Preparation method of fondaparinux sodium monosaccharide intermediate
A technology of sulfonic acid anhydride and sulfonyl halide, which is applied in the field of preparation of fondaparinux sodium monosaccharide intermediates, can solve the problems of unfavorable industrial production, many impurities, and high cost, and achieve less impurities, controllable reaction process, The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-1
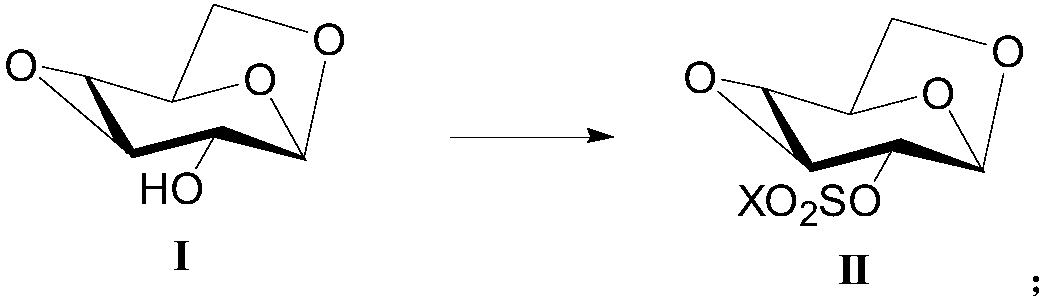
[0070] Example 1-1 : Preparation of 1,6:3,4-dianhydro-2-O-trifluoromethanesulfonyl-β-D-glucopyranose (compound shown in formula (II), X is trifluoromethyl):
[0071] Dissolve 1,6:3,4-dianhydro-β-D-glucopyranose (25.0g, compound represented by formula (I)), N,N-diisopropylethylamine (33.6g) in acetone (400mL), lower the temperature to -20°C, slowly add a solution of trifluoromethanesulfonic anhydride (58.7g) in acetone (100mL) dropwise, stir and react at -20°C for 1h, after the reaction is complete, add water dropwise to quench the reaction solution, and rotary evaporate under reduced pressure to dryness, extracted with dichloromethane, washed with salt water, dried over anhydrous sodium sulfate, and rotary evaporated to dryness under reduced pressure. The crude product was recrystallized from isopropanol to obtain 1,6:3,4-dianhydro-2-O-trifluoro Methylsulfonyl-β-D-glucopyranose, white solid (40.7g), yield 85%, purity 98.5%.
example 1-2
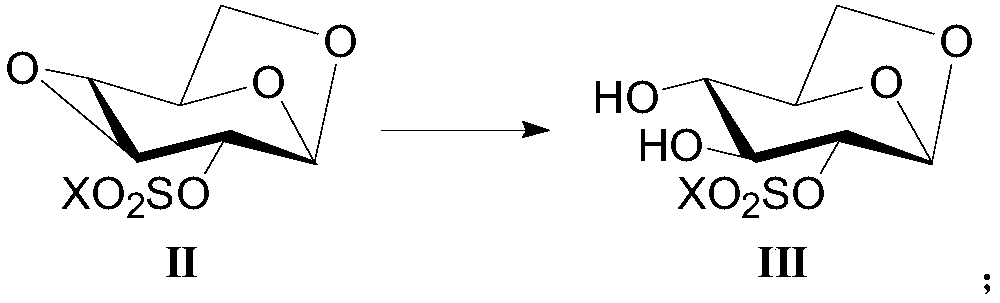
[0072] Example 1-2 : Preparation of 1,6-anhydro-2-O-trifluoromethanesulfonyl-β-D-glucopyranose (compound shown in formula (Ⅲ)):
[0073] Dissolve 1,6:3,4-dianhydro-2-O-trifluoromethanesulfonyl-β-D-glucopyranose (40.0g) in xylene (350mL), slowly add concentrated sulfuric acid (0.14g) dropwise , stirred at 40°C for 4 hours, after the reaction was completed, evaporated to dryness under reduced pressure, extracted with dichloromethane, washed with brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure, the crude product was recrystallized from a mixed solvent of ethyl acetate-petroleum ether , to obtain 1,6-anhydro-2-O-trifluoromethanesulfonyl-β-D-glucopyranose as a white solid (38.3 g), with a yield of 90% and a purity of 98.7%.
example 1-3
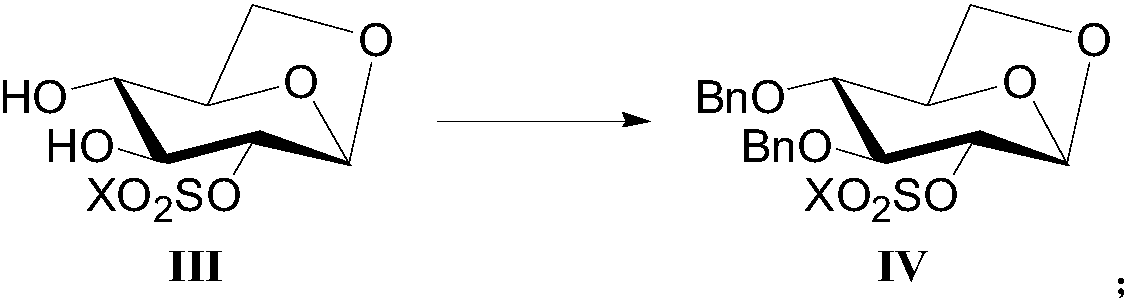
[0074] Example 1-3 : Preparation of 1,6-anhydro-2-O-trifluoromethanesulfonyl-3,4-bis-O-benzyl-β-D-glucopyranose (compound shown in formula (IV)):
[0075] Dissolve 1,6-anhydro-2-O-trifluoromethanesulfonyl-β-D-glucopyranose (35.0g), sodium hydroxide (19.0g) in N,N-dimethylacetamide (450mL) , add benzyl bromide (61.0g) in N,N-dimethylacetamide solution (50mL) dropwise, keep warm at 50°C for 8h until the reaction is complete, rotary evaporate to dryness under reduced pressure, extract with dichloromethane, wash with salt water, no Dry over sodium sulfate, evaporate to dryness under reduced pressure, and recrystallize the crude product from a mixed solvent of ethyl acetate-petroleum ether to obtain 1,6-anhydro-2-O-trifluoromethanesulfonyl-3,4-bis-O- Benzyl-β-D-glucopyranose, white solid (46.8g), yield 83%, purity 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com