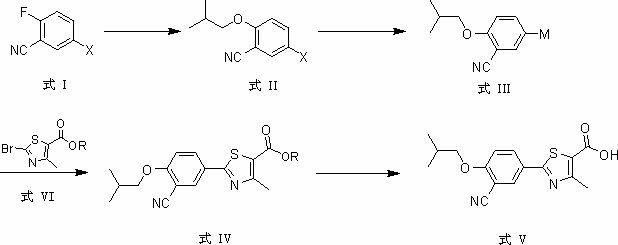
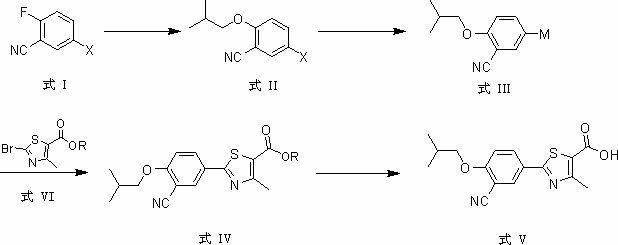
The synthetic method of febuxostat
A technology of febuxostat and a synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of complicated conversion, increase reaction steps, etc., and achieve the effects of high safety, reduced risk, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0028] Example: Synthesis of febuxostat—2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-5thiazolecarboxylic acid
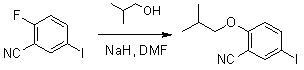
[0029] Synthesis of 5-iodo-2-isobutoxybenzonitrile
[0030]
[0031] Dissolve 2-methyl-1-propanol (2.8 mL, 30.2 mmol) in 50 mL DMF, cool to 0 °C, add NaH (1.21 g, 30.2 mmol), stir for 30 minutes, add 2-fluoro-5-iodine Benzonitrile (5.0 g, 20.2 mmol), raised to room temperature, stirred overnight. Add water (100 mL), extract with ethyl acetate (100 mL) three times, wash the organic phase twice with saturated sodium chloride solution (150 mL), dry over anhydrous sodium sulfate, filter, concentrate, and purify on a silica gel column (petroleum ether: Ethyl acetate = 20:1 elution), concentrated to give a light yellow liquid (5.78 g, 95% yield).
[0032] 1 H-NMR (d-DMSO, 400 MHz): 1.00(d, 6H, J=6.8), 2.05(m, 1H), 3.90(d, 2H, J=6.8), 7.06(d, 1H, J=8.8 ), 7.93(dd, 1H), 8.04(d, 1H, J=2.4).
[0033] Synthesis of 3-cyano-4-isobutoxyphenylboronic acid pina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com