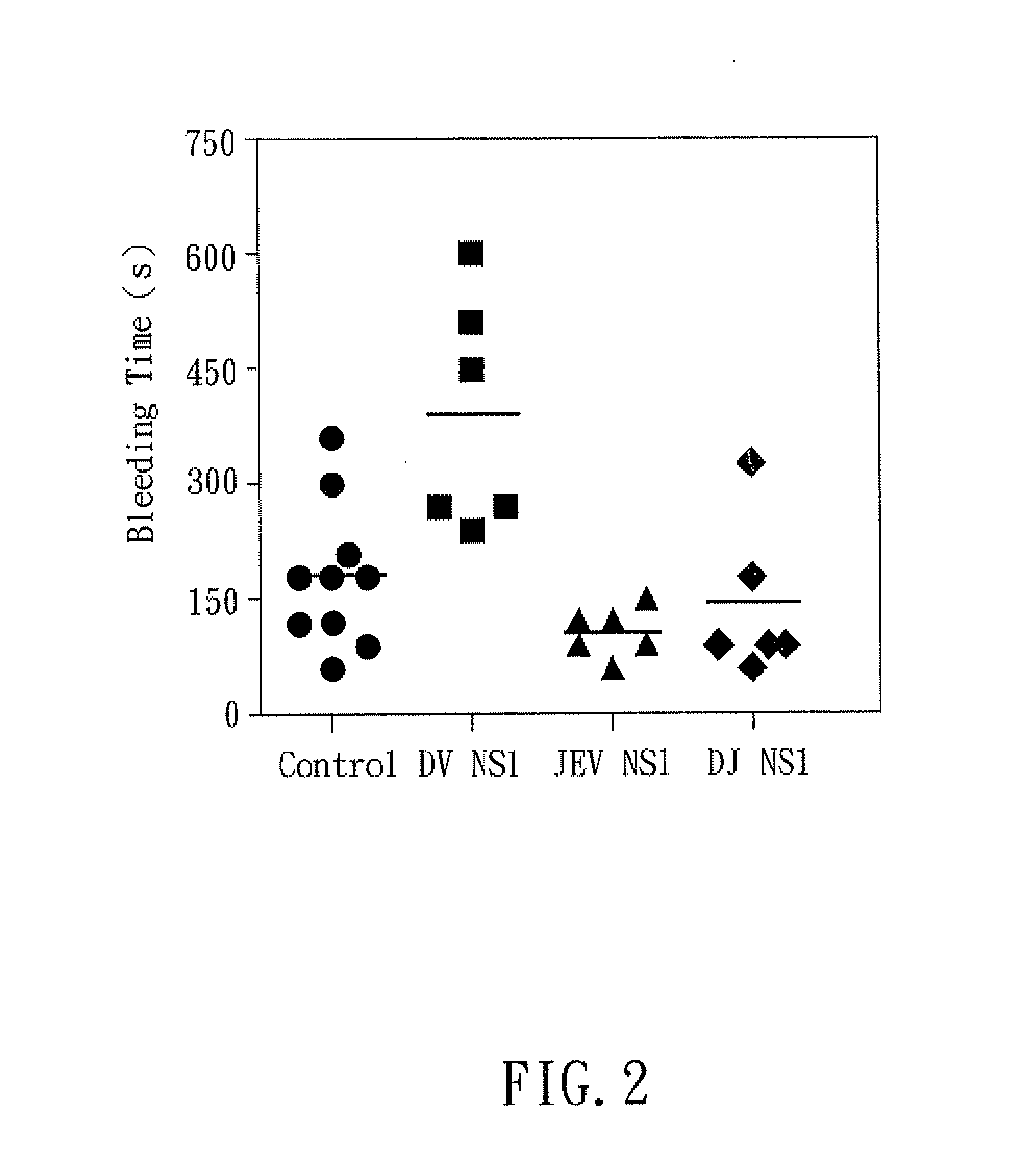
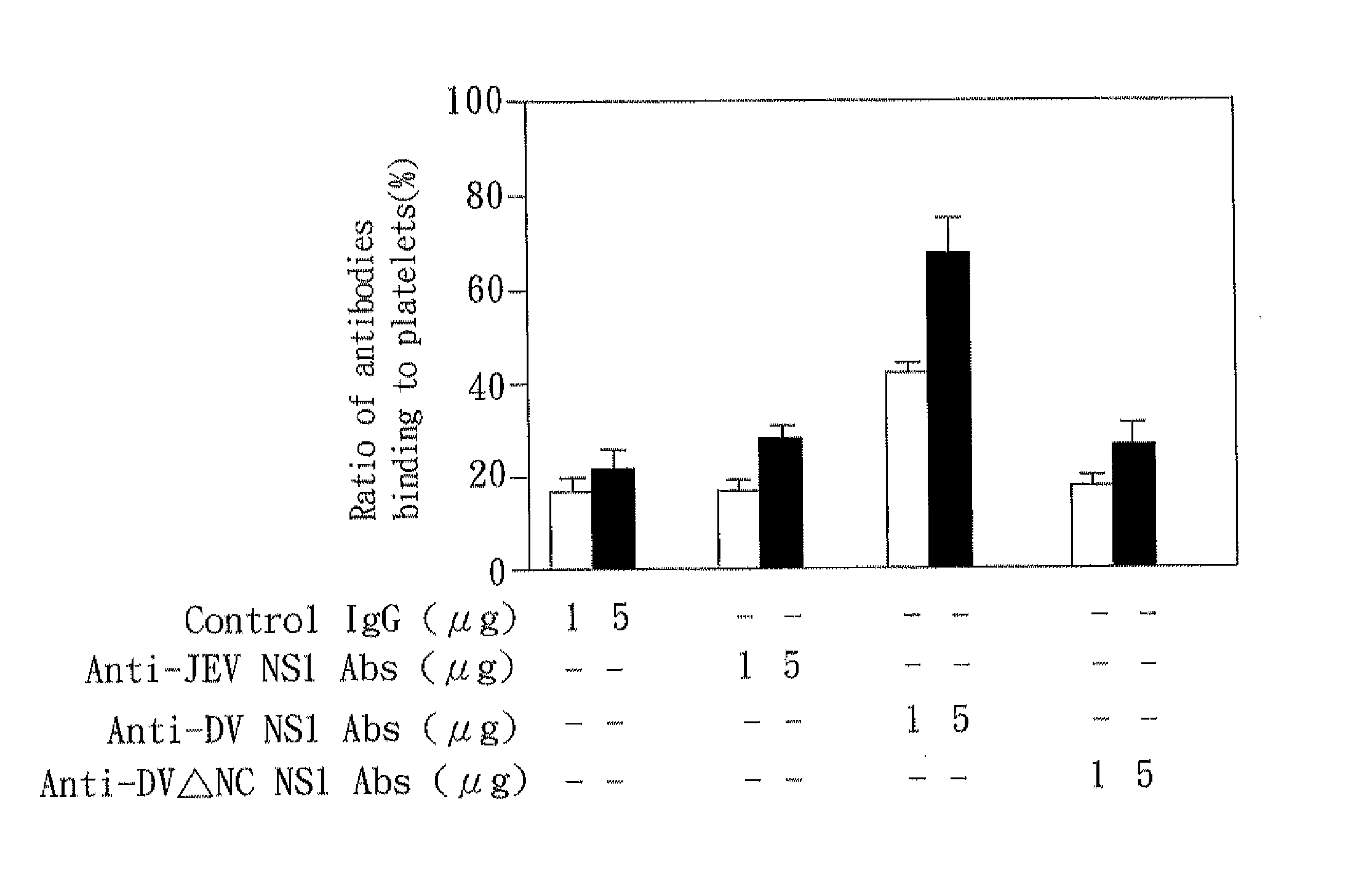
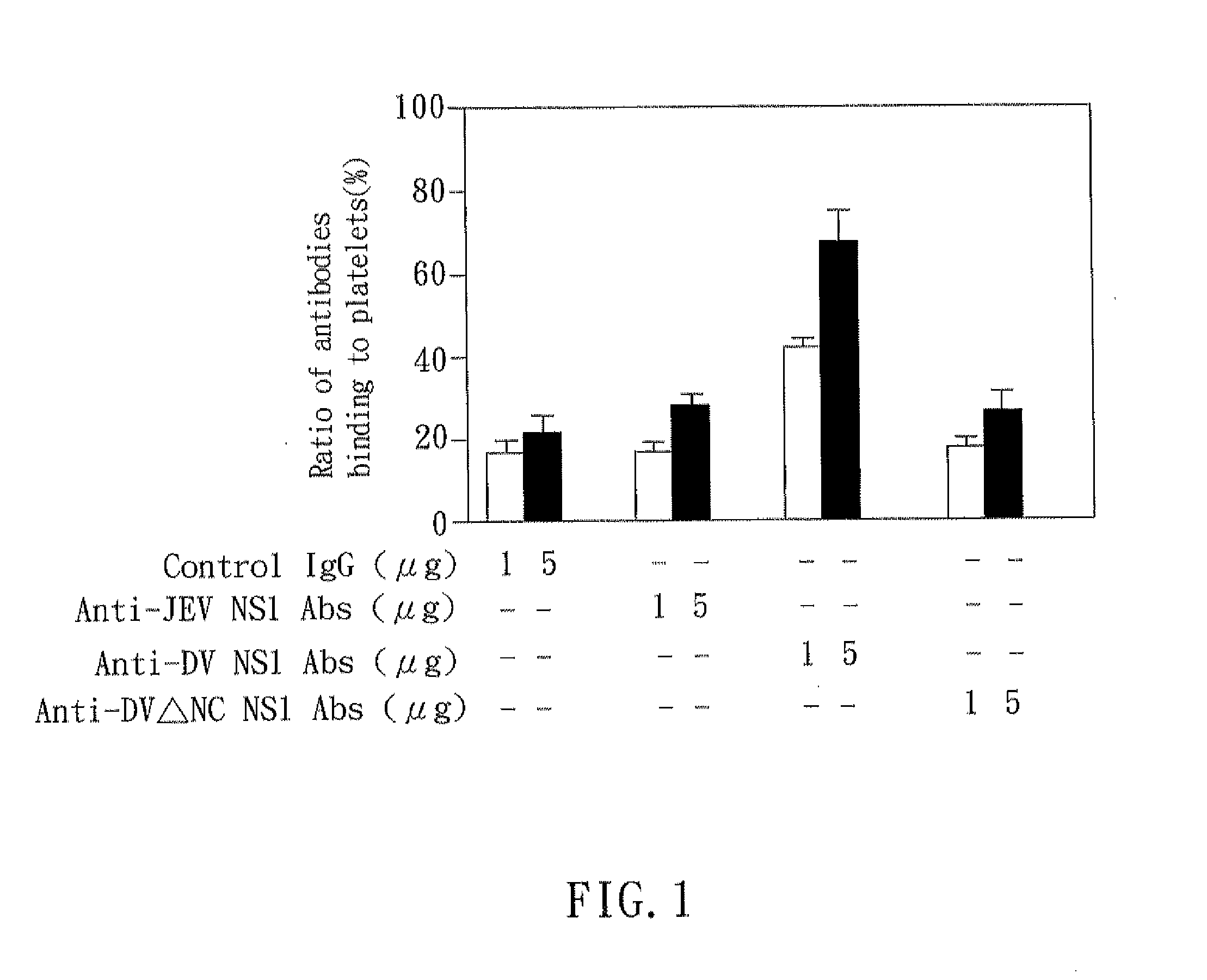
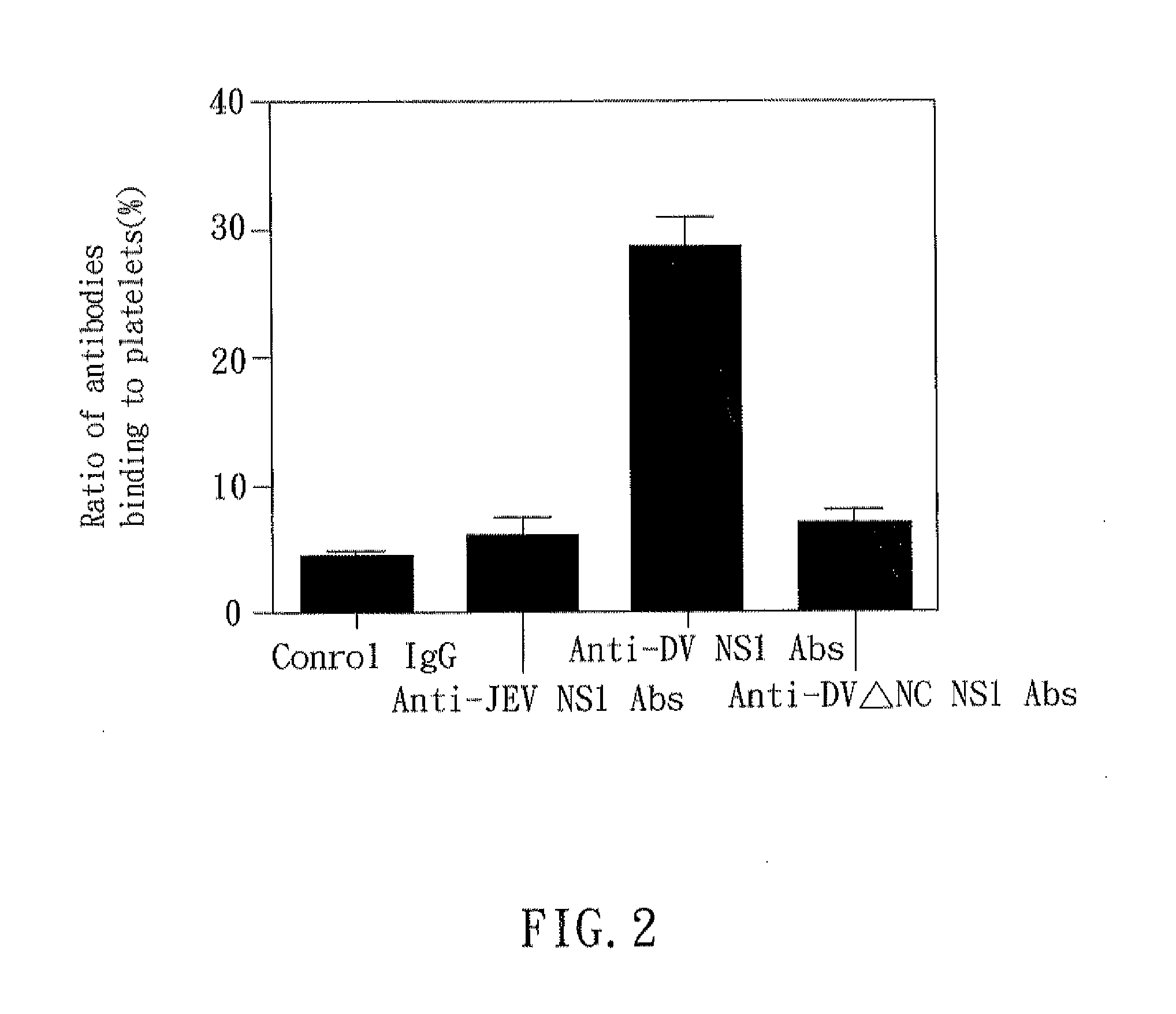
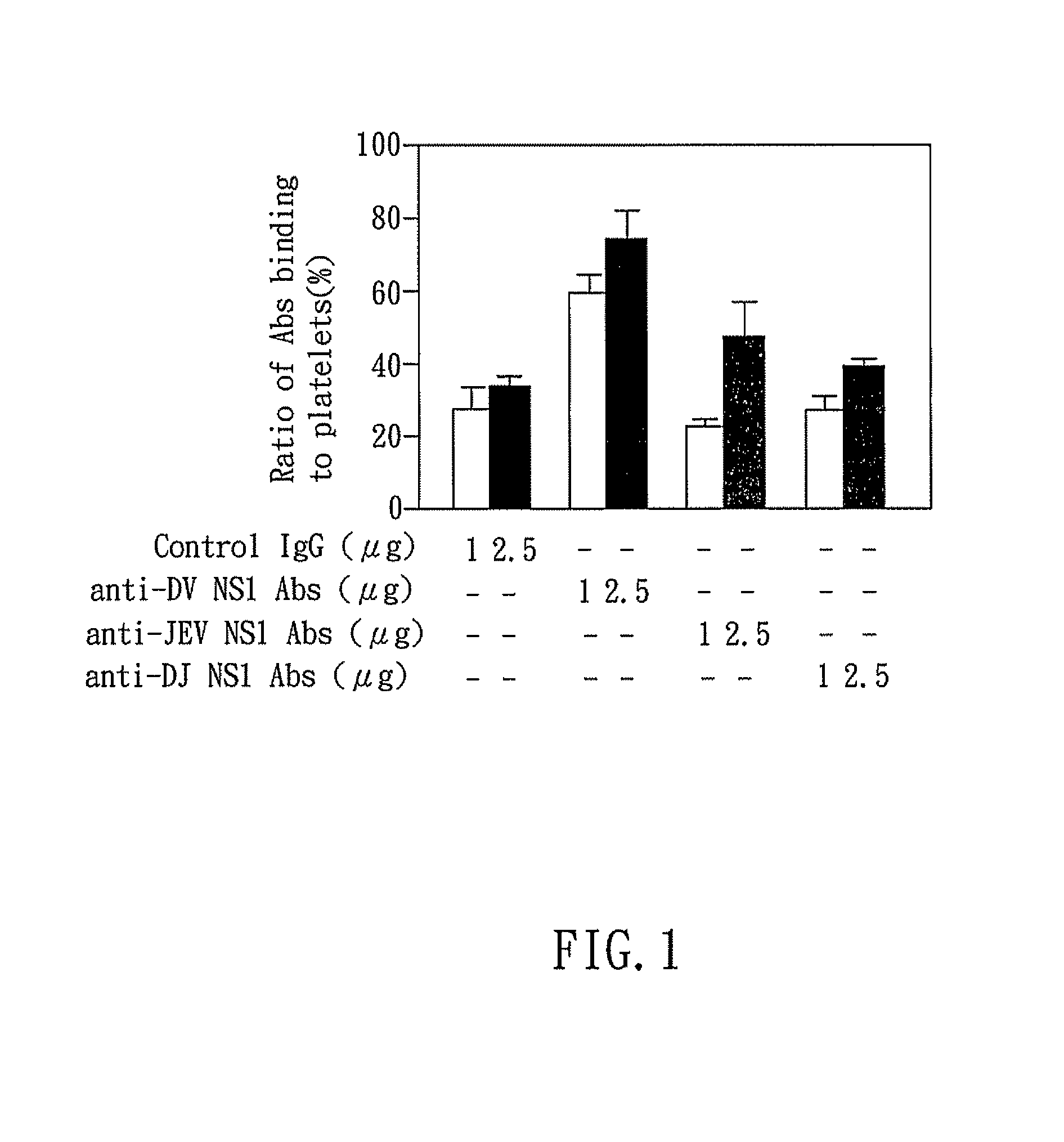
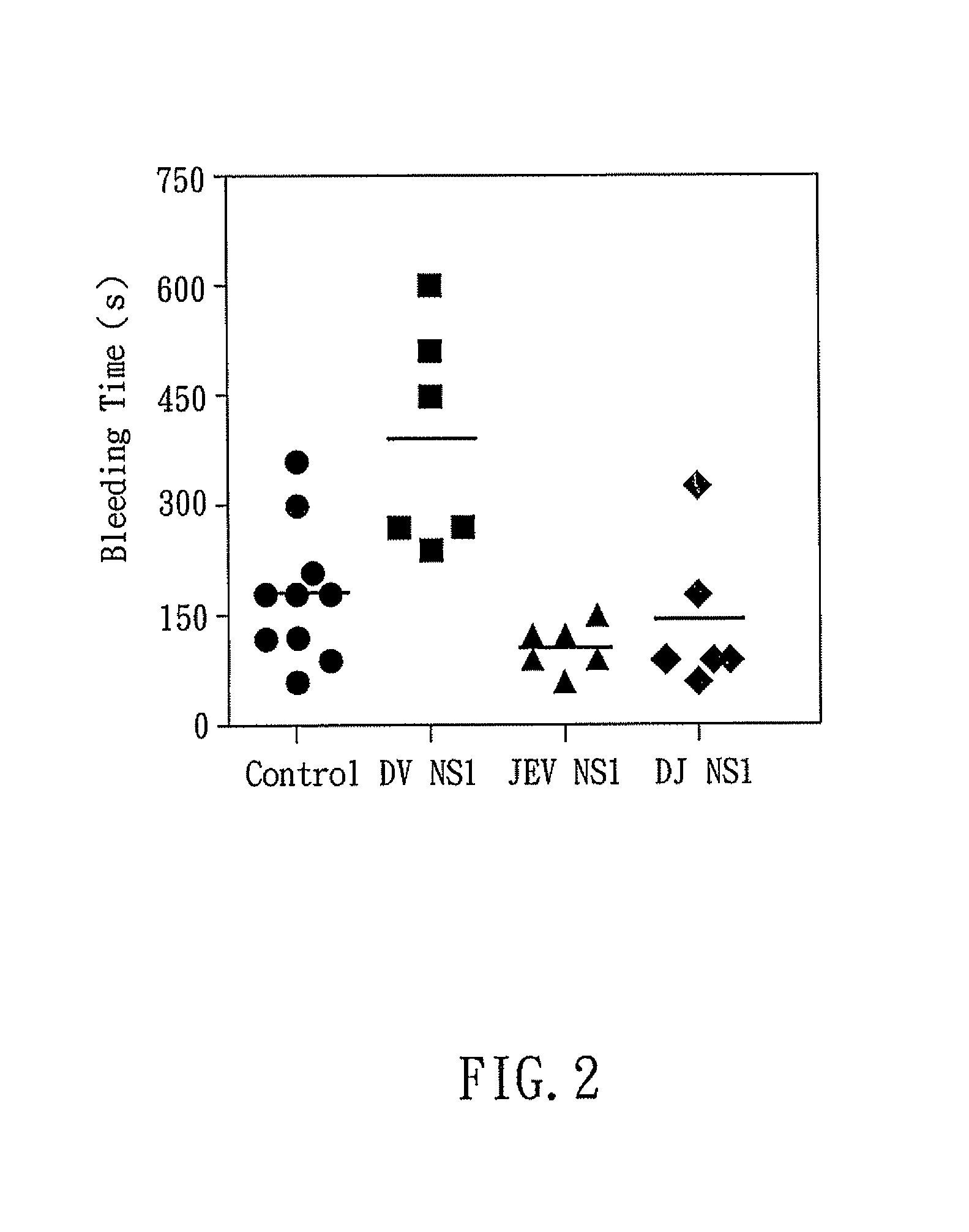
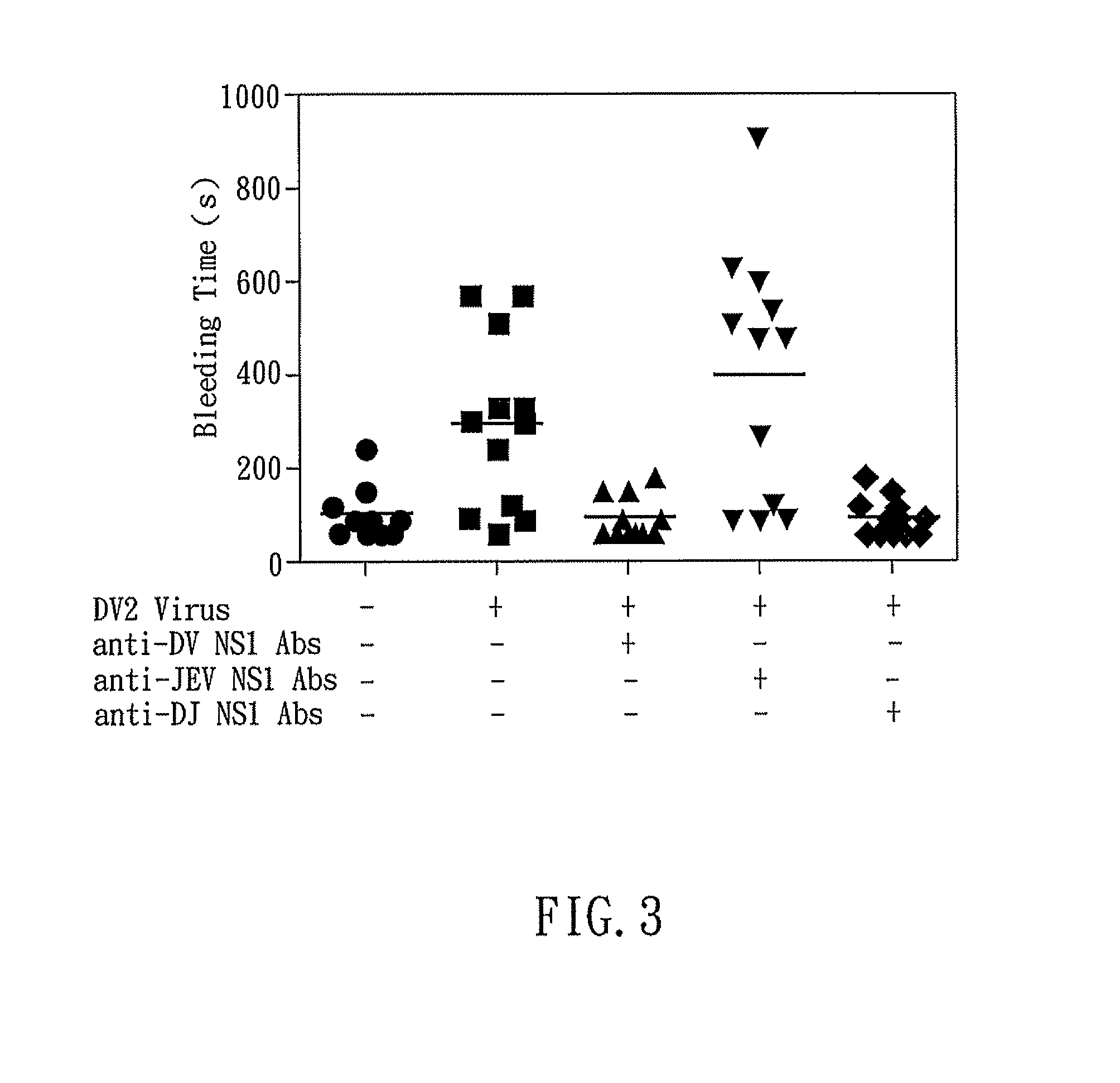
The present invention relates to a dengue vaccine, a pharmaceutical composition comprising the same, a nucleotide sequence, and an antibody composition. The dengue vaccine of the present invention includes chimeric nonstructural protein 1, which comprises N-terminus of DV NS1 from amino-acid residues 1 to 270 and C-terminus of JEV NS1 from amino-acid residues 271 to 352 (designated DJ NS1). Therefore, the dengue vaccine without autoimmunity according to the present invention is able to avoid cross-reactions with endothelial cells and platelets, and is able to shorten the bleeding time.
