Chimeric virus, and preparation method and application thereof
A technology of chimeric viruses and viruses, applied in biochemical equipment and methods, antiviral agents, virus antigen components, etc., can solve the problems that cells cannot be produced with human vaccines, the duration is short, and there is no cross-protection and weak, etc., to achieve good Application prospect, strong immunogenicity and high titer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
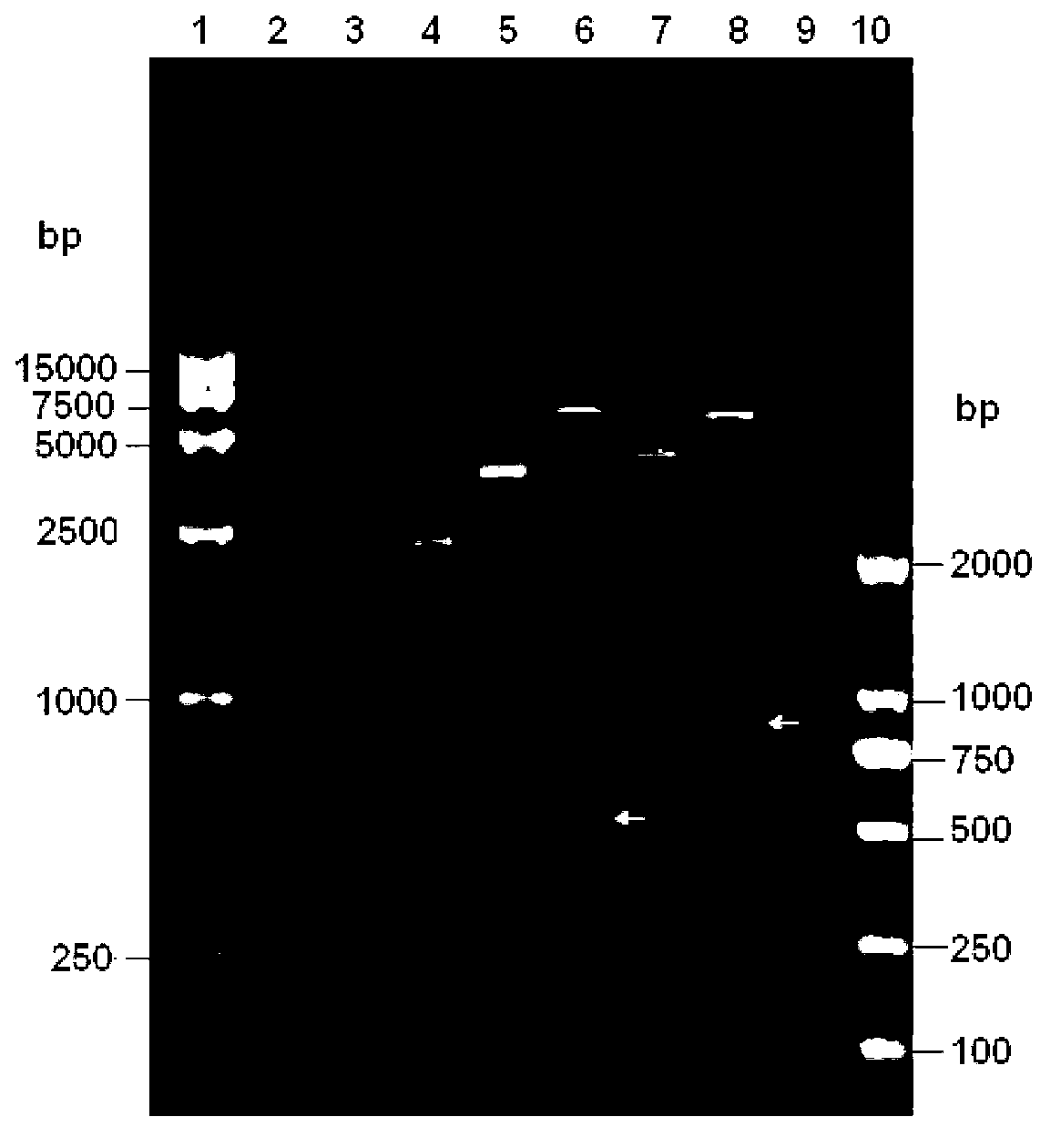
[0077] Example 1: Construction, identification and recovery of recombinant virus containing JEV vaccine strain SA14-14-2 full-length infectious full-length cDNA clone
[0078] 1. Transformation of the carrier
[0079] (1) Modification of low copy plasmid pACNR
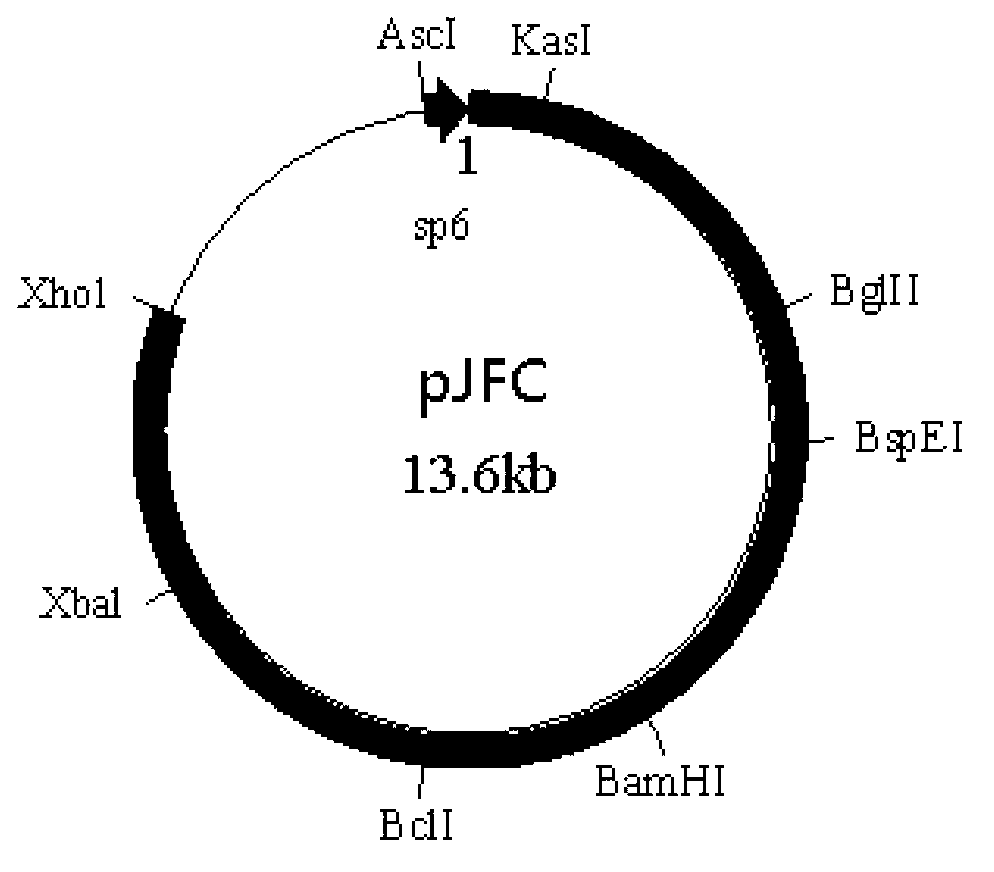
[0080] Dissolve primer Oligo-1 (5'-CGCGCCATTA GGCGCC TTAT AGATCT AATG TCCGGA TTAT GGATCC TGAT TGATCA TTAT TCTAGA ATTAC-3', underlined are KasI, BglII, BspEI, BamHI, BclI, XbaI recognition sites, artificially synthesized) and Oligo-2 (5'-TCGAGTAAT TCTAGA ATAA TGATCA ATCA GGATCC ATAA TCCGGA CATT AGATCT ATAA GGCGCC TAATGG-3', after forming complementary double strands with Oligo-1, the 5' and 3' ends respectively form sticky ends after Ascl and Xhol enzyme digestion, artificially synthesized) in 100 μl sterile double distilled water, mix 10 μl each, and heat to Cool naturally at 100°C, take 2 μl and connect with pACNR double-digested with AscI and Xhol at 4°C overnight, transform competent cell TOP...
Embodiment 2
[0139] Embodiment 2: Preparation and identification of Japanese encephalitis / dengue type 1 chimeric virus
[0140] 1. Preparation of JE / dengue type 1 chimeric virus
[0141] 1. Synthesis of dengue virus type 1 antigen gene prM-E fragment
