Oil-in-water type nanometer emulsion adjuvant and MRSA nanometer emulsion adjuvant vaccine and preparing method thereof
An oil-in-water type and nanoemulsion technology, which is applied in the field of biomedicine, can solve the problems of inconspicuous enhancement of vaccine immunogenicity, long protection period, and small side effects, and achieve enhanced bioavailability, low body irritation, and The effect of small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
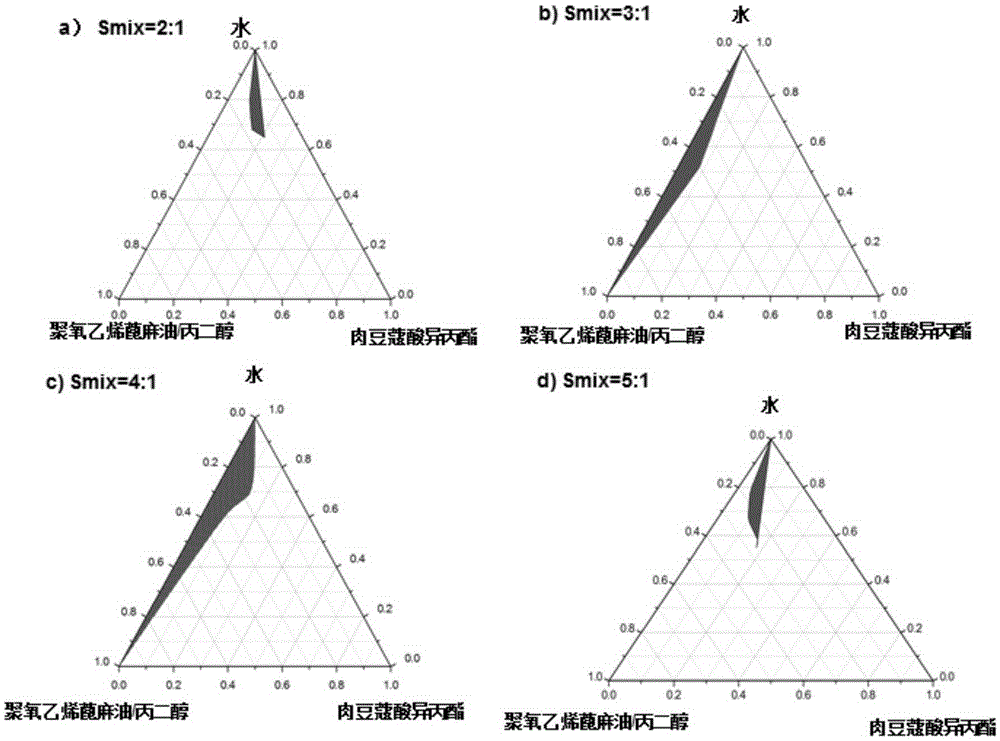
[0037] Example 1. Screening of nanoemulsion adjuvant prescription
[0038] For surfactants (SF), such as: Tween-80, Tween-85, Tween 60, Tween 20, polyoxyethylene castor oil (EL40, EL35), polyoxyethylene hydrogenated castor oil, etc., help surface activity (CoSF), such as Span 80, Span 85 and ethanol, glycerin, propylene glycol, absolute ethanol, 1,3-butanol, 1,3-propanediol, comprehensively design the experimental group, according to 9:1, 8:2 , 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 (the mass ratio of SF and CoSF), weigh SF and CoSF, add the selected oil phase, Such as: paraffin oil, ethyl acetate, isopropyl myristate, squalane, squalene, ethyl oleate, caprylic acid / decanoic acid triglyceride, etc., vortex and shake, add water dropwise, observe the changes in each group, Centrifugation at 13,000 rpm for 30 minutes, stability and emulsion droplet size were the main evaluation criteria to determine the SF and CoSF that form the nanoemulsion. Use surfactant / cosurfactant (mixed surfacta...
Embodiment 2
[0060] Example 2. Preparation of MRSA nanoemulsion adjuvant vaccine with oil-in-water nanoemulsion adjuvant
[0061] Due to the wide variety of vaccines, this example mainly uses the MRSA nanoemulsion adjuvant vaccine to prove the effect of the nanoemulsion adjuvant vaccine prepared by the present invention.
[0062] The preparation of MRSA protein antigens was carried out by reference (ZuoQF, YangLY, FengQ, LuDS, DongYD, CaiCZ, et al. Evaluation of the protective immunity ofanovel subunit fusionvaccineinamurine model of systemic MRSA infection. PloSone 2013; 8: e81212). The original concentration of the protein antigen is 1.5 mg / mL, the purity of the protein is 99.4%, and the prepared antigen is stored in a refrigerator at -70°C.
[0063] According to the oil-in-water nanoemulsion adjuvant screened in Example 1, a vaccine of the oil-in-water nanoemulsion adjuvant is prepared. The specific formula and preparation method are as follows:
[0064] Formula 1: 150μg / ml MRSA nanoemulsion ad...