Owner:NAT CHENG KUNG UNIV
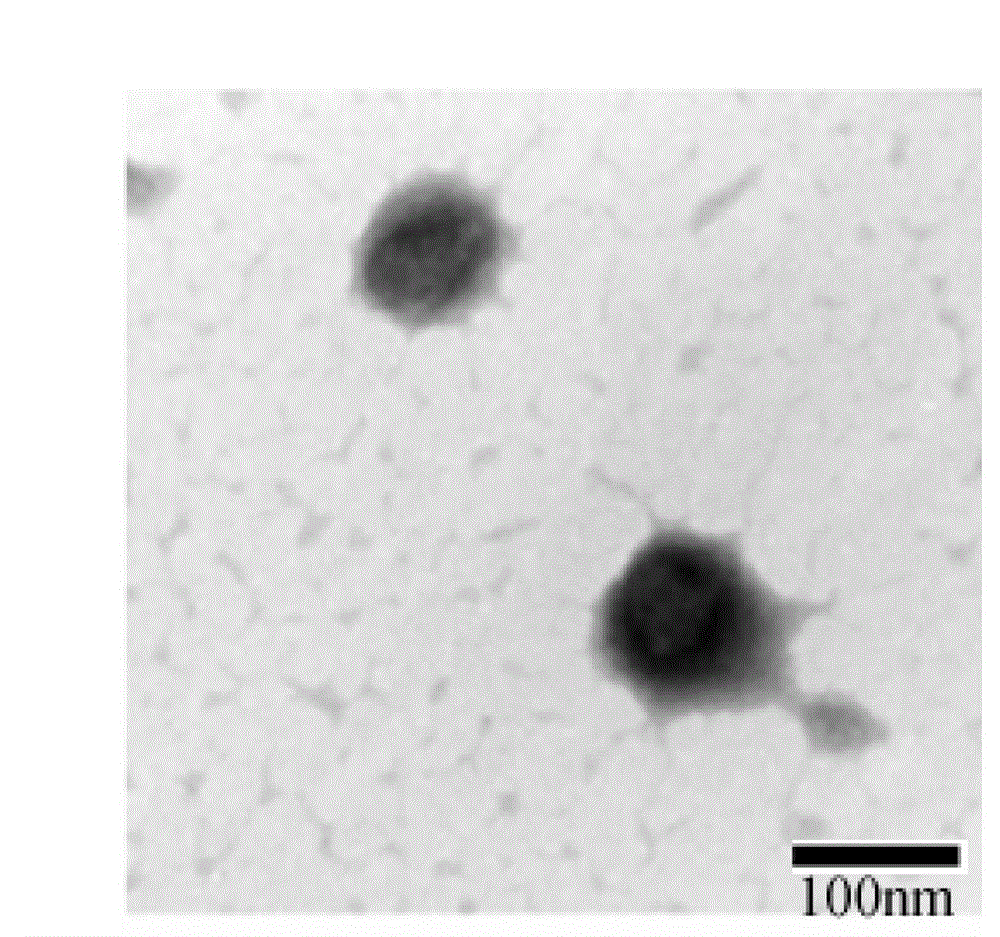
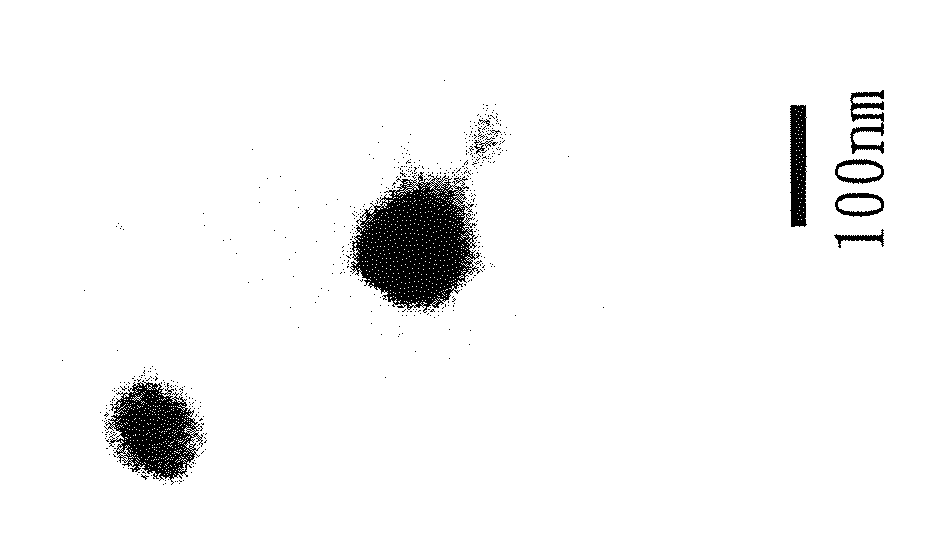
Biodegradable High-efficiency Dengue Vaccine, Method For Making The Same, And Pharmaceutical Composition Comprising The Same
InactiveCN103908662AReduce the number of timesReduce the cost of immunizationPowder deliverySsRNA viruses positive-senseAdjuvantVaccination
The present invention is related to a biodegradable high-efficiency dengue vaccine, a method for making the same, and a pharmaceutical composition comprising the same. The biodegradable high-efficiency dengue vaccine comprises a biodegradable nanocomplex with electric properties holding a dengue viral protein inside. An organism has antibody responses after vaccination with the biodegradable nanocomplex twice. Accordingly, in comparison with the Alum adjuvant and Ribi adjuvant used in the traditional dengue vaccine of the prior art, the vaccination times in the present invention is decreased to further reduce the vaccination cost, so the biodegradable high-efficiency dengue vaccine is good for being a commercial vaccine.
Owner:林以行
Biodegradable high-efficiency dengue vaccine, method for making the same, and pharmaceutical composition comprising the same
InactiveUS20140193446A1Enhance antibody productionEffective immune responsePowder deliverySsRNA viruses positive-senseBiological bodyAlum adjuvant
The present invention is related to a biodegradable high-efficiency dengue vaccine, a method for making the same, and a pharmaceutical composition comprising the same. The biodegradable high-efficiency dengue vaccine comprises a biodegradable nanocomplex with electric properties holding a dengue viral protein inside. An organism has antibody responses after vaccination with the biodegradable nanocomplex twice. Accordingly, in