[0142] Take the gene fragment prME (as shown in SEQID NO.4) encoding E protein of dengue virus type 1 ThD1000881 strain, and use Japanese encephalitis virus SA14-14-2 to encode 9 nucleotides of the carboxy-terminal protein of E protein in the fragment After the nucleotide replacement of the corresponding site of the strain, it is fused with 204 nucleotides at the amino terminal of the NS1 protein gene of the Japanese encephalitis virus SA14-14-2 strain.
[0143] Then insert the fragment into the pcDNA3.1 vector to obtain the plasmid clone pcDNA3.1-D1PrME of the dengue virus type 1 antigen gene prM-E.
[0144] 2. Construction of JE / dengue type 1 5' half molecule
[0145] The above-mentioned pcDNA3.1-D1prM-E plasmid containing the dengue vi...
Embodiment 3
[0179] Example 3 Construction of full-length cDNA plasmid of Japanese encephalitis / dengue type 2 chimeric virus, virus recovery and identification of important biological characteristics
[0180] 1. Construction of full-length cDNA plasmid of Japanese encephalitis / dengue type 2 chimeric virus
[0181] 1. Synthesis of DEN2prM / E protein coding gene
[0182] Get the gene fragment prM-E (as shown in SEQ ID NO.5) encoding the E protein of the DEN2PUO-218 strain, and use Japanese encephalitis virus SA14-14-2 to encode the 9 nucleotides of the carboxy-terminal protein of the E protein in the fragment After the nucleotide replacement of the corresponding site of the strain, it was fused with 204 nucleotides at the 5' end of the JE virus SA14-14-2NS1 protein gene, and inserted into the vector pMD18-T for the construction of a chimeric full-length clone .
[0183] 2. Construction of JE / DEN2 type 2 chimeric fragment 5' semi-molecular plasmid pJE / DEN2-5'
[0184] The synthetic gene fra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com