Embodiment 3
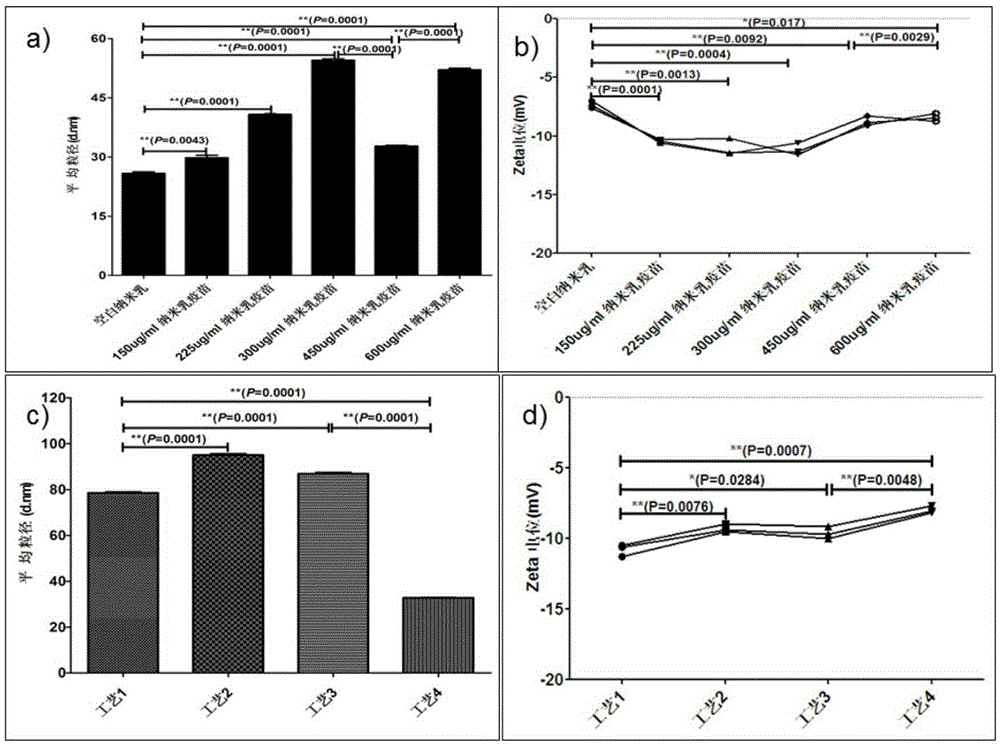
[0086] Example 3 Evaluation of the basic quality characteristics of MRSA nanoemulsion adjuvant vaccine
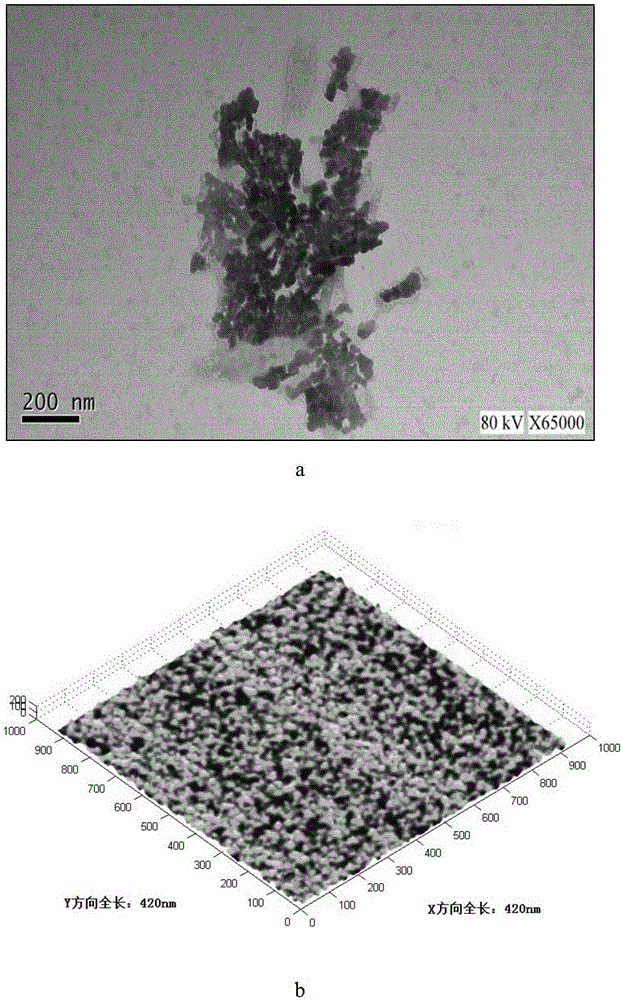
[0087] A small amount of MRSA nanoemulsion adjuvant vaccine is appropriate, diluted 100 times with water, and dropped on a copper net covered with a supporting film. After standing for 10 minutes, it is blotted dry with a filter paper, and then 2% phosphotungstic acid (pH 7.4) ) The solution was negatively dyed on a copper mesh for 3 minutes, and then evaporated to dryness. Observed by a transmission electron microscope and photographed. A small amount of MRSA nanoemulsion adjuvant vaccine is appropriate, diluted 100 times with water, then air-dried naturally, and directly observed with the atomic mechanics microscope IPC-208B (Chongqing University). All images are automatically smoothed to eliminate low-frequency noise in the slow-scan direction. The measurement is done under the following conditions: tungsten probe (force constant, 0.06N·m); scanning range, 10.5×10.5nm; ima...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com