comparison with the Alum adjuvant and Ribi adjuvant used in the traditional dengue vaccine of the prior art, the vaccination times in the present invention is decreased to further reduce the vaccination cost, so the biodegradable high-efficiency dengue vaccine is good for being a commercial vaccine.
Owner:NAT CHENG KUNG UNIV
Chimeric virus, and preparation method and application thereof
ActiveCN103205460AHigh titerImproving immunogenicityViral antigen ingredientsMicroorganism based processesFull length cdnaSerotype
The invention discloses an infectious Japanese encephalitis virus clone which is a recombinant vector including full-length cDNA of genome of a Japanese encephalitis virus attenuated strain SA14-14-2. The invention further discloses a chimeric virus and application thereof and a bacterial artificial chromosome as represented by SEQ ID No. 1. The chimeric virus provided by the invention can grow and proliferate on a plurality of cells used for production of human vaccines and show similar growth characteristics, is applicable to production of four serotype Dengue vaccines and has the advantages of strong immunogenicity, high antibody titer, good application prospects and a high industrial application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD +1
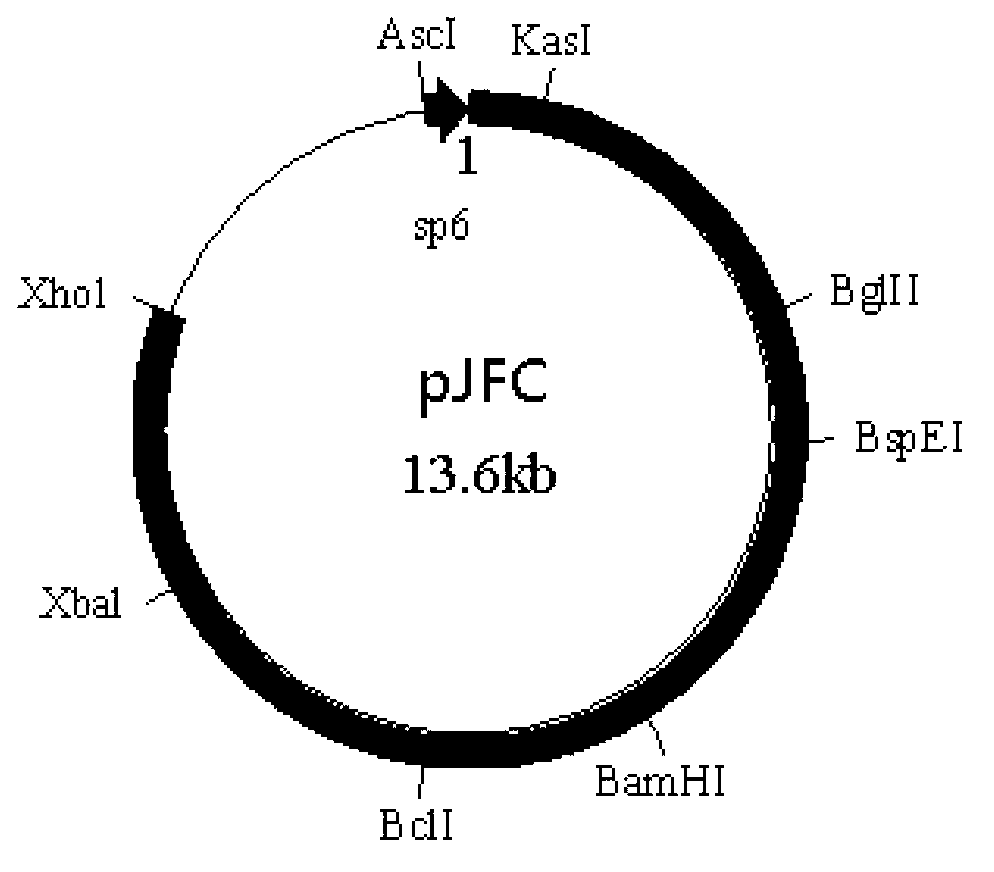
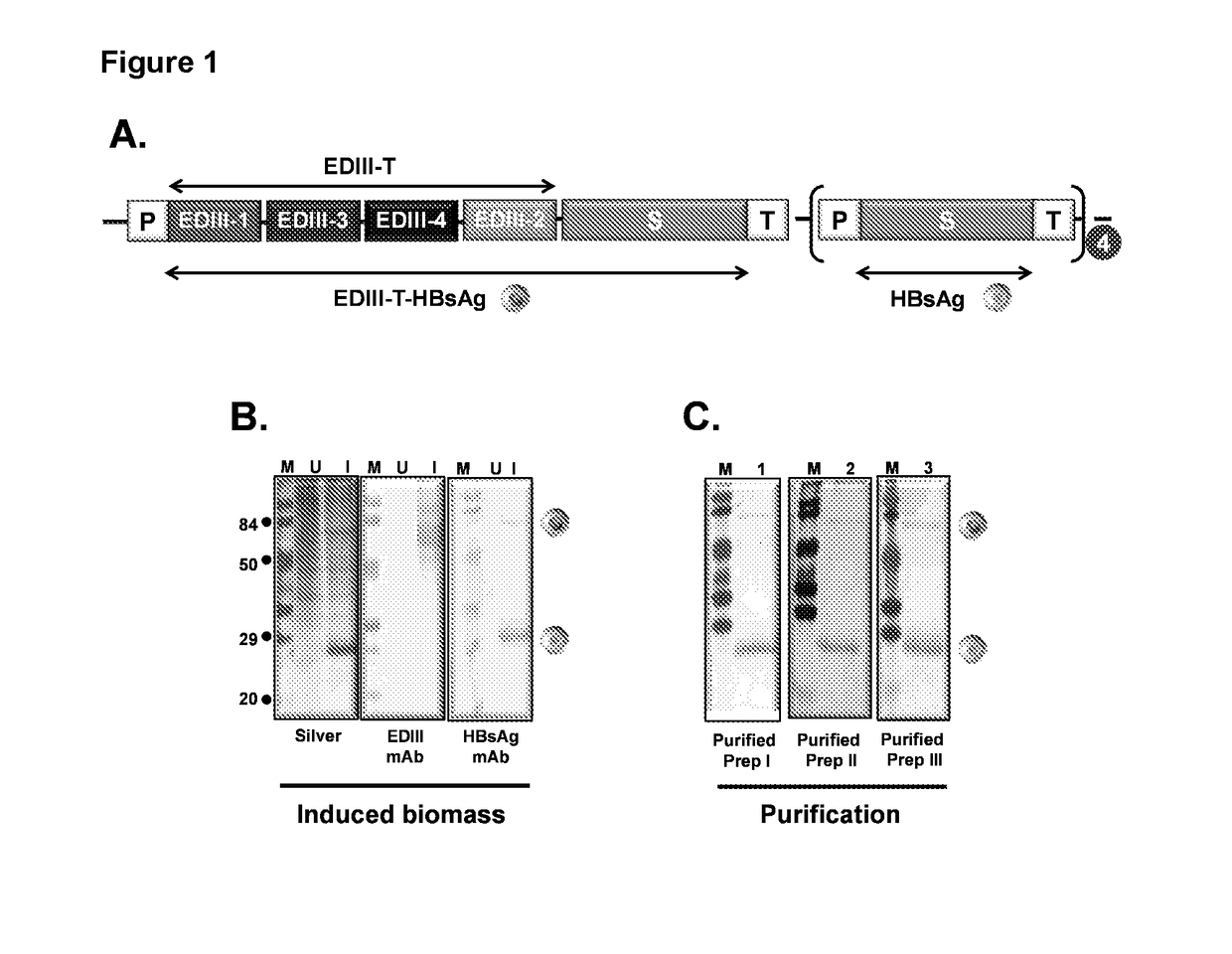
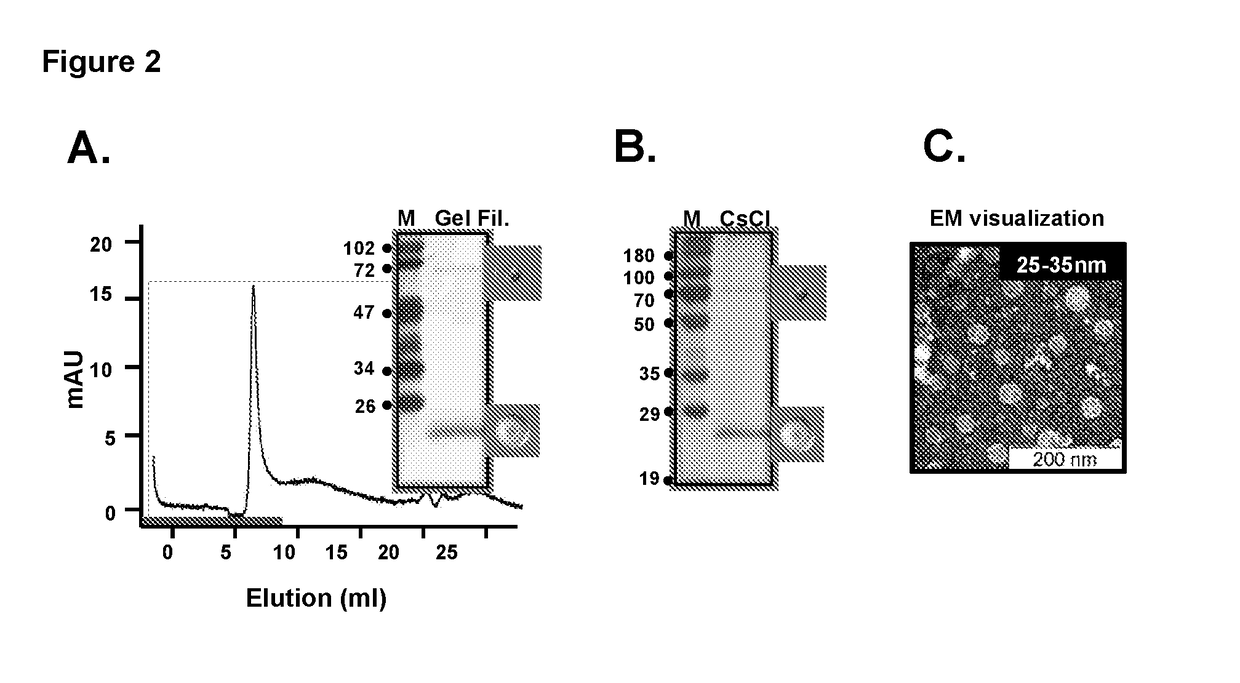
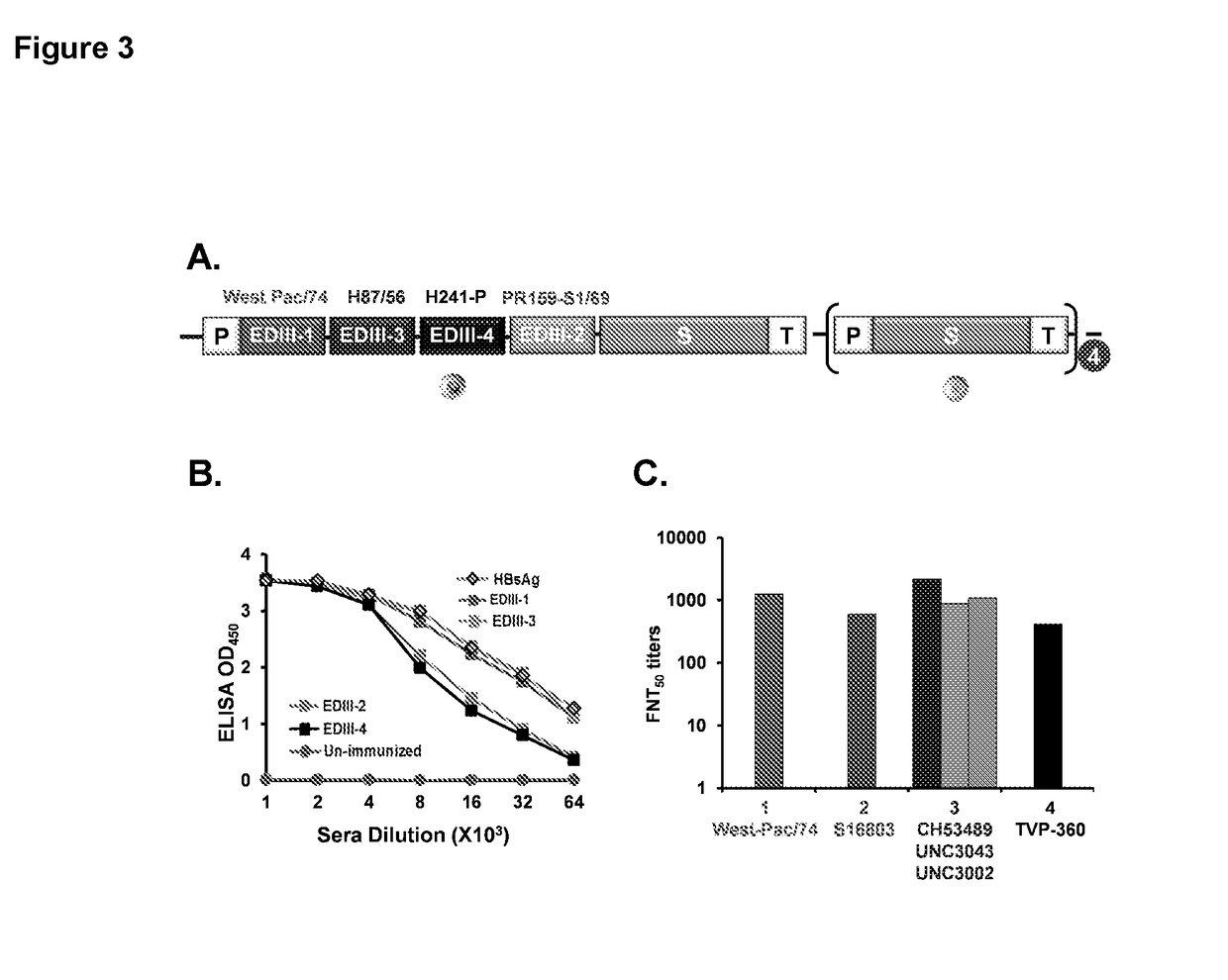
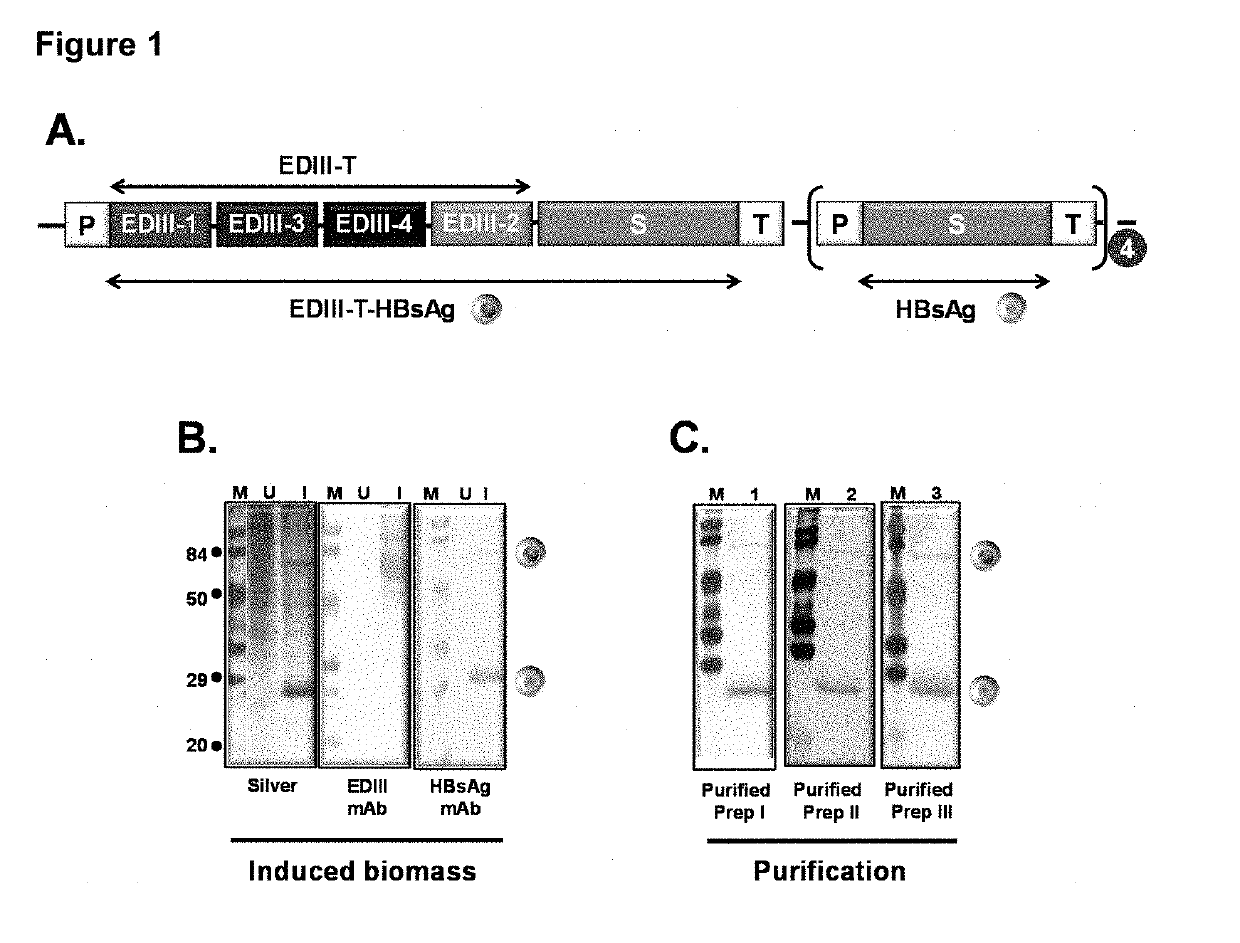
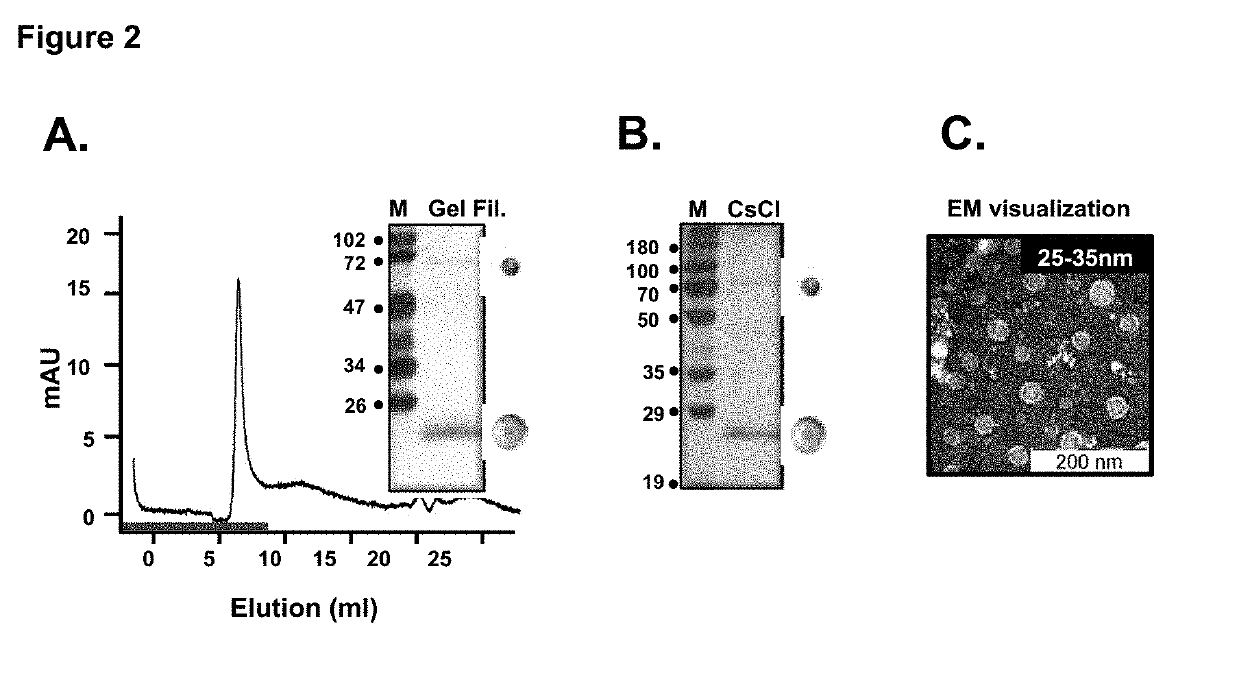
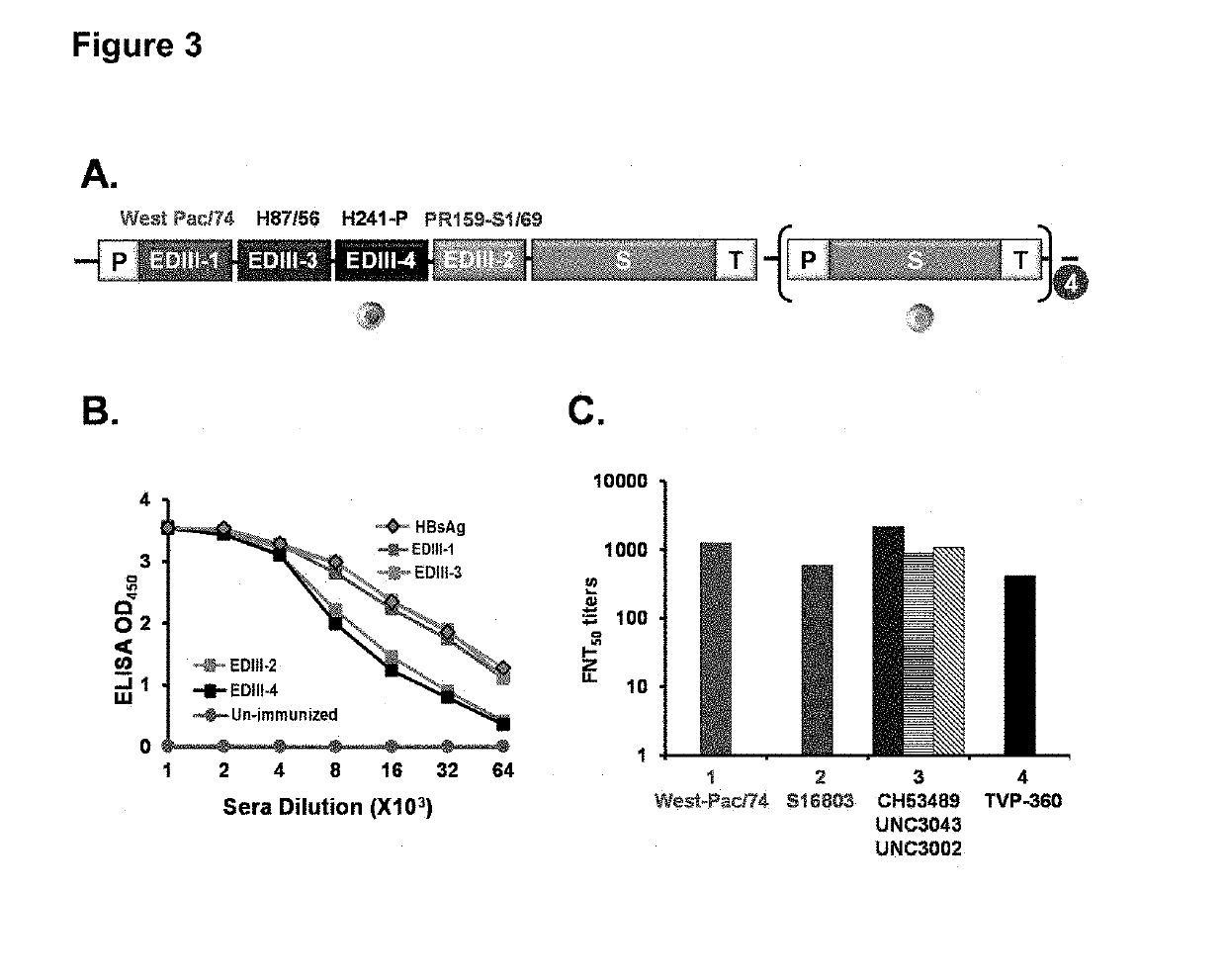
Tetravalent dengue vaccine
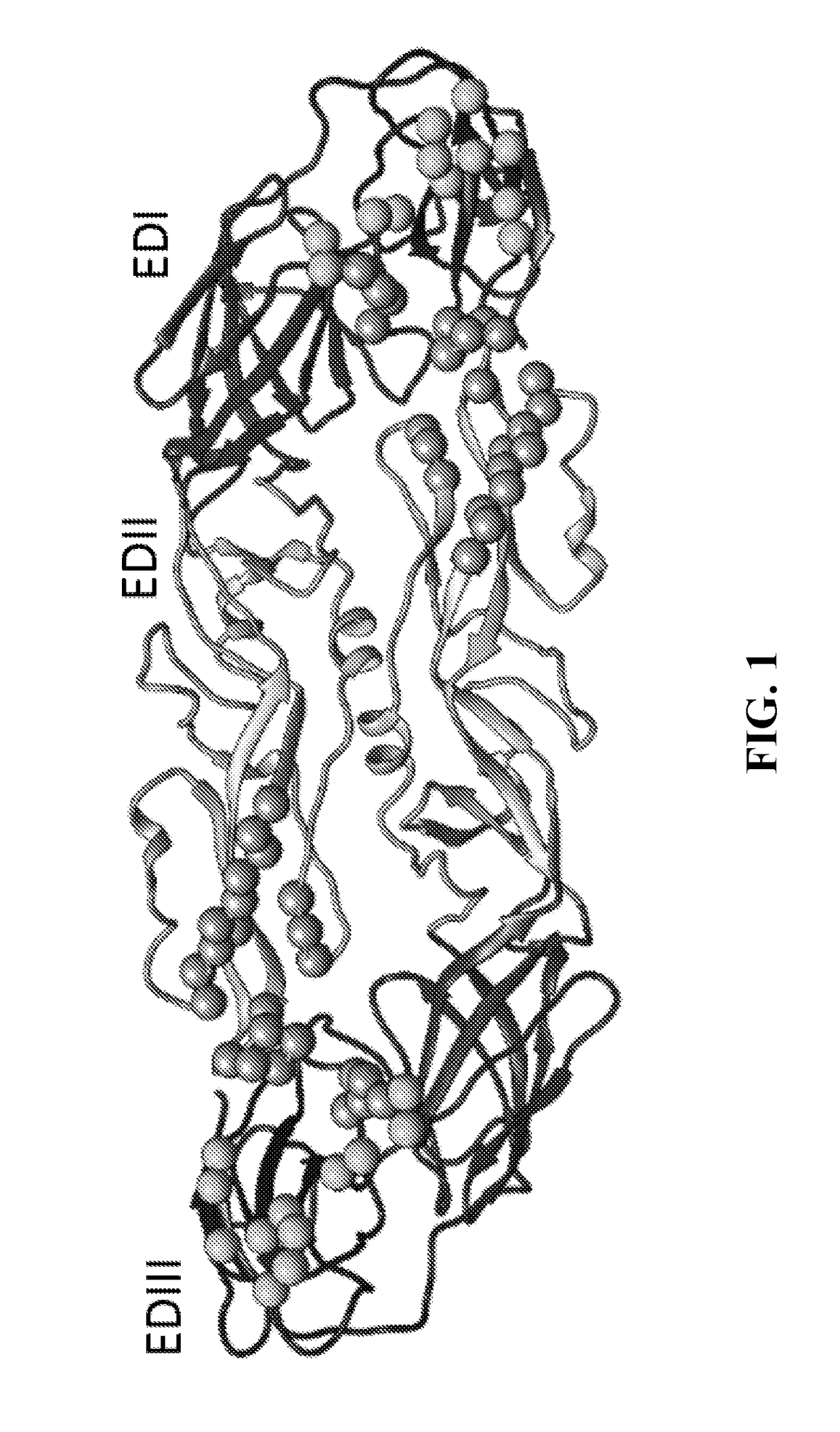
The invention provides a recombinant polypeptide comprising the EDIII domain of each of Dengue virus serotype DENV-1, DENV-2, DENV-3, and DENV-4 linked to the N-terminal of HBsAg.
