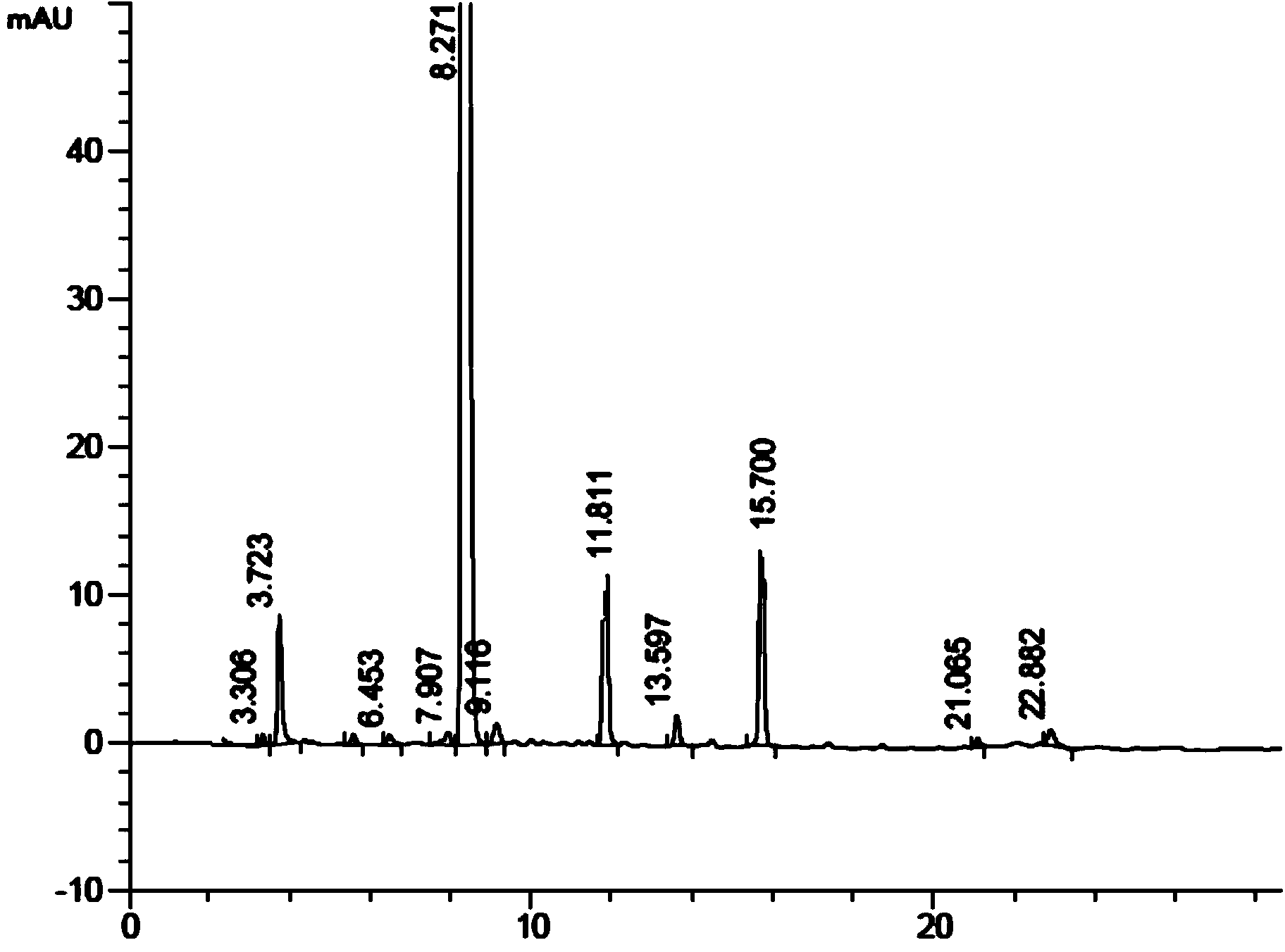
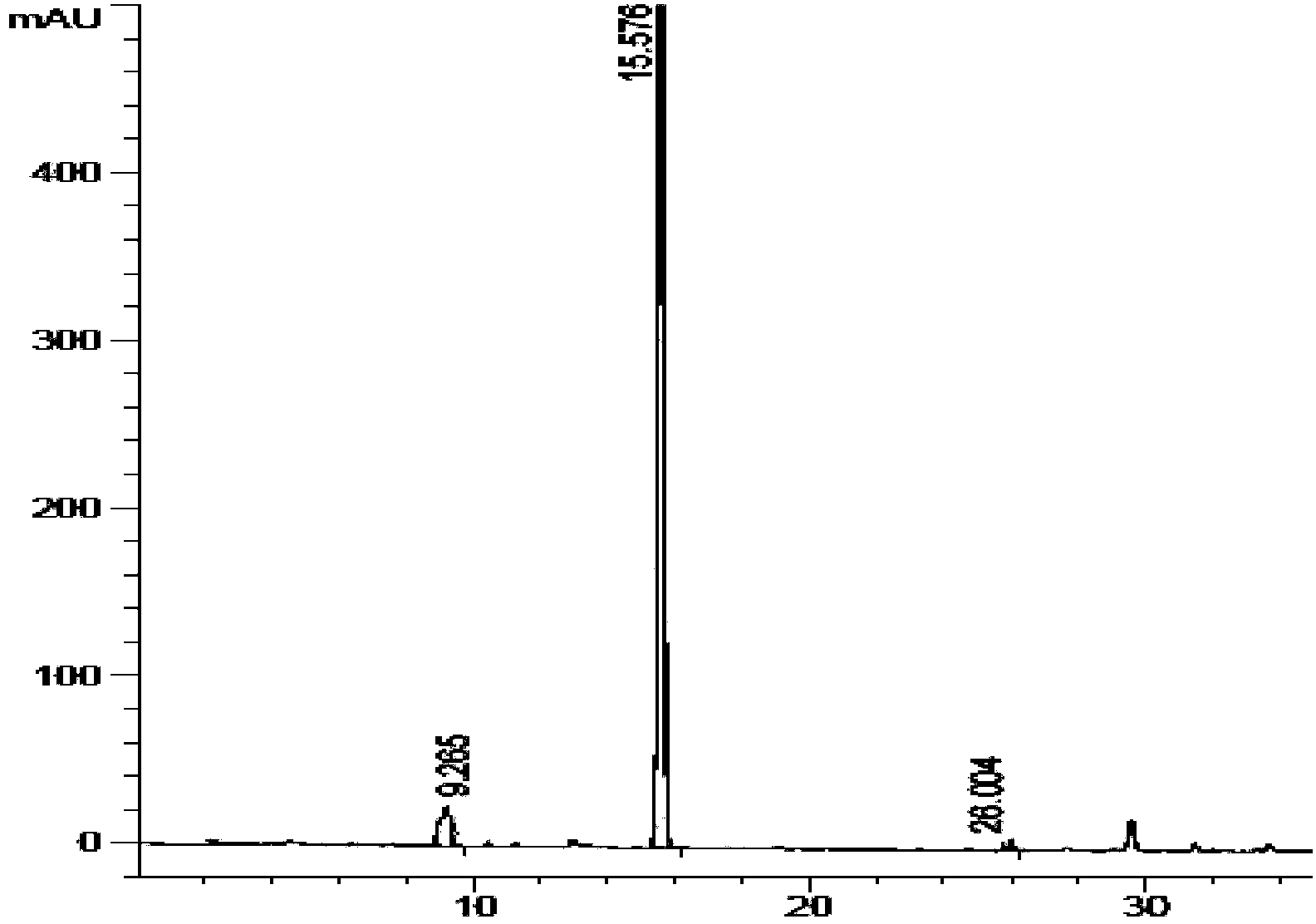
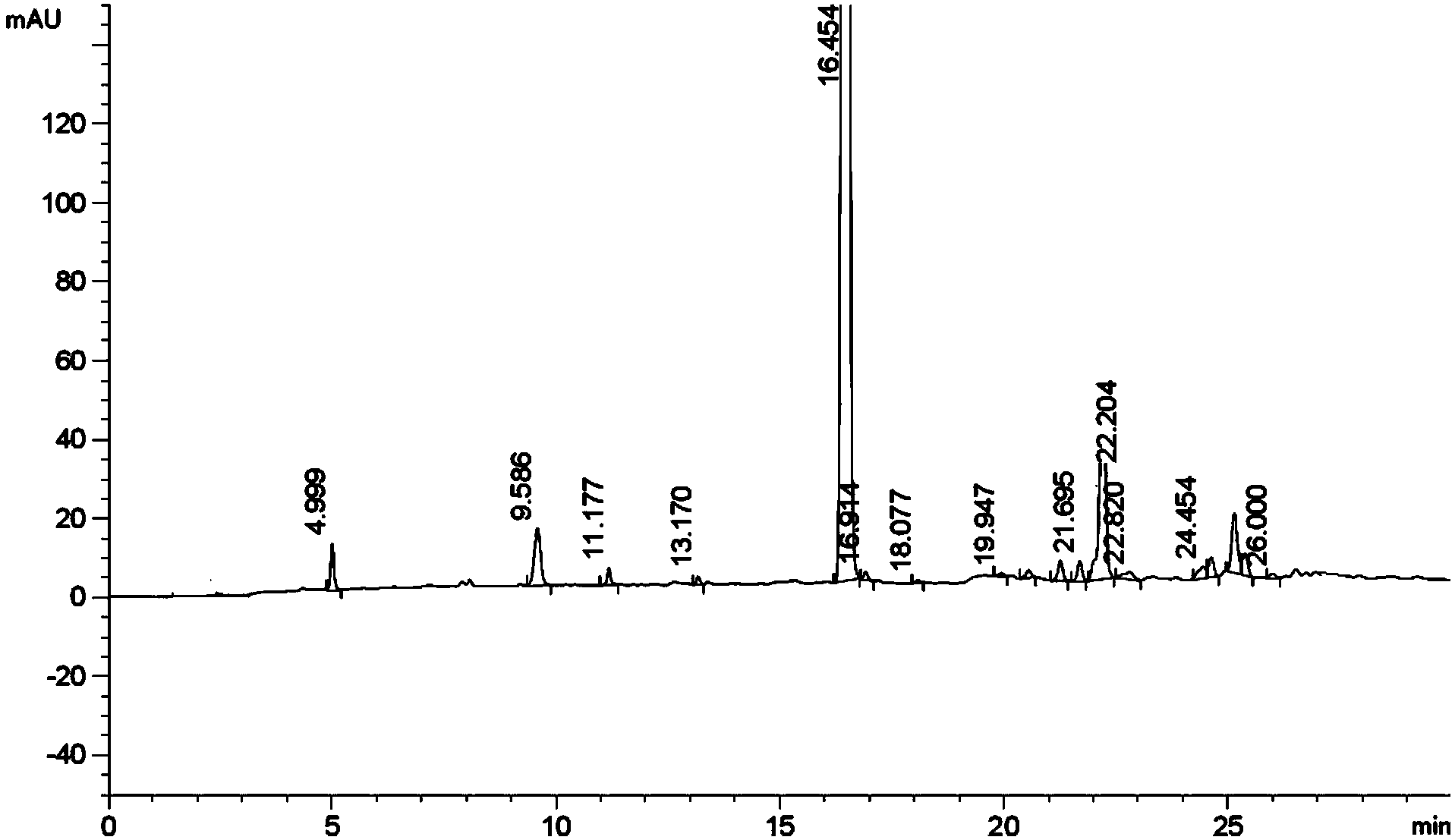
Preparation method of kyprolis
A technology of carfilzomib and compounds, which is applied in the field of preparation of carfilzomib, can solve the problems of inapplicability to large-scale industrial production, complex and diverse reaction conditions, and low yield of final products, and achieve low price and few reaction steps , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The invention provides a kind of preparation method of carfilzomib, comprises the following steps:
[0045] Step 1. Under the action of a condensing agent and an organic base, a condensation reaction occurs between the compound shown in formula (II) and the compound shown in formula (III) to obtain the compound shown in formula (IV), which is then protected by deamination to generate formula (V ) shown in the compound;
[0046]
[0047] Among them, R 1 is a carboxyl protecting group;
[0048] Step 2. Under the action of a condensing agent and an organic base, a condensation reaction occurs between the compound shown in formula (V) and the compound shown in formula (VI) to obtain the compound shown in formula (VII), which is then protected by decarboxylation to generate formula ( IX) the compound indicated;
