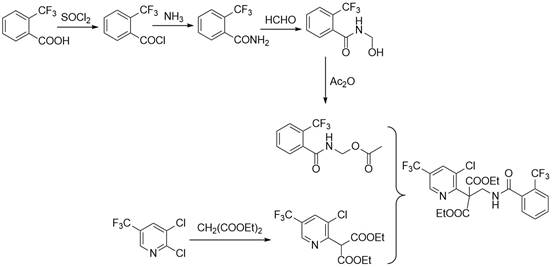
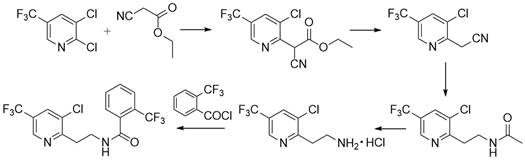
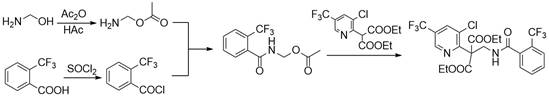
Synthesis method of fluopyram intermediate
A technology of fluopyram and its synthetic method, which is applied in the field of preparation of pesticide intermediates, can solve the problem of increased unit consumption of o-trifluoromethylbenzoyl chloride, high activity of o-trifluoromethylbenzoyl chloride, and poor stability of intermediate products and other issues, to achieve high product yield, reduce raw material costs, and facilitate post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 47.0 g (1.00 mol) methanolamine was added to 6.6 g (0.10 mol) acetic acid, 0.9 g (2%) tetrabutylalonyl chloride, 140.0 g of 1,2-dichloroethane to add mechanical stirring, condensed tube, thermometer In the 1000 ml of three flasks, 112.3 g (1.10 mol) acetic anhydride was dripped over 30 minutes, and the reaction endpoint was determined according to liquid chromatography analysis. After the reaction was completed, the reaction was used to regulate the system pH. = 7, the water layer, the water wash the reaction liquid once, and the organic phase often pressed back to flow water to obtain an amino acetate 1,2-dichloroethane solution to carry out the next reaction.
[0030] 157.8 g (0.83 mol) was added to 2000 ml of three-port flasks with mechanical stirring, condensed tubes, and thermometer, and 108.6 g (0.91 mol) in 30 minutes. The sulfoxide, 80 to 85 ° C for 2 to 3 hours, determined according to the liquid chromatographic analysis results, after the reaction is completed, th...
Embodiment 2
[0034]47.0 g (1.00 mol) methanolamine was added to 7.8 g (0.13 mol) acetic acid, 2.2 g (5%) tetrabutylammonium chloride, 185.0 g 1, 2-dichloroethane to add a mechanical stirring, condensation, and thermometer. In a 1000 ml of three flasks, 122.5 g (1.2 mol) acetic anhydride was dripped over 30 minutes, and the reaction endpoint was determined according to liquid chromatography analysis. After the reaction was completed, the reaction was used to adjust the hydrogencarbonate aqueous solution to regulate the system pH. = 7, the water layer, the water wash the reaction liquid once, and the organic phase often pressed back to flow water to obtain an amino acetate 1,2-dichloroethane solution to carry out the next reaction.
[0035] 157.8 g (0.83 mol) was added to the 2000 ml of the three-port flask with mechanical stirring, condensed tubes, and thermometer, and 108.6 g (0.91 mol) was added to 30 minutes in 30 minutes. The sulfoxide, 80 to 85 ° C for 2 to 3 hours, determined according to...
Embodiment 3
[0039] 47.0 g (1.00 mol) methanolamine was added to 9.6 g (0.16 mol) acetic acid, 3.6 g (8%) tetrabutylammonium chloride, 230.0 g of 1,2-dichloroethane with mechanical stirring, condensed tube, thermometer In the 1000 ml of three flasks, 132.7 g (1.30 mol) acetic anhydride was added over 30 minutes, and the reaction endpoint was determined according to liquid chromatography analysis. After the reaction was completed, the hydrogencarbonate aqueous solution was used to regulate the system pH. = 7, the water layer, the water wash the reaction liquid once, and the organic phase often pressed back to flow water to obtain an amino acetate 1,2-dichloroethane solution to carry out the next reaction.
[0040] 157.8 g (0.83 mol) was added to 2000 ml of three-port flask with mechanical stirring, condensed tubes, and thermometer, 30 minutes (0.91 mol) in 30 minutes, 780 g 1,2-dichloroethane. Chloride, 80 to 85 ° C for 2 to 3 hours, determined according to the liquid chromatographic analysis r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com