Owner:INT CENT FOR GENETIC ENG & BIOTECH
Dengue vaccine, pharmaceutical composition comprising the same, and nucleotide sequence
InactiveUS20120065373A1Shorten bleeding timeDepleted of cross-reactivitySsRNA viruses positive-senseSugar derivativesNucleotideGenetics
The present invention relates to a dengue vaccine, a pharmaceutical composition including the same, and a nucleotide sequence. The dengue vaccine includes N and C termini-deleted nonstructural protein ΔNC NS1 with a peptide fragment from amino acids 36 to 270, which is derived from dengue virus nonstructural protein 1 (DV NS1) with deletions of N-terminal region from amino acids 1 to 35 and C-terminal region from amino acids 271 to 352. The dengue vaccine of the present invention is depleted of cross-reactivity with endothelial cells and platelets, and can shorten the bleeding time.
Owner:NAT CHENG KUNG UNIV
Methods and compositions for dengue virus vaccines and diagnostics
ActiveUS20180280494A1Avoid infectionGood effectSsRNA viruses positive-senseViral antigen ingredientsDengue vaccineImmunotherapy
The present invention provides compositions directed to recombinant flavivirus E glycoprotein ectodomain dimers for use in diagnostic and immunotherapeutic methods.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
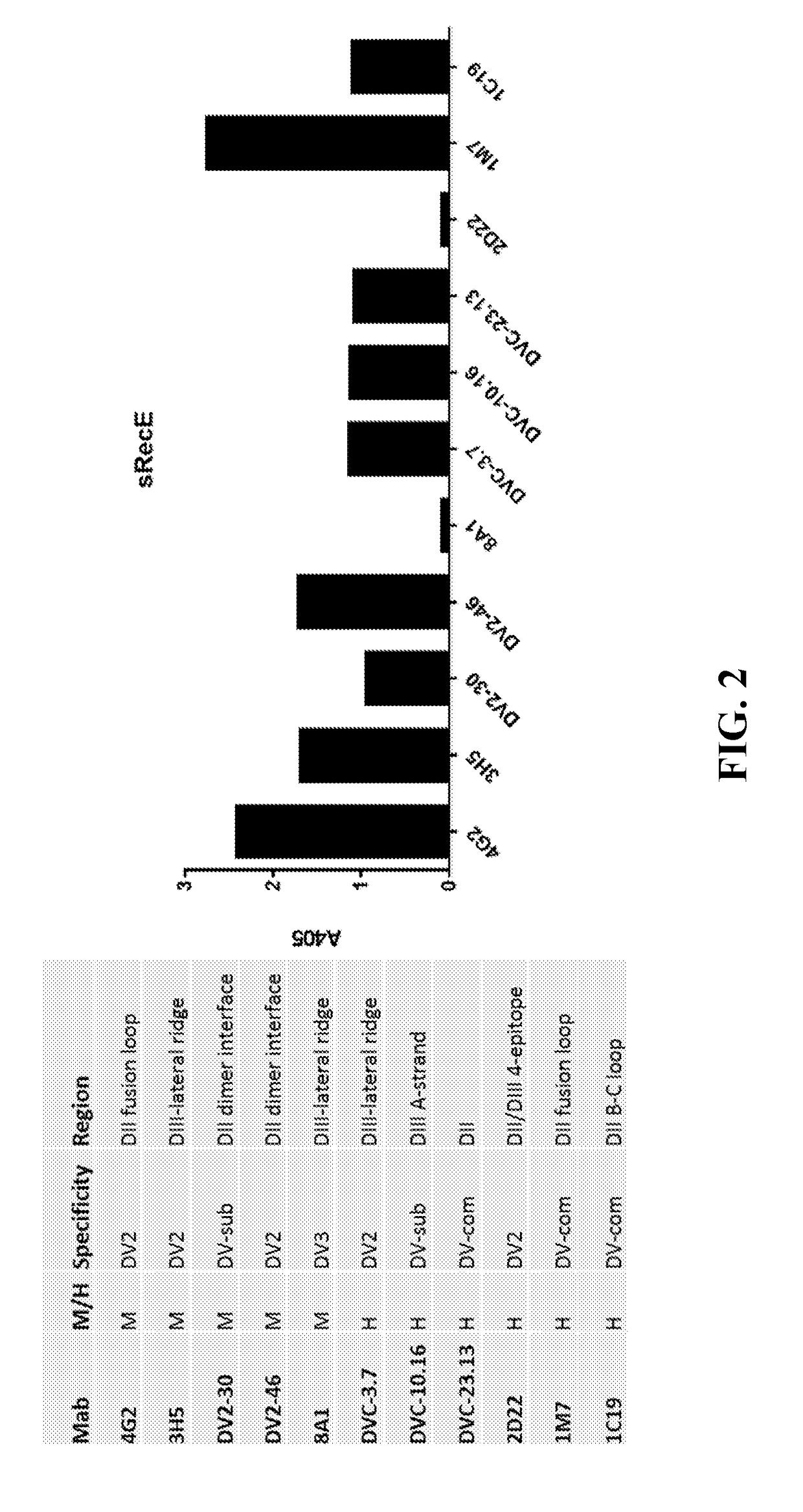
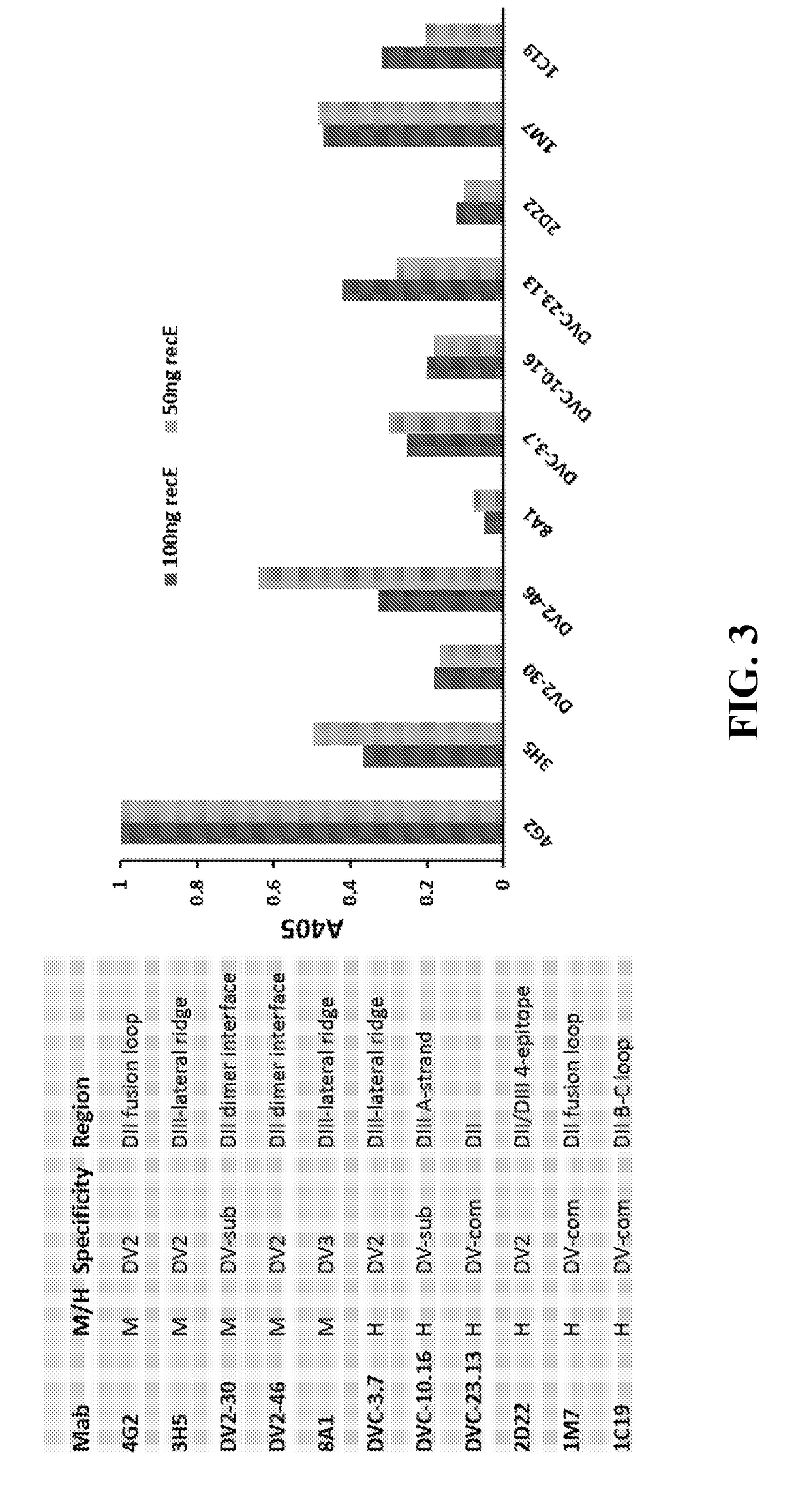
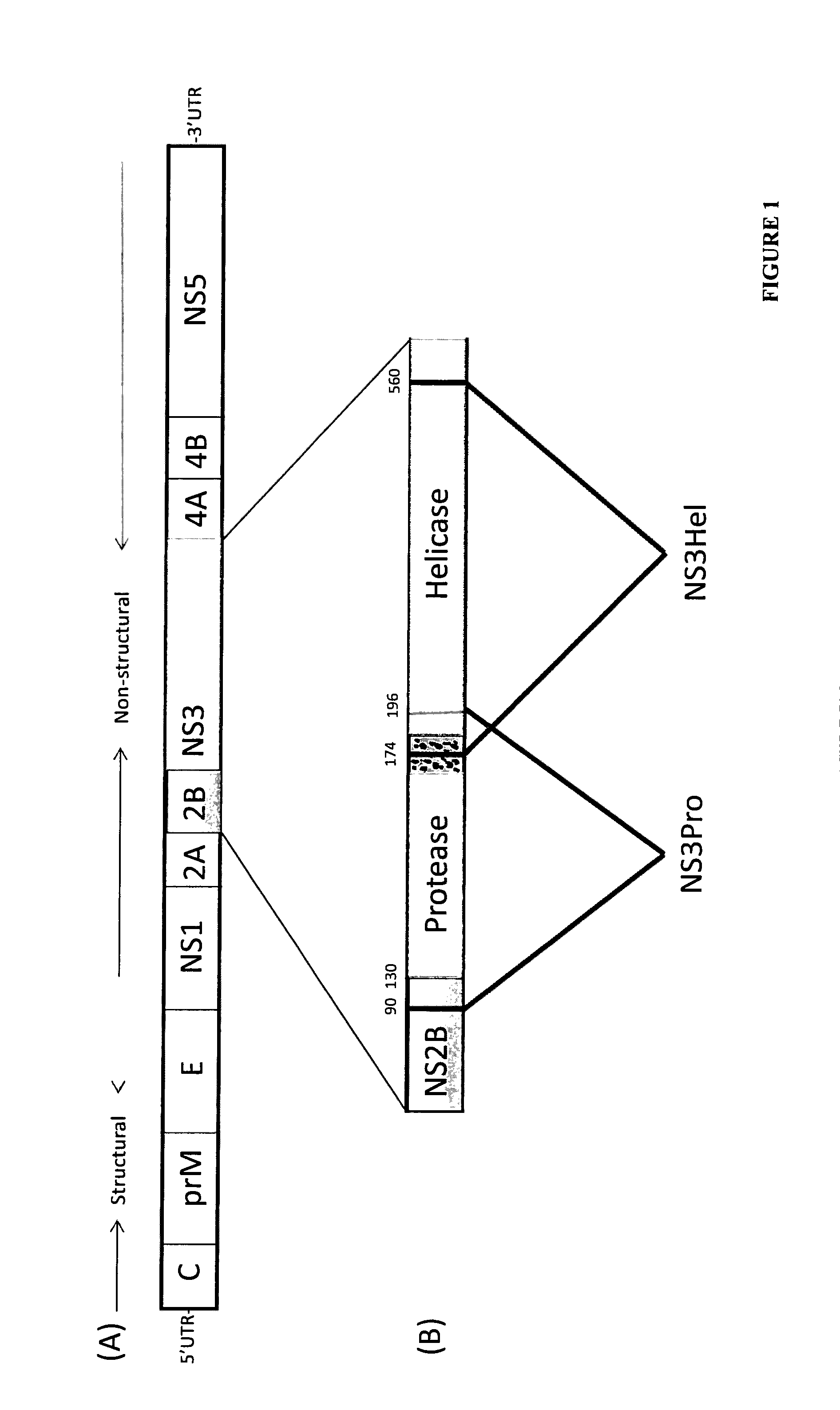
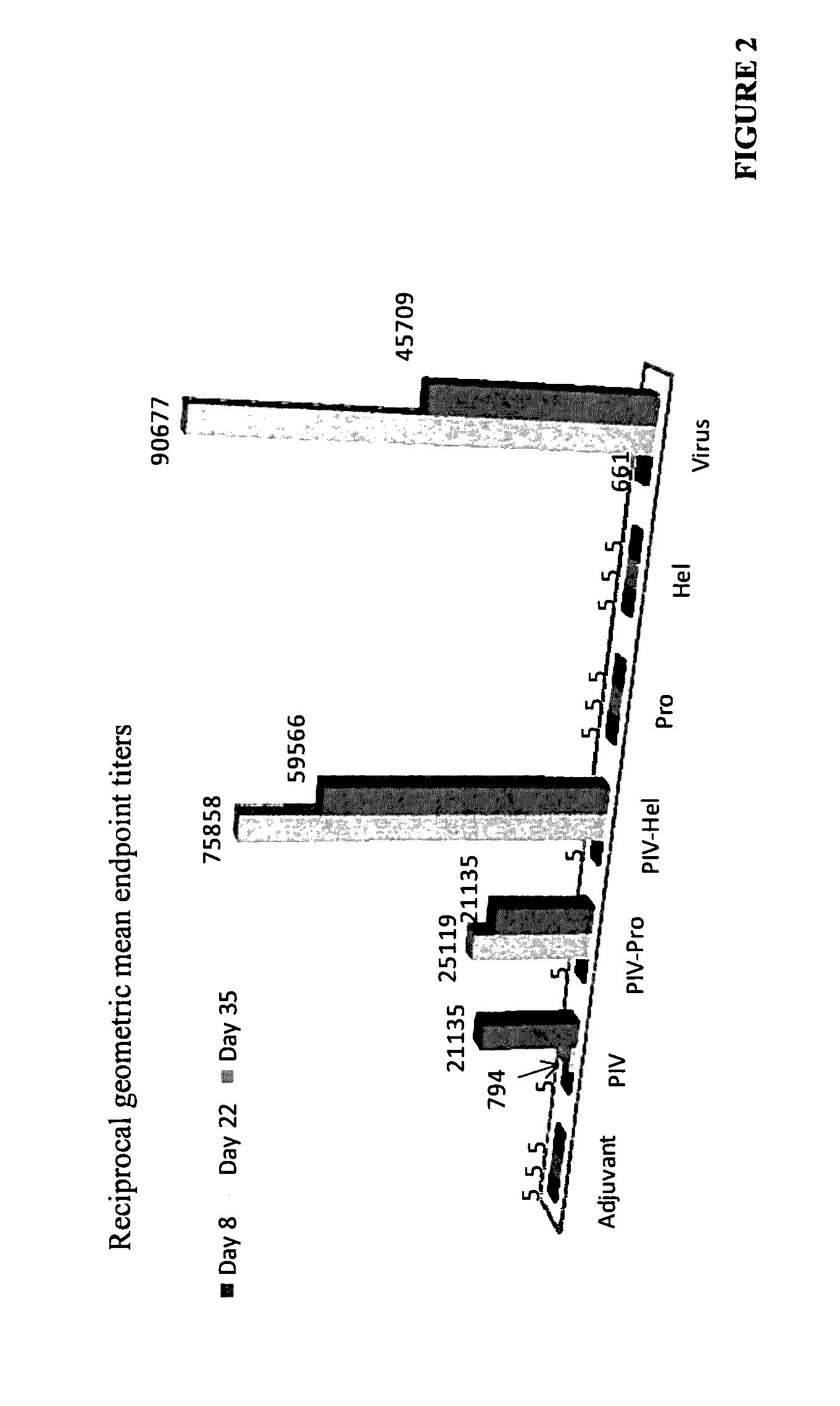
Immune Enhancing Recombinant Dengue Protein
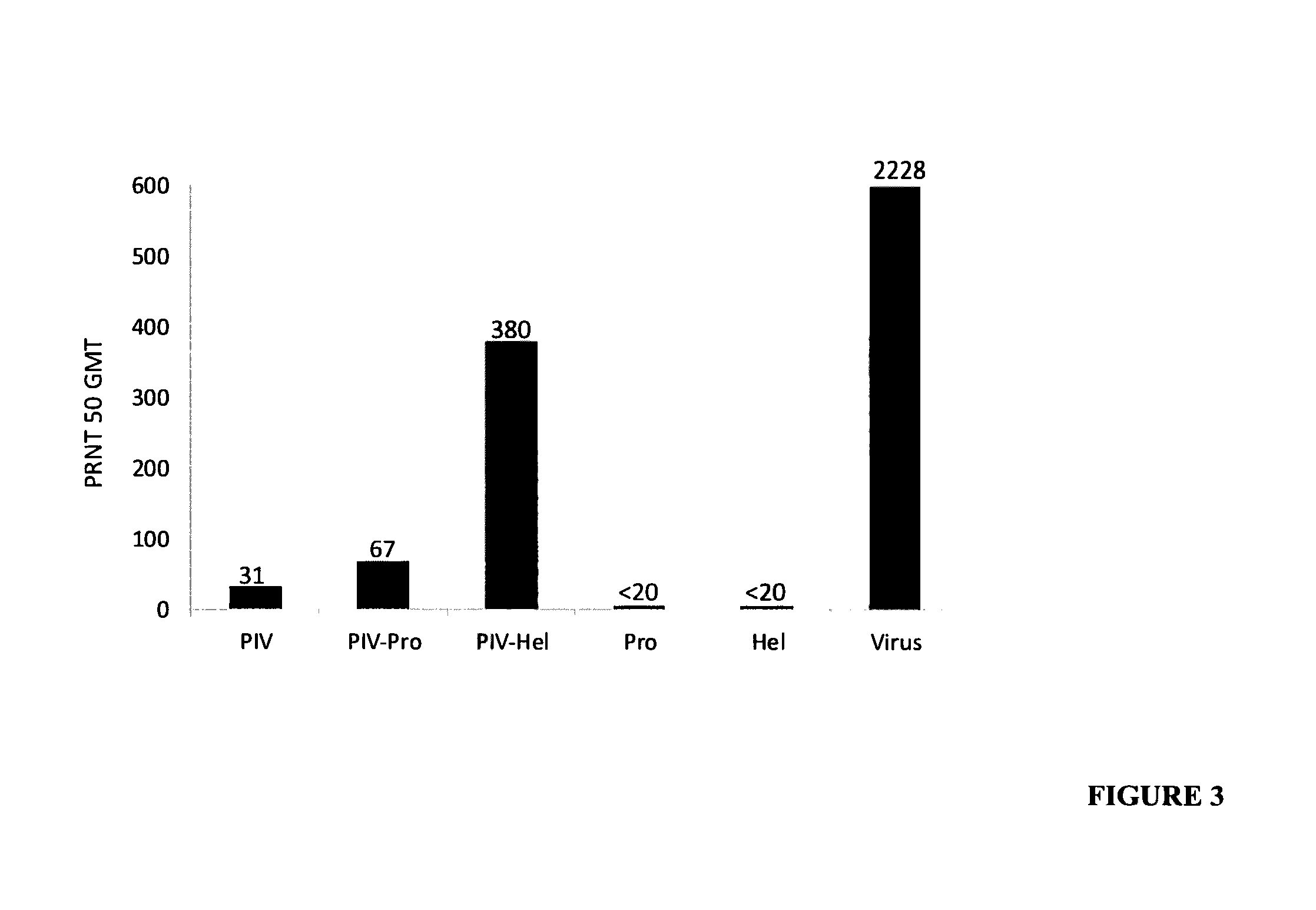
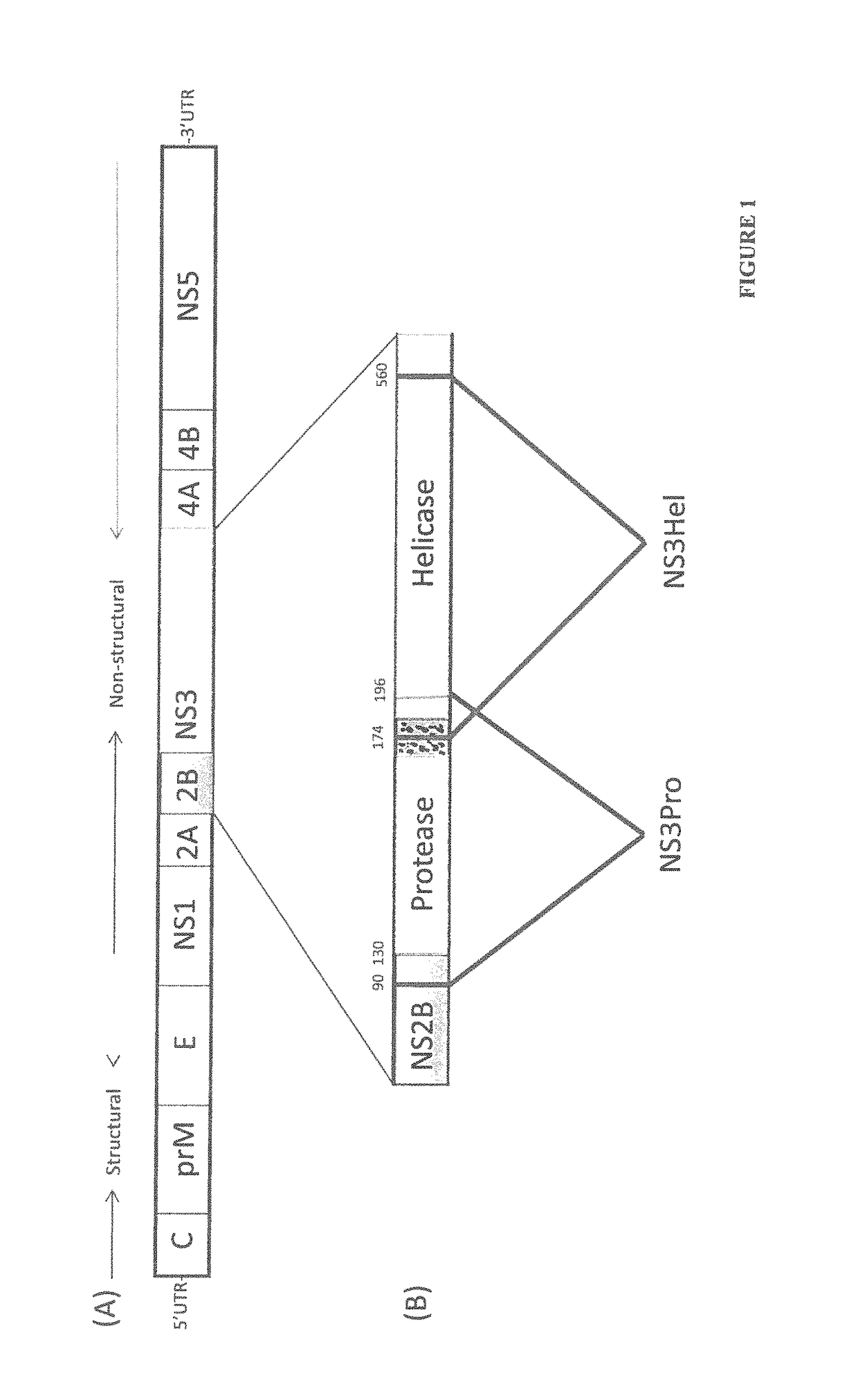
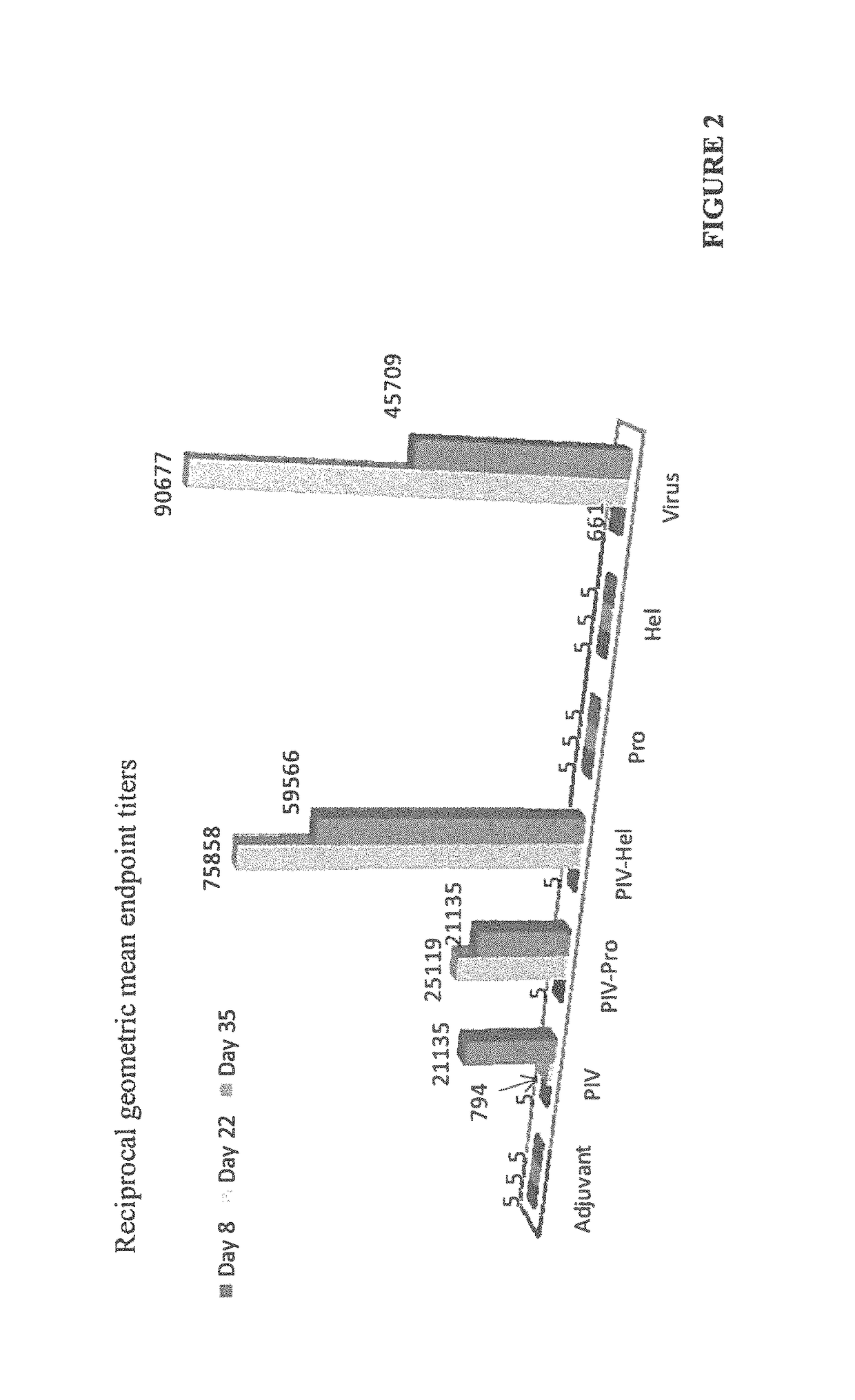
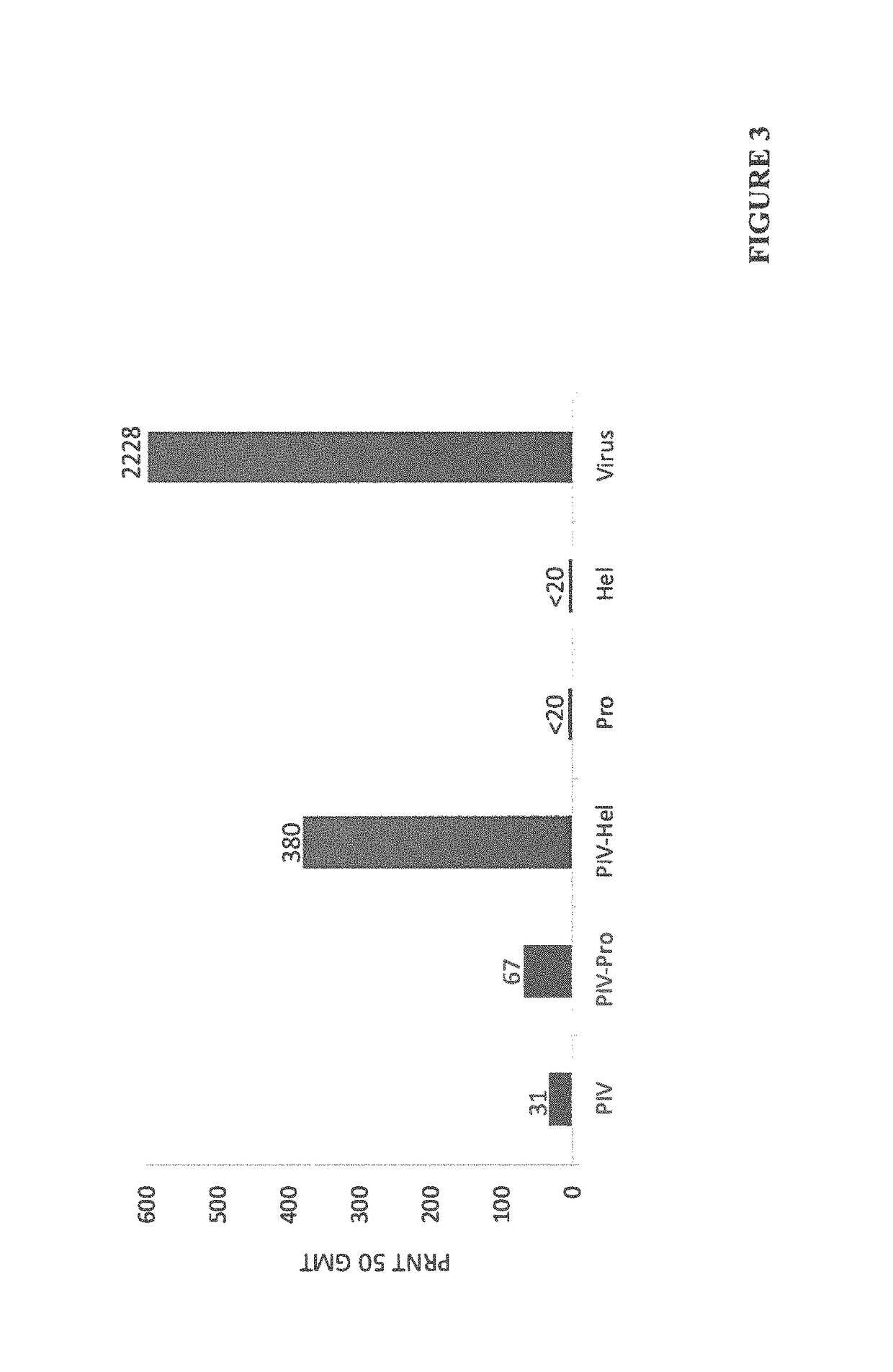
The invention relates to a method for preventing, ameliorating or treating disease caused by dengue virus in a subject in need thereof comprising administering to the subject a dengue vaccine formulation in combination with a NS3 helicase polypeptide and / or fragment(s) thereof, wherein said method comprises stimulating humoral as well as cell-mediated immunity to the dengue virus in the subject.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Immune enhancing recombinant dengue protein
The invention relates to a method for preventing, ameliorating or treating disease caused by dengue virus in a subject in need thereof comprising administering to the subject a dengue vaccine formulation in combination with a NS3 helicase polypeptide and / or fragment(s) thereof, wherein said method comprises stimulating humoral as well as cell-mediated immunity to the dengue virus in the subject.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Stable live attenuated recombinant dengue vaccine
