Preparation method of fondaparinux sodium injection composition
A technology of fondaparinux sodium and injection, applied in the field of medicine, can solve the problems of easy crystallization and poor clarity, and achieve the effects of improving stability, reducing the generation of impurities, and reducing crystallization and turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
--
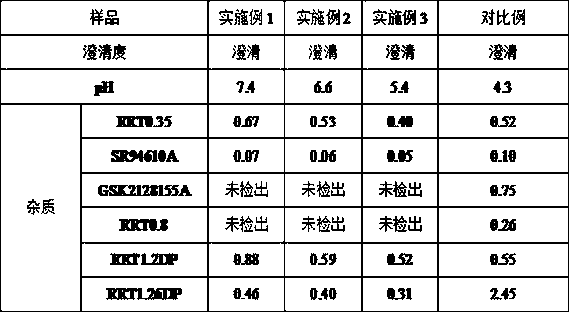
[0022] In the present embodiment, the preparation method of the fondaparinux sodium injection composition comprises the following steps:
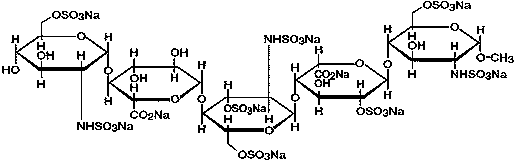
[0023] 1) According to the prescription of fondaparinux sodium injection composition, each raw material is weighed according to the prescription amount, and the fondaparinux sodium injection composition is composed of the following raw materials: fondaparinux sodium, sodium chloride, pH Regulator, water for injection, wherein, the pH regulator is sodium hydroxide or hydrochloric acid;
[0024] 2) Take fondaparinux sodium and sodium chloride, combine them, add 75% water for injection in total, stir and dissolve, adjust the pH to 7-8 with a pH regulator, then add the remaining water for injection, stir Mixing to obtain a medicinal solution, wherein the stirring is carried out at 10°C;
[0025] 3) Filling the prepared medicinal solution, after high-temperature sterilization and cooling, light inspection, packaging and inspection, t...
Embodiment 2
--
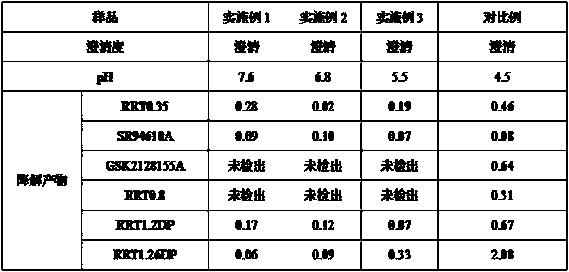
[0028] In the present embodiment, the preparation method of the fondaparinux sodium injection composition comprises the following steps:
[0029] 1) According to the prescription of fondaparinux sodium injection composition, each raw material is weighed according to the prescription amount, and the fondaparinux sodium injection composition is composed of the following raw materials: fondaparinux sodium, sodium chloride, pH Regulator, water for injection, wherein, the pH regulator is sodium hydroxide or hydrochloric acid;
[0030] 2) Take fondaparinux sodium and sodium chloride, combine them, add 80% of the total amount of water for injection, stir and dissolve, use a pH regulator to adjust the pH to between 6-7, then add the remaining water for injection, and stir Mixing to obtain a medicinal solution, wherein the stirring is carried out at 20°C;
[0031] 3) Filling the prepared medicinal solution, after high-temperature sterilization and cooling, light inspection,...
Embodiment 3
--
[0034] In the present embodiment, the preparation method of the fondaparinux sodium injection composition comprises the following steps:
[0035] 1) According to the prescription of fondaparinux sodium injection composition, each raw material is weighed according to the prescription amount, and the fondaparinux sodium injection composition is composed of the following raw materials: fondaparinux sodium, sodium chloride, pH Regulator, water for injection, wherein, the pH regulator is sodium hydroxide or hydrochloric acid;
[0036] 2) Take fondaparinux sodium and sodium chloride, combine them, add 85% water for injection in total, stir and dissolve, adjust the pH to 5-6 with a pH regulator, then add the remaining water for injection, stir Mixing to obtain a medicinal solution, wherein the stirring is carried out at 30°C;
[0037] 3) Filling the prepared medicinal solution, after high-temperature sterilization and cooling, light inspection, packaging and inspection, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com