Total synthesis method of optically pure tetrandrine
A total synthesis technology of tetrandrine, which is applied in the field of total synthesis of optically pure tetrandrine, can solve the problems of low biological activity of racemic tetrandrine, limited production, high pressure on environmental protection, etc., and achieve optimal synthesis Effects of process parameters, reduction of synthesis steps, reduction of reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Preparation of compound 3
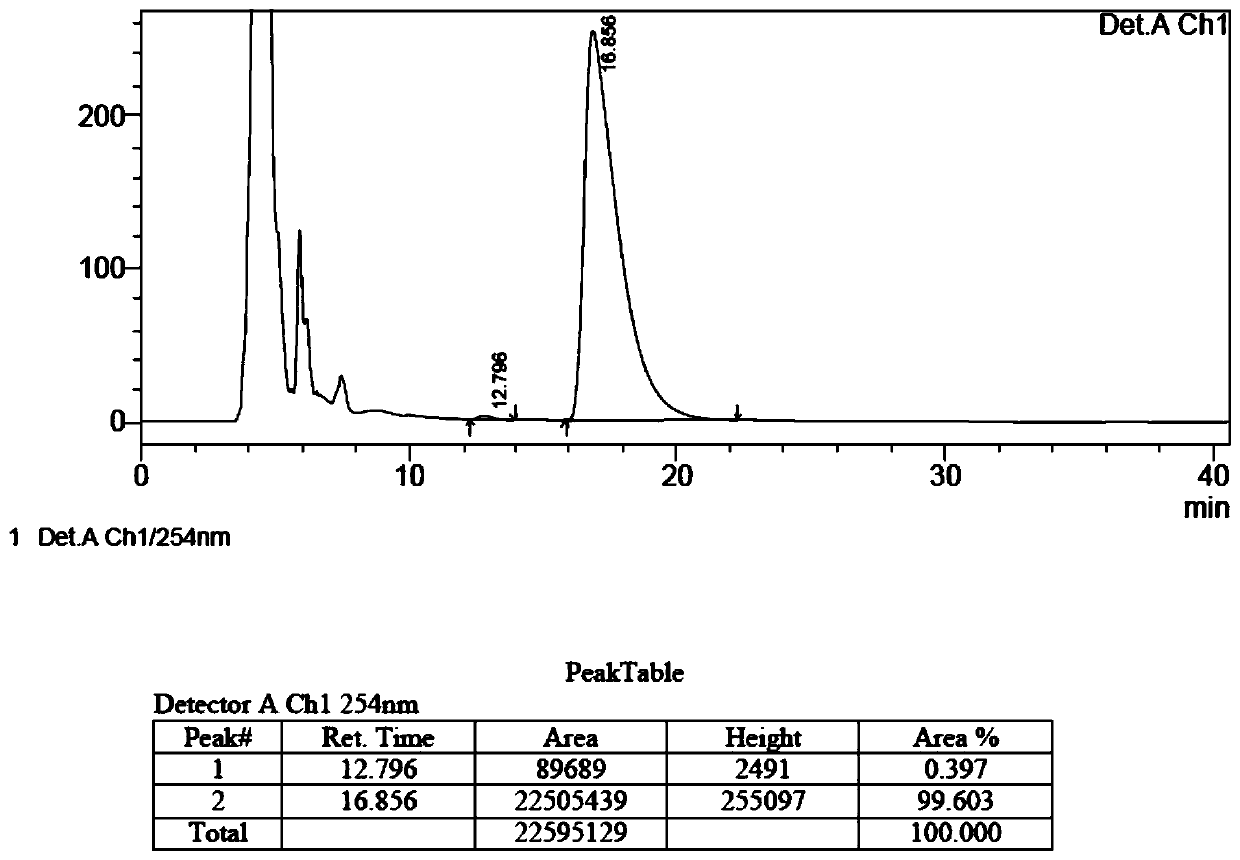
[0054] Add 4.2g of compound 1, 4.6g of compound 2, 0.36g of cuprous bromide, 9.8g of potassium carbonate and 84g of toluene into a 250mL three-necked flask, heat to 100°C for 48 hours under the protection of nitrogen, and the reaction of the raw materials is complete; the reaction solution is concentrated under reduced pressure To dryness, add 42g of water, adjust pH=3 or so with 10% dilute hydrochloric acid, and then extract twice with 82g of dichloromethane; combine the dichloromethane phases, wash twice with 82g of water, and dry over anhydrous sodium sulfate; Concentrate under pressure to dryness to obtain a brown solid, which is purified by column chromatography to obtain 5.5 g of a light yellow solid, which is compound 3, with a yield of 69.7%, and an ee value of 99.2%. The chromatographic conditions for detecting its optical purity by HPLC are: mobile phase Ratio: 0.1% diethylamine-n-hexane: ethanol = 80:20; flow rate: 1.0mL / min; c...