The present invention relates to field of live attenuated recombinant tetravalent dengue vaccines and methods of producing stable compositions. Present invention specifically relates to a stable composition and methods of using such a stable composition comprising live attenuated recombinant dengue virus, stabilizer, bulking agent and optionally buffering agents, wherein the live attenuated dengue virus is generated from Δ 30 and or Δ 31 deleted or mutated dengue strains. More specifically, the present invention relates to stabilizers for freeze-dried live attenuated immunogenic and / or vaccine compositions that may comprise, inter alia, dengue virus(es). The invention further relates to stabilized, freeze-dried live attenuated immunogenic and / or vaccine compositions of, dengue virus(es), which may contain these stabilizers. Other aspects of the invention are described in or are evident from the following disclosure, and are within the ambit of the invention.
Owner:PANACEA BIOTEC
Method for evaluating immune effect of dengue vaccine
Owner:ARMY MEDICAL UNIV
Concomitant Dengue and Yellow Fever Vaccination
ActiveUS20180193447A1SsRNA viruses positive-senseViral antigen ingredientsYellow fever vaccinationYellow fever immunisation
The present invention relates to a yellow fever (YF) vaccine for use in a method for inducing a protective immune response against yellow fever, wherein said method comprises concomitantly administering said yellow fever vaccine to a human subject together with a tetravalent dengue vaccine which comprises a live attenuated dengue virus of each of serotypes 1 to 4. This invention also pertains to a tetravalent dengue vaccine which comprises a live attenuated dengue virus of each of serotypes 1 to 4 for use in a method of inducing a protective immune response against yellow fever, wherein said method comprises concomitantly administering said tetravalent dengue vaccine to a human subject together with a yellow fever (YF) vaccine.
Owner:SANOFI PASTEUR SA
Composition of subunit dengue vaccine
PendingCN113876939AFeatures are obvious and easy to understandSsRNA viruses positive-senseViral antigen ingredientsDengue vaccineTGE VACCINE
The present invention relates to a composition of a subunit dengue vaccine comprising a fusion protein of conjugating or connecting delta C nonstructural protein 1 (NS1[delta]C or truncated NS1[delta]C) to at least one polypeptides of NS3c (or truncated NS3c) and / or consensus envelope protein domain III (cEDIII), thereby enhancing better protection against DENV challenge and alleviating associated pathological effects.
Owner:林以行
Attenuated dengue virus vaccine containing adaptive mutation from mrc-5 cells
InactiveUS20120269853A1SsRNA viruses positive-senseViral antigen ingredientsWild typeSite-directed mutagenesis
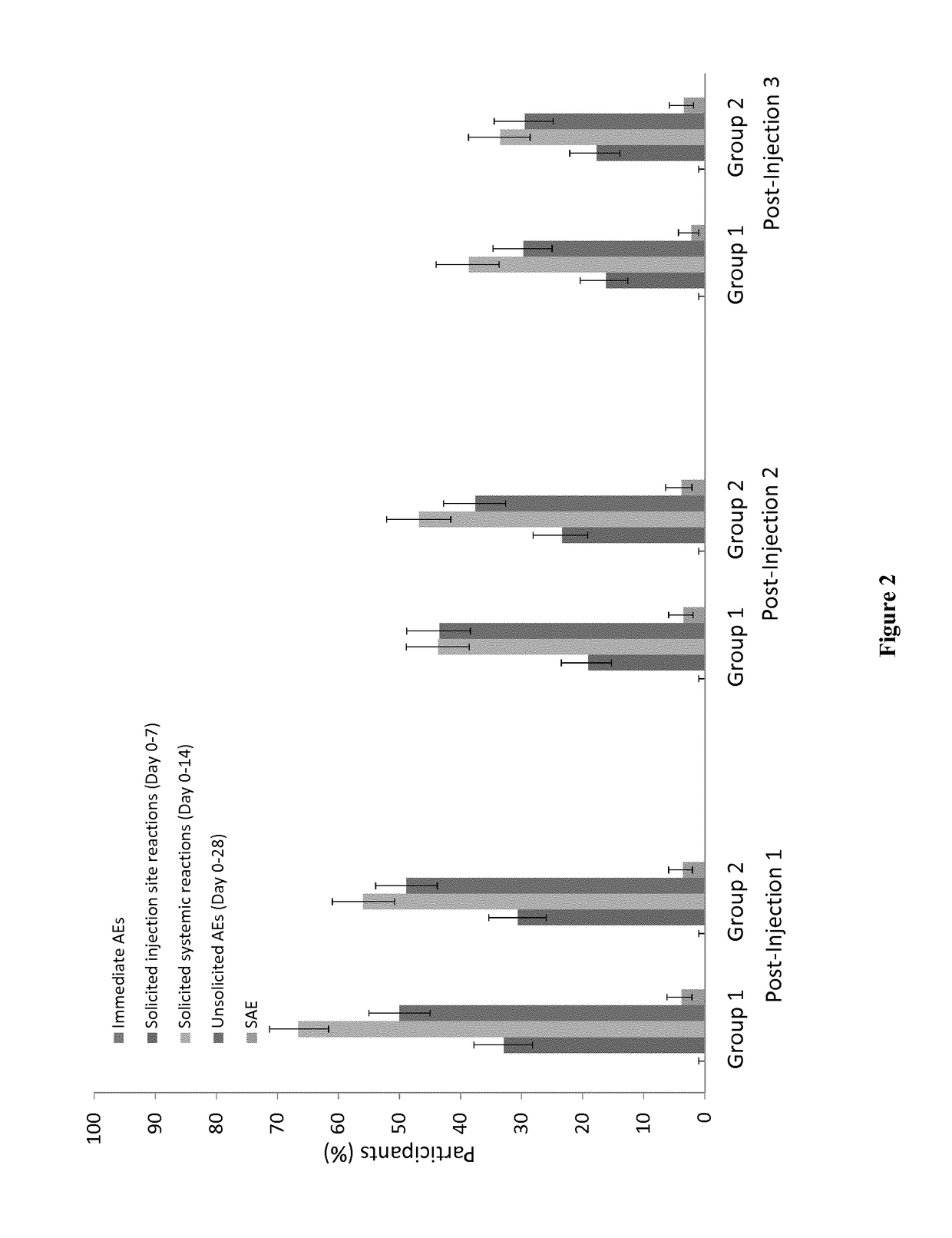
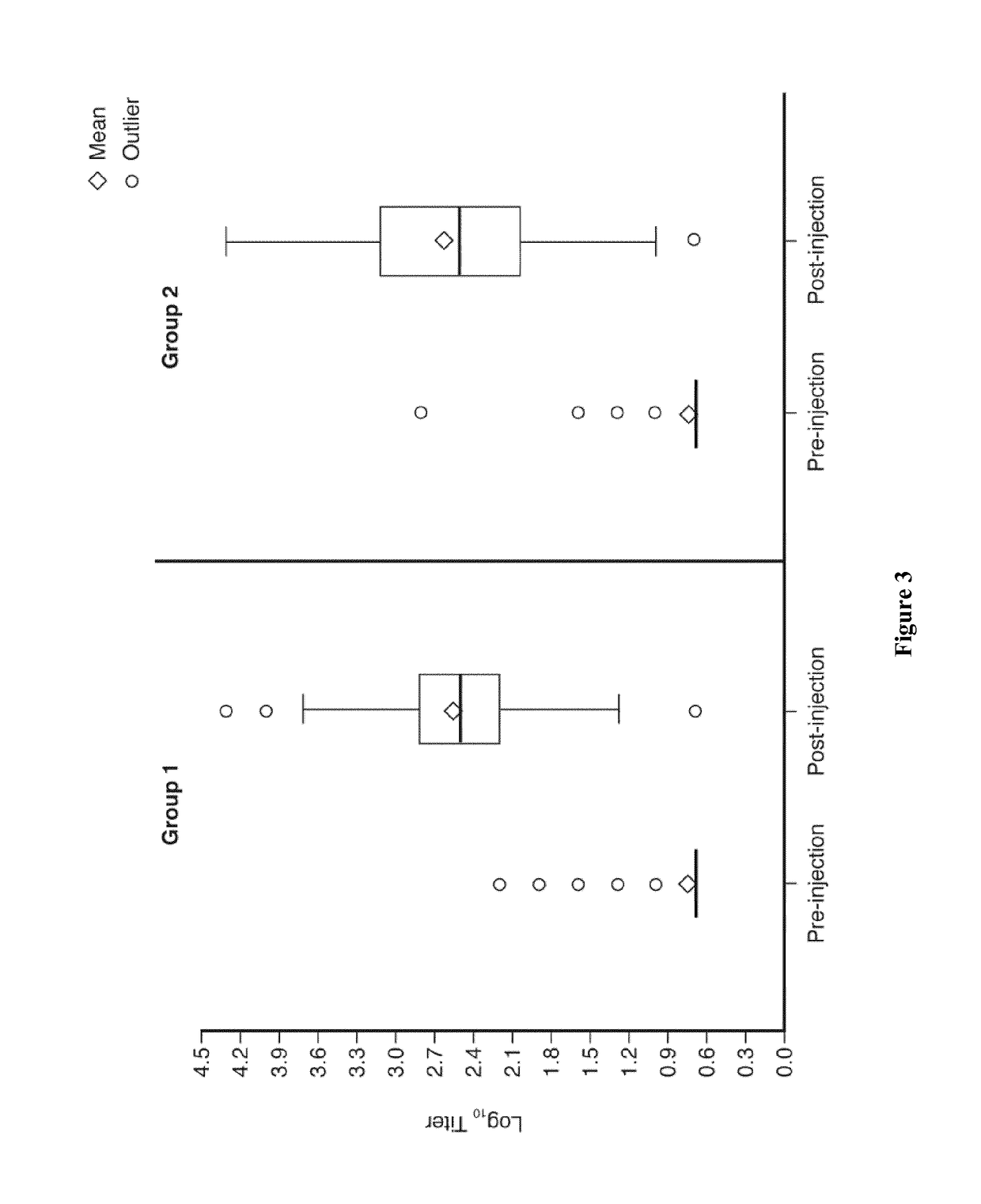
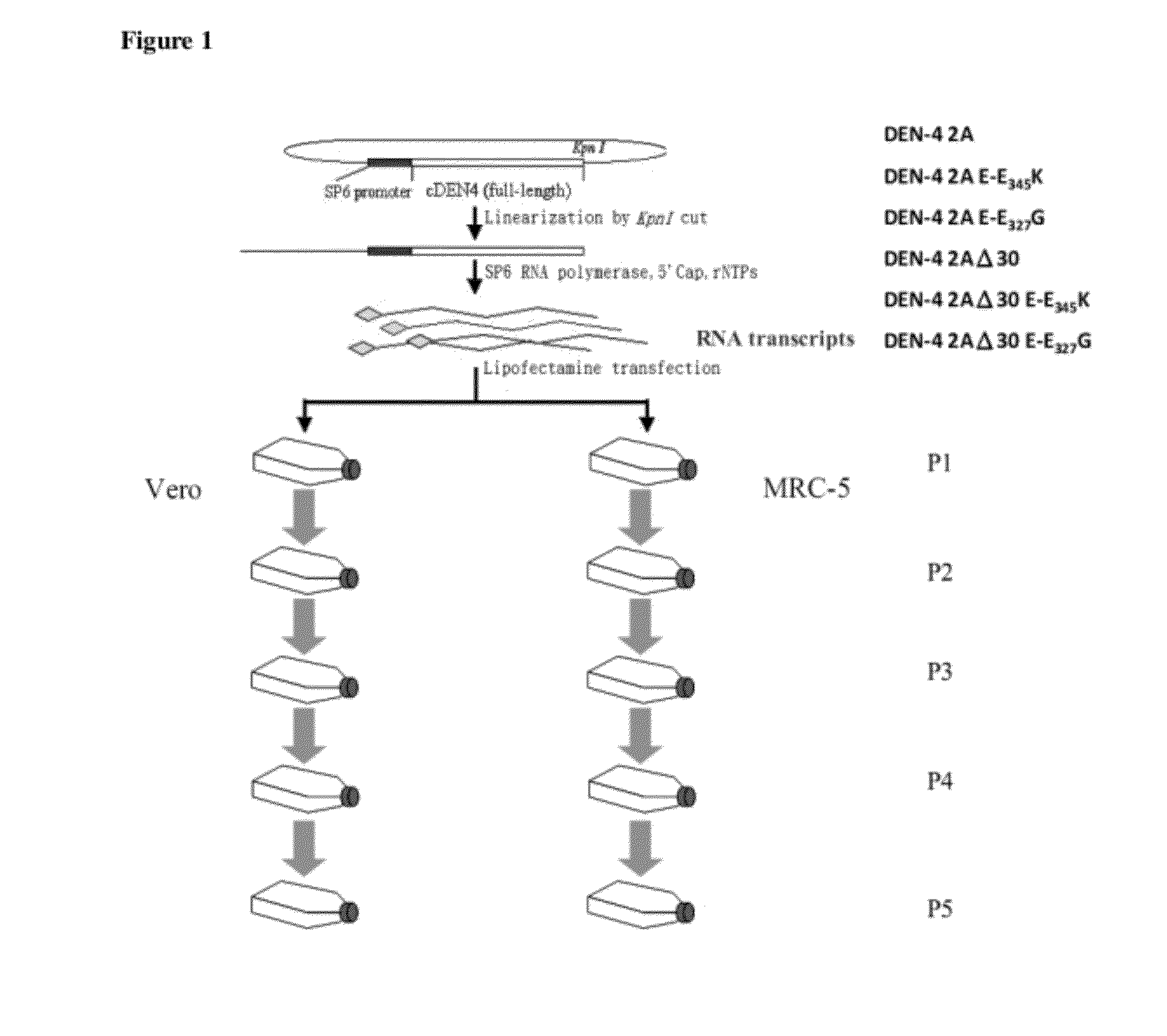
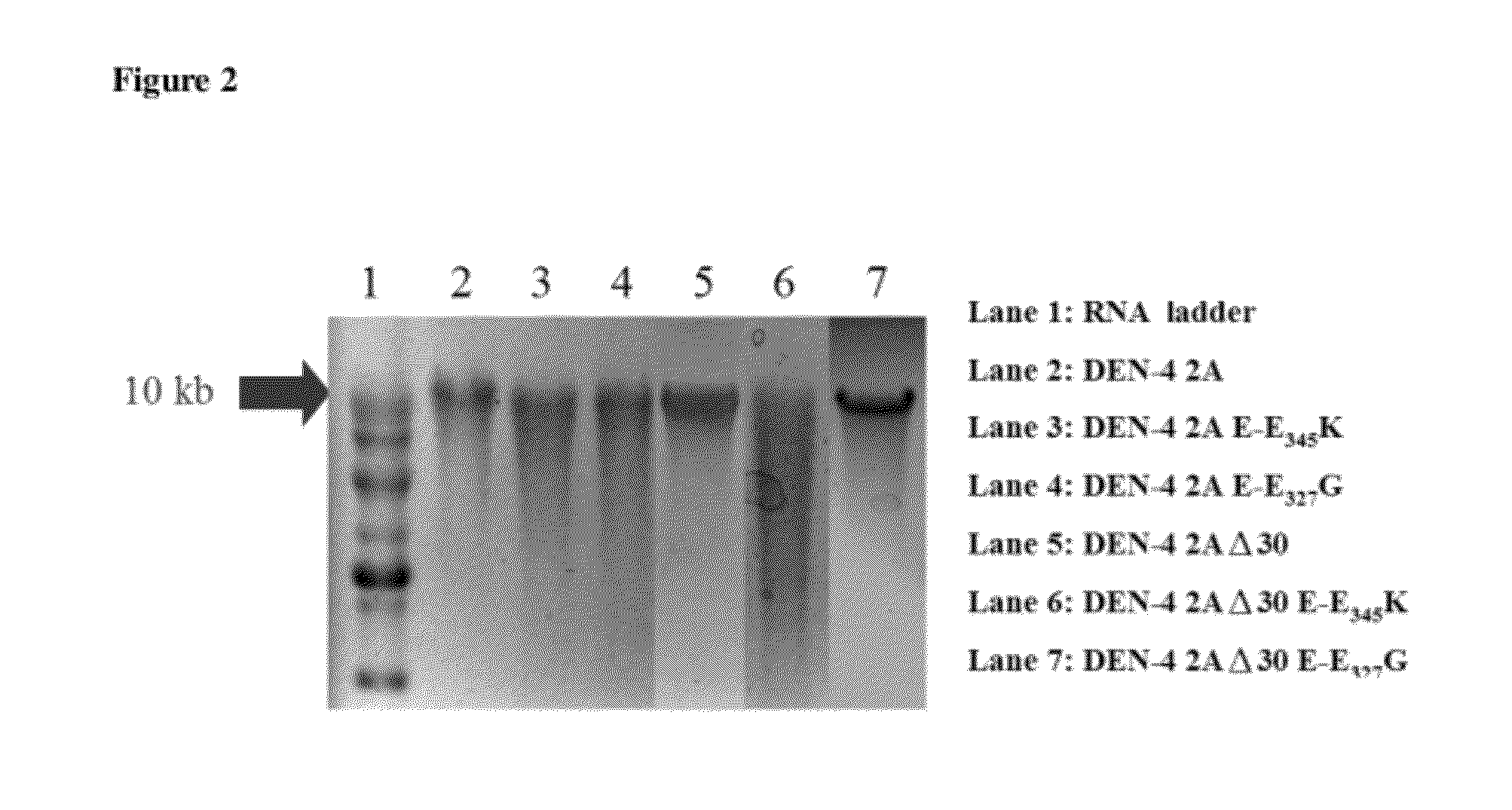
