Preparation method of fluorocalciferol
A technology for calcidol and its compounds, which is applied in the field of preparation of fluorocalcidol, can solve the problems of unsuitability for industrial production, long reaction route, complicated operation, etc., and achieve the effect of short synthesis route, less by-products and simple purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
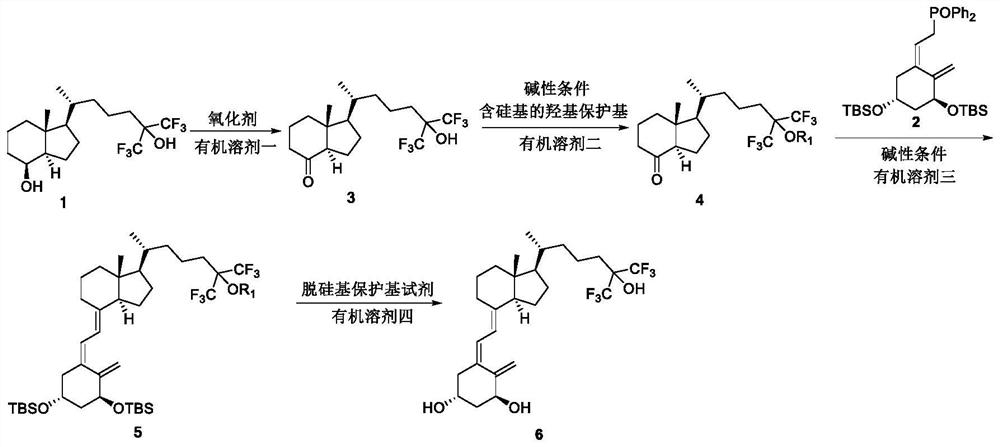
[0038] Refer figure 1 The reaction route for the preparation method of fluorobyl alcohol, comprising the steps of:
[0039] Step 1: Compound 1 reacts compound 3 in the organic solvent 1 to the oxidant.
[0040]
[0041] Step 2: Compound 3 reacts compound 4 under an organic solvent 2 in alkaline conditions to obtain compound 4;
[0042]
[0043] Step 3: Compound 4 is reacted with the compound 2 in an organic solvent three in alkaline conditions to obtain a compound 5;
[0044]
[0045] Step 4: Compound 5 Deep off in an organic solvent 4 with an acidic reagent or a fluoride-containing ion reagent condition to obtain a compound 6: fluororonyl alcohol
[0046]
[0047] In the step one, the oxidant is one or more of dichloro chloric acid pyridine, chlorocyanic acid pyridine, chromium chromium, trioxide pyridine, and recombationate, and its molecular feed is Compound 1. 1.5 ~ 5 times; one or more of the organic solvent is one of dichloromethane, chloroform, n-heptane, acetone, i...
Embodiment 1
[0054] Example 1: Preparation of Compound 3
[0055]
[0056] Under room temperature conditions, Compound 1 (10 g, 25.6 mmol) and dichloromethane (200 mL) were added to nitrogen-protected three reaction bottles, stirred and dissolved, and PDC (24.1 g, 64.0 mmol) was added, and the system became dark brown, 25 ~ 30 ° C reaction for 3 h, TLC monitoring (expander: PE / EA = 2: 1) raw material reaction is complete. The reaction liquid was filtration, diatomalifoil to filter, washable with dichloromethane, concentrated filtrate, column chromatography purified, eluent: n-hexane / EA = 10: 1 → 5: 1, 25 ~ 30 ° C The vacuum was dried for 30 min to give a white granular solid 9.45 g, and the yield was 95%.
Embodiment 2
[0057] Example 2: Compound 4 (R 1 Preparation of = TMS)
[0058]
[0059] EtOAc (EtOAc) (2.3 g, 15.42 mmol) was added after the addition of 10 to 30 ° C for 12h, TLC monitoring (expandant: PE: EA = 5: 1) raw material substantially. Dilutina (100 mL) diluted reaction solution was added, and the water (50 ml × 2) was washed sequentially washed sequentially with a 20% aqueous solution of hydrochloric acid (50 mL), saturated water (50 ml × 2), and anhydrous sulfate was dried. EtOAc (EtOAc)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com