Embodiment 2
[0062] (1) Preparation of Compound 3
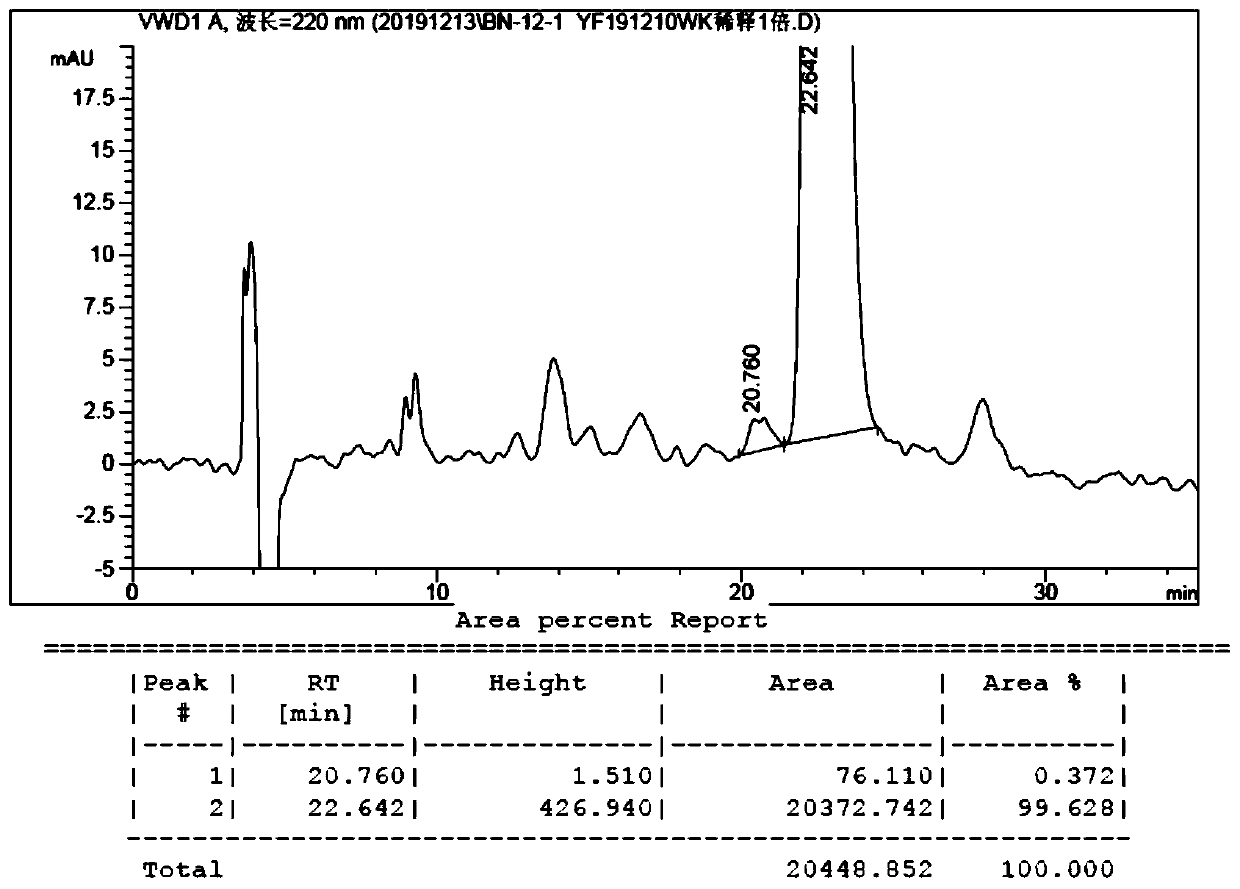
[0063] Add 4.8g of compound 1, 4.0g of compound 2, 1.8g of cuprous bromide dimethyl sulfide mixture, 8.4g of cesium carbonate and 80g of o-xylene into a 250mL three-necked flask, heat to reflux for 48 hours under the protection of nitrogen, and the raw materials are completely reacted . The reaction solution was concentrated to dryness under reduced pressure, 50 g of water was added, the pH was adjusted to about 3 with 10% dilute hydrochloric acid, and extracted twice with 100 g of dichloromethane. The dichloromethane phases were combined, washed twice with 100 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain a brown solid, which was purified by column chromatography to obtain 5.1 g of a light yellow solid, namely compound 3, with a yield of 56.9% and an ee value of 99.3%;
[0064] (2) Preparation of Compound 4
[0065] Add 3.0 g of compound...