The present invention relates to an attenuated dengue virus vaccine. In present invention, target mutagenesis at Glu345Lys was constructed in two infectious cDNA clones of a recombinant version of wild type virus DEN-4 2A and its derived 3′ NCR deletion mutant vaccine candidate virus DEN-4 2AΔ30. Using PCR-mediated site-directed mutagenesis method, the infectious cDNA clone-derived Glu345-Lys mutants of DEN-4 2A and DEN-4 2AΔ30 were passaged in Vero cells and MRC-5 cells for five consecutive times. The results shows that single point mutation E-Glu345Lys of DEN-4 2A and DEN-4 2AΔ30 were found stably existed when passaged in MRC-5 cells, which means mutagenesis at Glu345Lys of DEN-4 2A and DEN-4 2AΔ30 are both suitable to be probagated in MRC-5 cell for producing virulence attenuated dengue virus vaccine.
Owner:NATIONAL TSING HUA UNIVERSITY
Process for preparing an attenuated tetravalent dengue vaccine
ActiveUS10004795B2High productImprove productivityPowder deliveryBacterial antigen ingredientsSerotypeDengue vaccine
The present invention refers to a process for preparing an attenuated tetravalent dengue vaccine and its product. The present invention also refers to a process for preparing a tetravalent dengue vaccine for administration to a subject, to a method for inducing an immune response to virus dengue serotype 1, 2, 3 and 4 in a patient and to a tetravalent dengue vaccine kit.
Owner:FUNDACAO BUTANTAN
A kind of chimeric virus and its preparation method and application
ActiveCN103205460BHigh titerImproving immunogenicityViral antigen ingredientsMicroorganism based processesFull length cdnaSerotype
The invention discloses an infectious Japanese encephalitis virus clone which is a recombinant vector including full-length cDNA of genome of a Japanese encephalitis virus attenuated strain SA14-14-2. The invention further discloses a chimeric virus and application thereof and a bacterial artificial chromosome as represented by SEQ ID No. 1. The chimeric virus provided by the invention can grow and proliferate on a plurality of cells used for production of human vaccines and show similar growth characteristics, is applicable to production of four serotype Dengue vaccines and has the advantages of strong immunogenicity, high antibody titer, good application prospects and a high industrial application value.
Owner:CHENGDU INST OF BIOLOGICAL PROD +1
Dengue vaccine, pharmaceutical composition comprising the same, nucleotide sequence and antibody composition
InactiveUS8771704B2Avoid potential risksImprove stabilitySsRNA viruses positive-senseSugar derivativesNucleotideAutoimmune responses
The present invention relates to a dengue vaccine, a pharmaceutical composition comprising the same, a nucleotide sequence, and an antibody composition. The dengue vaccine of the present invention includes chimeric nonstructural protein 1, which comprises N-terminus of DV NS1 from amino-acid residues 1 to 270 and C-terminus of JEV NS1 from amino-acid residues 271 to 352 (designated DJ NS1). Therefore, the dengue vaccine without autoimmunity according to the present invention is able to avoid cross-reactions with endothelial cells and platelets, and is able to shorten the bleeding time.
Owner:NAT CHENG KUNG UNIV
Dengue virus vaccine
