Method for evaluating immune effect of dengue vaccine
A technology for dengue vaccine and immune effect, applied in the field of immunology, can solve the problems of high cost, easy death, and inability to accurately simulate the immune effect of vaccines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A kind of immune effect evaluation method of dengue vaccine, it comprises the following steps:
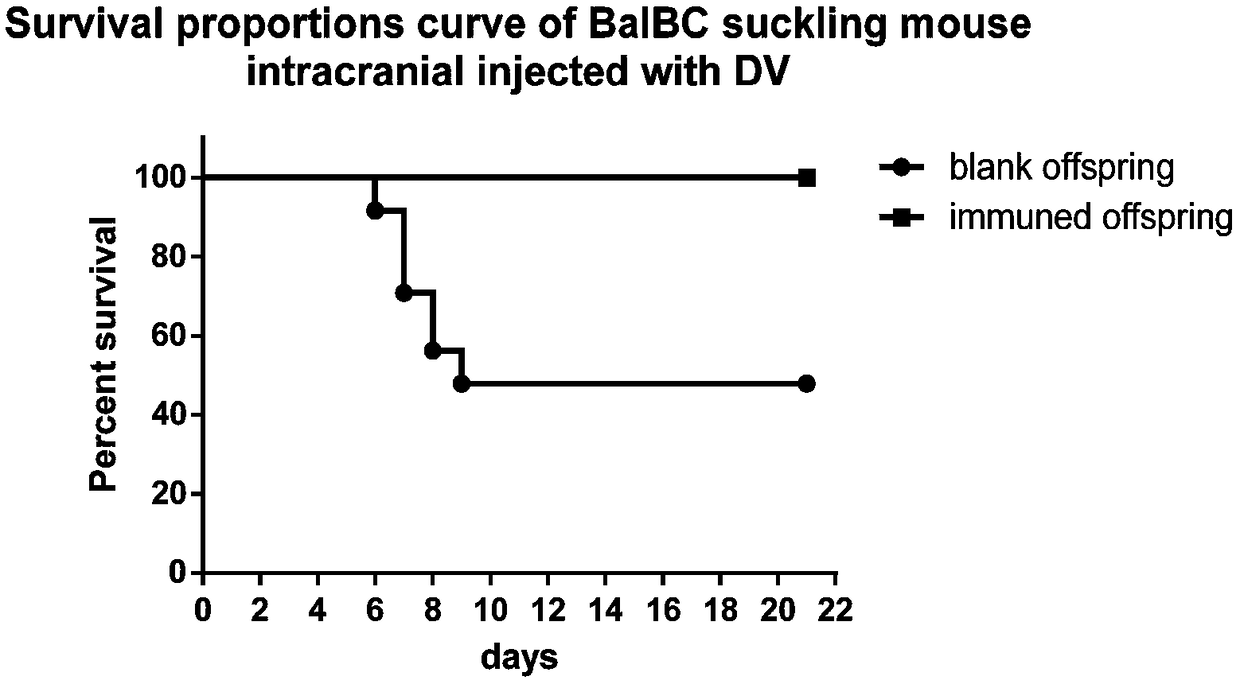
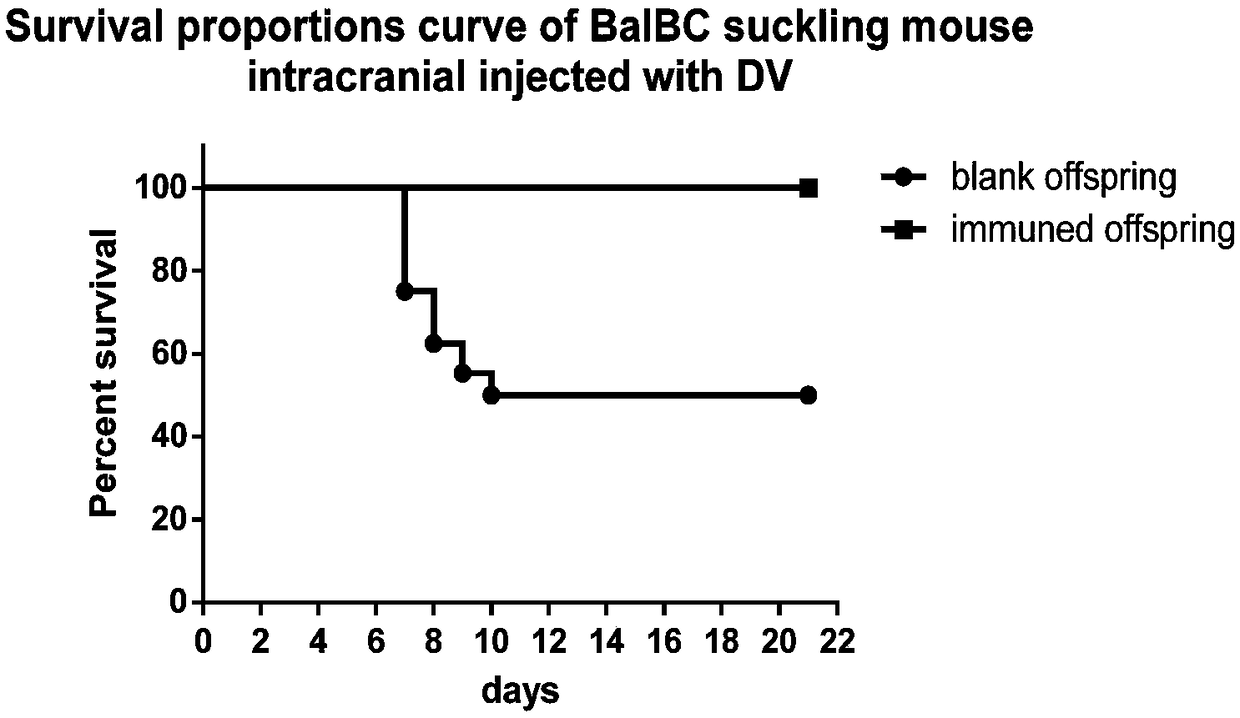
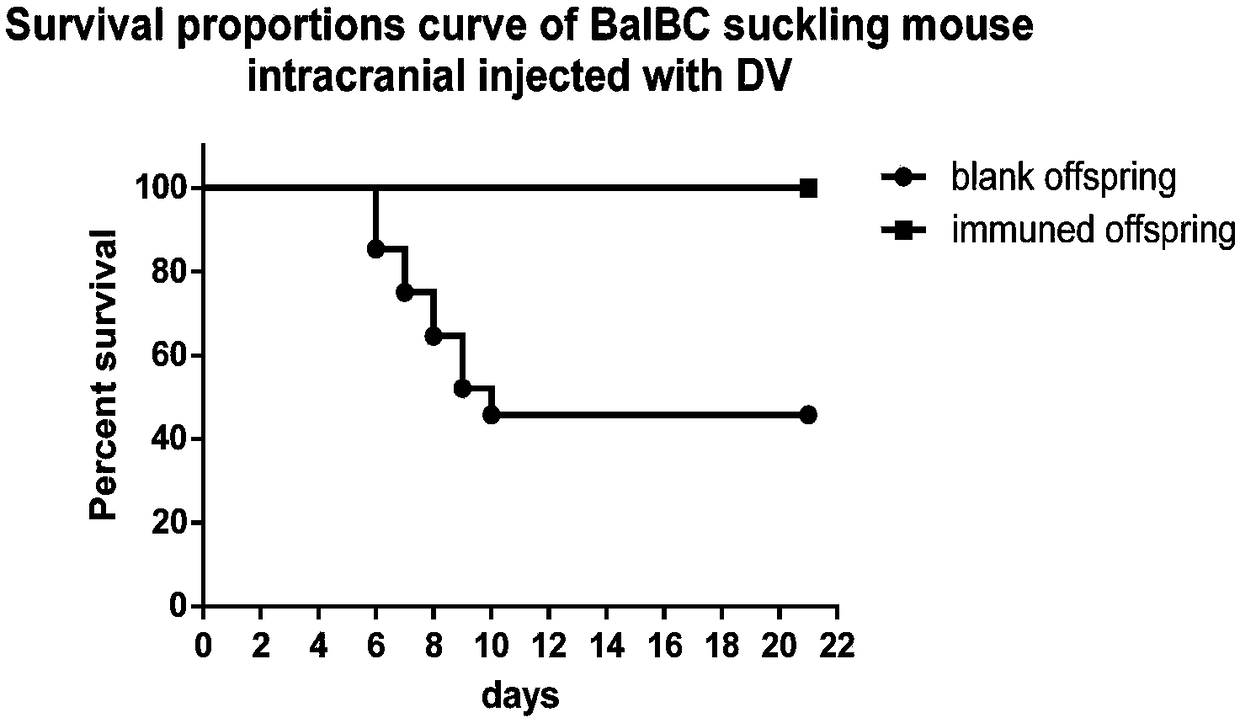
[0043] 1) At first utilize monolayer vero cell to cultivate dengue virus, the virus liquid that obtains after poisoning is measured titer by plaque test, the result is (6.25×10 5 ); then the dengue virus liquid was divided into 200 μl / tube, 20 μl / tube, 4 μl / tube and 2 μl / tube, 50 tubes each, and stored at -80°C for later use;
[0044] 2) Prepare a number of pregnant Balb / c mice, and use the virus in step 1) to challenge the newborn blank offspring intracranially; in order to measure the virus titer when 50% of the suckling mice die, it is necessary to test the blank newborn milk Rat intracranial challenge dengue virus of