[0049]
[0050] Step 3, the compound represented by formula (IX) and the compound represented by formula (VIII) undergo a condensation reaction to generate...
Embodiment 1
[0090] Preparation of compound shown in embodiment 1 formula (V-a)
[0091] Under nitrogen protection, add 27.9g (0.10mol) of N-Boc-L-homophenylalanine and 26.0g (0.105mol) of L-leucine methyl ester trifluoroacetate into a 1000mL three-necked flask, and add 500mL Tetrahydrofuran, and 51.7 g (0.4 mol) of DIEA were added thereto, and the mixture was cooled to 0°C while stirring. To this mixture was added HOBT 14.9 g (0.11 mol). The reactant was placed under nitrogen, stirred for 2 h, distilled under reduced pressure, and the residue was dissolved in 300 mL of dichloromethane, and washed twice with saturated sodium bicarbonate, water and saturated saline, each time 150 mL, organic The layer was evaporated to dryness under reduced pressure to obtain 36.5 g of the compound of formula (IV), to which 120 mL of a mixed solution of trifluoroacetic acid: dichloromethane = 4:1 was added, stirred and reacted at 15°C for 7 h, and after concentration, 29.3 g of off-white solid was obtained...
Embodiment 2
[0095] Preparation of compound shown in embodiment 2 formula (V-b)
[0096] Under argon protection, add 27.9g (0.10mol) of N-Boc-L-homophenylalanine and 39.2g (0.2mol) of L-leucine ethyl ester hydrochloride into a 1000mL three-necked flask, and add 600mL of acetonitrile , and 158.2 g (2.0 mol) of pyridine was added thereto, and the mixture was cooled to -5°C while stirring. Add HBTU113.8g (0.3mol) to this mixture, place the reactant under argon, stir the reaction for 18h, distill under reduced pressure, dissolve the residue in 300mL ethyl acetate, wash with saturated sodium bicarbonate, water and saturated salt in sequence Wash twice with water, 150 mL each time, evaporate the organic layer to dryness under reduced pressure to obtain 37.2 g of the compound of formula (IV), add it to HCl-ethyl acetate (about 1.5 mol / L) solution, and react at 20 °C After 10 hours, it was concentrated to dryness, and after purification, 26.6 g of off-white solid was obtained. According to HPLC d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com