InactiveCN108159410AOvercome limitationsAvoid double digestionSsRNA viruses positive-senseViral antigen ingredientsSimian AdenovirusesEnzyme digestion
The invention provides a dengue virus vaccine. The invention starts from the currently rarely reported T-cell immunity of the dengue virus, a simian adenovirus carrier is used to construct the vaccineto break through the limitations of the human adenovirus carrier in the practical application, an Ad carrier vaccine C9-NS1 for the dengue virus II is successfully constructed, the carrier clones a PCR (Polymerase Chain Reaction) product rapidly through the clone technology, the double enzyme digestion operations of a plasmid and a target fragment are avoided, and the carrier is relatively convenient and efficient. A histidine tag is arranged at the end C, expressing the fusion protein, of the carrier, so that the affinity chromatography and purification of the recombinant protein is facilitated, and the protein of the histidine tag is relatively small and barely produces effects on the activity and the function of the expression protein. The immunologic function of the vaccine from the protein NS 1 of the dengue virus is evaluated comprehensively from multiple aspects, and a foundation is laid for the further research and development of the efficient spectrum dengue gene vaccine.
Owner:重庆卓诺生物技术有限公司
Tetravalent dengue vaccine
Owner:INT CENT FOR GENETIC ENG & BIOTECH
A kind of Zika/dengue vaccine and its application
ActiveCN112194712BAvoid it happening againADE effect is reduced or eliminatedSsRNA viruses positive-senseVirus peptidesEpitopeZika virus
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
JE/Dengue Chimeric Virus Using Attenuated JE Virus Strain as Gene Skeleton and Its Application
ActiveCN102796749BImprove securityStable attenuation characteristicsFungiBacteriaDengue vaccineTGE VACCINE
The invention discloses a pidemic encephalitis B / dengue chimeric virus with an epidemic encephalitis B virus attenuated strain as a gene framework and application of the epidemic encephalitis B / dengue chimeric virus. The invention provides a deoxyribonucleic acid (DNA) molecule, and the DNA molecule is recombinant DNA which is obtained by replacing an encoding gene of protein A in complementary DNA (cDNA) corresponding to genome ribonucleic acid (RNA) of an epidemic encephalitis B virus by using an encoding gene of protein B; the protein A consists of an amino acid sequence shown as a sequence 1 in a sequence table; and the protein B consists of an amino acid sequence shown as a sequence 2 in the sequence table. The pidemic encephalitis B / dengue chimeric virus with the epidemic encephalitis B virus attenuated strain as the gene framework is a recombinant virus containing the DNA molecule. The pidemic encephalitis B / dengue type II chimeric virus also has a good application prospect when taken as a dengue vaccine and used for preventing dengue virus infection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com