Embodiment 3
[0070] (1) Preparation of compound 3
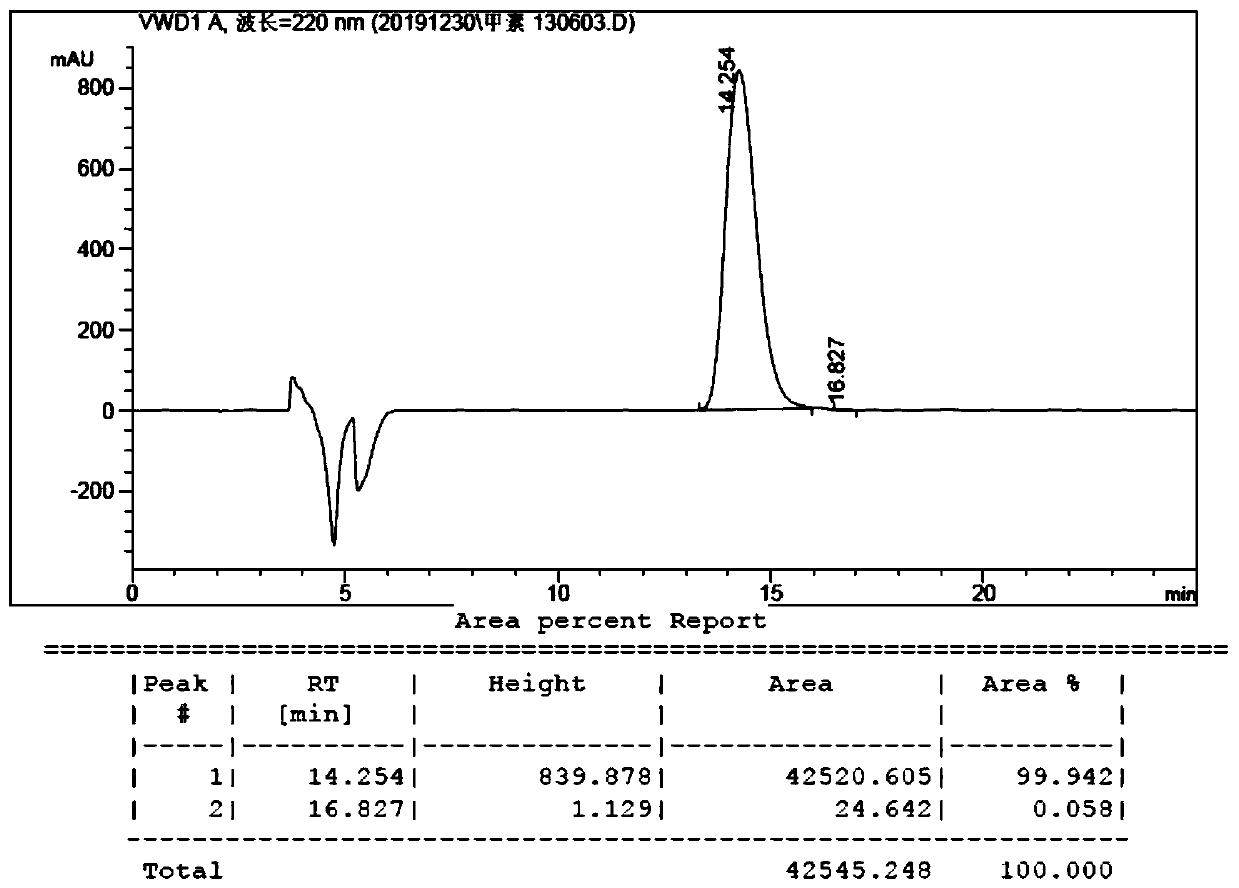
[0071] Add 4.8g of compound 1, 4.0g of compound 2, 0.3g of cuprous chloride, 2.0g of sodium hydroxide and 80g of toluene into a 250mL three-neck flask, and heat to reflux for 60 hours under the protection of nitrogen to react the raw materials completely. The reaction solution was concentrated to dryness under reduced pressure, 50 g of water was added, the pH was adjusted to about 3 with 10% dilute hydrochloric acid, and extracted twice with 100 g of dichloromethane. The dichloromethane phases were combined, washed twice with 100 g of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain a brown solid, which was purified by column chromatography to obtain 4.9 g of a light yellow solid, namely compound 3, with a yield of 54.1% and an ee value of 98.6%.
[0072] (2) Preparation of Compound 4
[0073] Add 3.0 g of compound 3, 60 g of hydrochloric acid and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com