different titer groups, that is, culture stock solution group, 10-fold dilution group, 50-fold dilution group and 100-fold dilution group, of which 10-fold dilution group, 50-fold dilution group and 100-fold dilution group Add 180 μl, 196 μl and 198 μl of s...
Embodiment 2
[0058] A kind of immune effect evaluation method of dengue vaccine, it comprises the following steps:
[0059] 1) Firstly, a monolayer of vero cells was used to culture dengue virus, and the virus liquid obtained after harvesting the virus was tested for titer by plaque test (6.25×10 5 ), then the dengue virus solution was divided into 200 μl / tube, 20 μl / tube, 4 μl / tube and 2 μl / tube, 50 tubes each, and frozen at -80°C for later use;
[0060] 2) Prepare several pregnant Balb / c mice, and use the virus in 1) to challenge the newborn blank offspring intracranially. Intracranial challenge dengue virus of different titer groups, that is, culture stock solution group, 10-fold dilution group and 100-fold dilution group, in which 10-fold dilution group and 100-fold dilution group were added with 180 μl, 196 μl and 198 μl of no Bacteria PBS solution, every group intracranial injection dosage is 20 μ l each, the virus titer group that causes 50% suckling mice to die repeats 5 times, an...
Embodiment 3
[0073] A kind of immune effect evaluation method of dengue vaccine, it comprises the following steps:
[0074] 1) Firstly, a monolayer of vero cells is utilized to cultivate dengue virus, and the virus liquid obtained after receiving the virus is measured by a plaque test for titer (6.25×10 5 ), then the dengue virus solution was divided into 200 μl / tube, 20 μl / tube, 4 μl / tube and 2 μl / tube, 50 tubes each, and frozen at -80°C for future use.
[0075] 2) Prepare several pregnant Balb / c mice, and use the virus in 1) to challenge the newborn blank progeny mice intracranially. In order to measure the virus titer when 50% of suckling mice die, it is necessary to challenge dengue virus of different titer groups intracranially to blank neonatal suckling mice, i.e. culture stock solution group, 10-fold dilution group and 100-fold dilution group, wherein The 10-fold dilution group and the 100-fold dilution group were added with 180 μl and 198 μl sterile PBS solution before use, respec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com