Japanese encephalitis/dengue chimeric virus and application thereof
A dengue virus, replicon technology, applied in the direction of antiviral agents, virus/phage, virus antigen components, etc., can solve the problem of no dengue vaccine and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Construction of JEV full-length cDNA clone
[0036] 1. Cultivation of JEV SA14-14-2 strain and extraction of total cellular RNA
[0037] The JEV SA14-14-2 strain is a product of Chengdu Institute of Biological Products, and it has been subcultured in this laboratory for no more than 3 generations. Inoculate the JEV SA14-14-2 virus seed solution on the adherent and overgrown BKH-21 cells (Stratagene, USA), and when the cell lesion reaches +++~++++, the total RNA of the cells is extracted by the Trizol reagent method. LS Reagent is a product of Invitrogen Corporation of the United States.
[0038] 2. Integrate the full-length JEV cDNA into the plasmid pBR322 to prepare a full-length JEV cDNA clone
[0039] Using ThermoScript TM RT-PCR System (Invitrogen, USA) reverse-transcribed JEV RNA to synthesize the first strand of cDNA. The Elongase Amplification System (Invitrogen, USA) was used to amplify the whole JEV genome in two segments (the 5' end half mo...
Embodiment 2
[0042] Embodiment 2: Construction and identification of JEV replicon
[0043] 1. Construction of JEV replicon
[0044] On the basis of the above-mentioned JEV full-length cDNA clone pBRF, the structural protein gene PrM / E was deleted and replaced by a multiple clone site (MCS) for convenient cloning of foreign genes. The principle of multiple cloning site selection: After comprehensive analysis of the full sequence of the plasmid pBR322 and JEV SA14-14-2 strain, and the prM / E sequence of the DV2NGC strain, the cleavage sequence of 6 bases was selected based on the principle of no frame shift. Age I, BSSH II, Xho I, Xma I are multiple cloning sites. The restriction site analysis software is the SeqBuilder module in Lasergene. In order to avoid lethal deletion, two replicons were constructed at the same time, one completely deleted the PrM / E gene; the other retained the C-terminal 213 bp of the E gene, because the inventors considered that this part of the sequence may be impo...
Embodiment 3
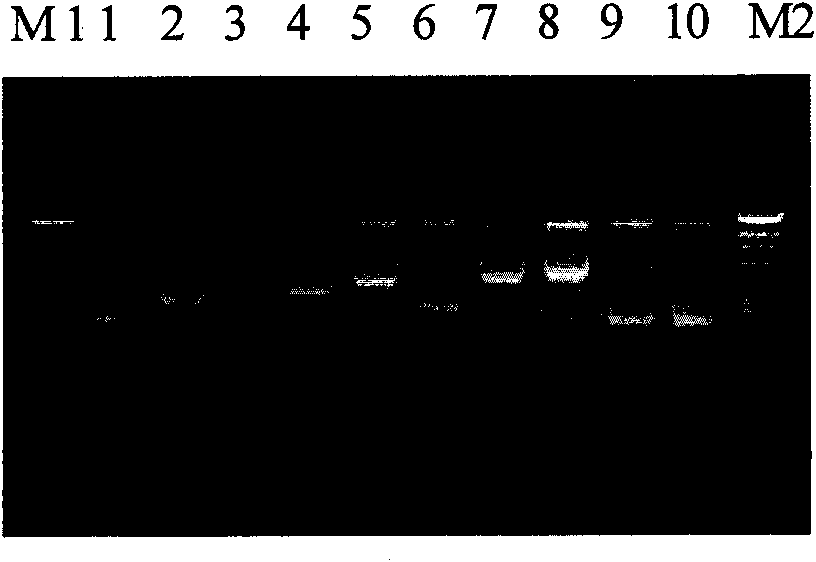
[0061] Embodiment 3: Preparation and identification of Japanese encephalitis / dengue type 2 chimeric virus
[0062] 1. Construction of JE / dengue type 2 chimera clone
[0063] Using DV2 virus cDNA as a template, the prM / E full-length gene (DV2-prM / E, 1983bp) of DV2 (NGC strain, Institute of Viral Diseases, Chinese Center for Disease Control and Prevention) and the intercepted prM / E gene were obtained by RT-PCR technology A 213nt gene fragment at the 3' end (DV2-1770, 1770bp) (amplified using Platinum Pfx DNA polymerase from Invitrogen). The primers for amplifying DV2-prM / E are: upstream primer 5' accggt TTCCATTTAACC ACA-3' (the underline is the introduced Age I restriction site), downstream primer 5'- cccggg GGCCTGCACCATGAC-3 (the underline is the introduced Xma I restriction site); the primers for amplifying DV2-1770 are: the upstream primer is 5'- accggt TTCCATTTAACCACA-3' (the underline is the introduced Age I restriction site), the downstream primer is 5'- ccccgggg G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com