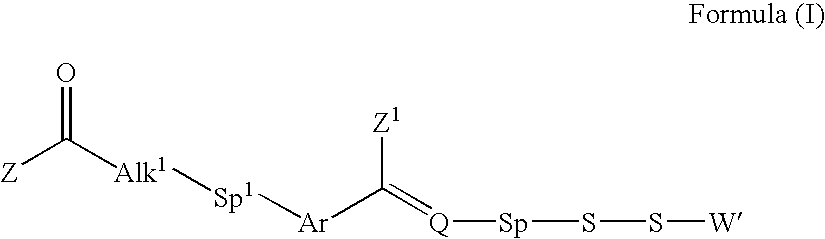
Processes for the convergent synthesis of calicheamicin derivatives
a technology of convergent synthesis and calicheamicin, which is applied in the field of process for convergent synthesis of calicheamicin derivatives, can solve the problems of low overall yield, complex synthetic methods for constructing calicheamicin derivatives, and inherently toxic calicheamicin moiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
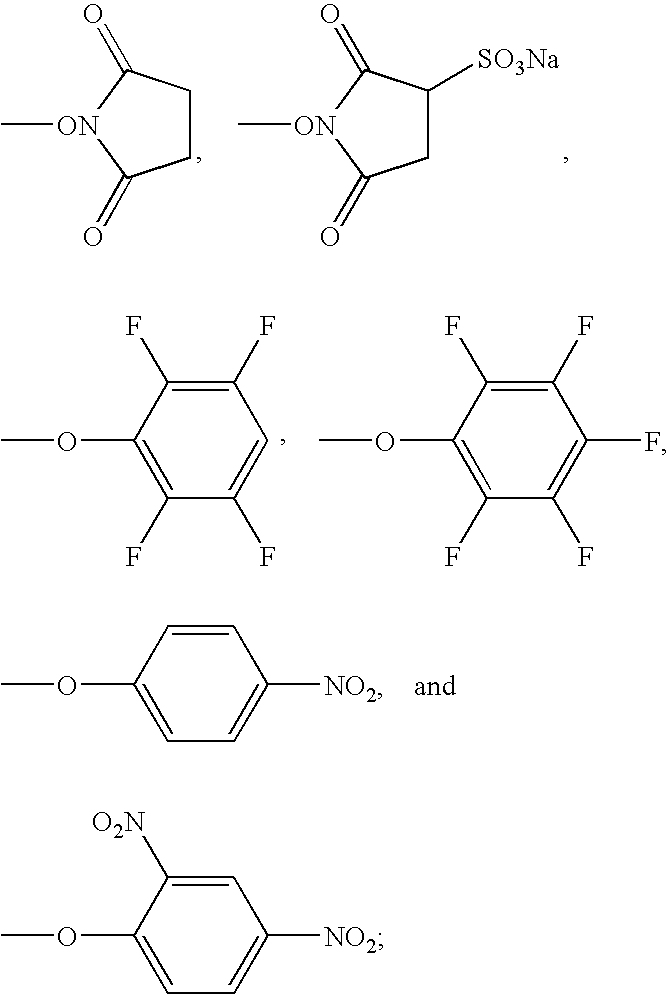
Image
Examples
example 1
Butanoic acid, 3-mercapto-3-methyl-,2-[(E)-1-[4-(4-hydroxy-4-oxobutoxy)phenyl]ethylidene]hydrazide
[0076]
[0077]To a stirred mixture of 0.5 g [3.4 mmol] of 3-methyl-3-mercapto-butanoic acid hydrazide in 5.0 ml of methanol is rapidly added 0.91 g [4.1 mmol] of 4-(4-acetylphenoxy)-butanoic acid followed by an additional 10 ml of methanol and 1.5 ml of acetic acid and stirring continued for 24 hours. The reaction mixture is filtered and the solid washed with 100 ml of methanol to give 0.78 g of the title compound as a solid.
example 2
Butanoic acid, 3-mercapto-3-methyl-,2-[(E)-1-[4-(4-hydroxy-4-oxobutoxy)phenyl]ethylidene]hydrazide
[0078]
[0079]A mixture of 3-methyl-3-mercapto-butanoic acid hydrazide (4.0 g, 27 mmol), 4-(4-acetylphenoxy)-butanoic acid (5.0 g, 22.5 mmol) and acetic acid (7.5 mL) in methyl alcohol (75 mL) is heated at about 45° C. for about 7 h. The mixture is allowed to cool to room temperature. The white solid (7.12 g, 90%) is collected on a Buchner funnel and washed with MeOH (2×10 mL). 1H NMR (DMSO-d6): δ (ppm) 12.14 (s, 1H), 10.37 and 10.21 (s, 1H), 7.74-7.70 (m, 2H), 6.97-6.95 (m, 2H), 4.04 (t, 2H), 3.09 and 3.04 (s, 2H), 2.66 (s, 1H), 2.41-2.37 (t, 2H), 2.22 and 2.20 (s, 3H), 1.97-1.93 (m, 2H), 1.8 (s, 3H), 1.47 (s, 3H). MS: 375 (M++Na).
example 3
Butanoic acid, 3-mercapto-3-methyl-,2-[(E)-1-[4-[4-[(2,5-dioxo-1-pyrrolidinyl)oxy]-4-oxobutoxy]phenyl]ethylidene]hydrazide
[0080]
[0081]To a mixture of 0.5 g [1.42 mmol] of butanoic acid, 3-mercapto-3-methyl-,2-[(E)-1-[4-(4-hydroxy-4-oxobutoxy)phenyl]ethylidene]hydrazide (Example 1 or 2) and 0.22 g [1.89 mmol] of N-hydroxysuccinimide is added 10 ml of N,N-dimethylformamide followed by the rapid addition of 0.70 g [3.65 mmol] of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and the mixture stirred at room temperature for 3 hours. The reaction mixture is concentrated in vacuo to a residue which is partitioned between ethyl acetate and water. The separated organic layer is washed with water, saturated sodium chloride and dried (MgSO4). The organic layer is evaporated in vacuo to give an oily residue which crystallized from ethyl acetate-hexane affording 0.21 g of the title compound as a colorless solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| liquid chromatography | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com