Fondaparinux sodium disaccharide intermediate fragment BA and synthetic method thereof
A technology of fondaparinux sodium and a synthetic method is applied in the field of synthesis and preparation of disaccharide fragment BA, can solve problems such as unfavorable drug promotion and popularization, unfavorable large-scale production, high product price, etc., and achieves optimized synthetic route and product purification. The effect of convenience and few synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
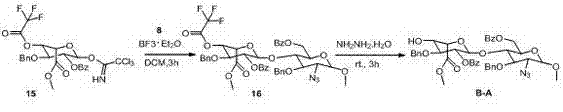
[0033] A Fondaparinux sodium disaccharide intermediate fragment BA, characterized in that: the structural formula of the Fondaparinux sodium disaccharide intermediate fragment BA is: ,among them, R 1 For: -CH 3 ;-C 2 H 5 . R 2 For: -OH; -OAc; -OBz. R 3 For: -OH; -OAc; -OBn. R 4 For: -N 3 ; -NHAc; -NHCBz. R 5 For: -COOH; -COOCH 3 ;-COOC 2 H 5 .
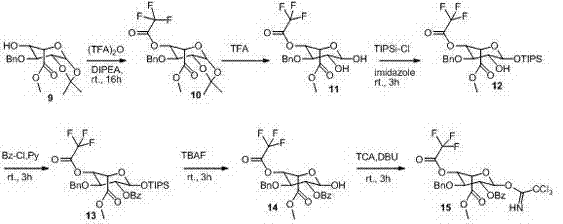
[0034] A method for synthesizing Fondaparinux sodium disaccharide intermediate fragment BA, firstly synthesize ring A and ring B respectively, where ring A is the compound: , Ring B is the compound: ; The A ring and the B ring are coupled into a disaccharide derivative under the action of Lewis acid; the disaccharide derivative is under the action of a base to remove the carboxylic acid group on the B ring to obtain the Fondaparinux sodium disaccharide intermediate Body fragment BA.
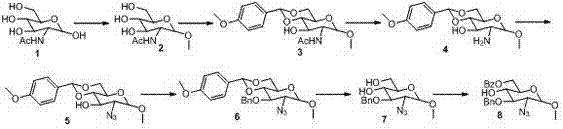
[0035] Such as figure 1 As shown, the synthesis process of ring A is as follows:
[0036] Step 1: Preparation of 1-O-methyl 2-acetylamino-2-deoxy-α-D-glucopy...
specific Embodiment , example 2
[0069] The difference between this embodiment and the first embodiment is: the starting material N-acetyl-glucosamine is charged at 200g; 3-O-benzyl-1,2-O-isopropylidene-6-methyl-α The feed amount of -L-idonic acid is 318 g, and the feed amount of other substances is correspondingly enlarged according to the mass ratio, referring to the operation of each step described in Example 1, to obtain 208 g of Fondaparinux disaccharide intermediate fragment BA.
specific Embodiment , example 3
[0071] The difference between this embodiment and the first embodiment is that: the starting material N-acetyl-glucosamine dosage is 1kg; 3-O-benzyl-1,2-O-isopropylidene-6-methyl-α -The dosage of L-idonic acid is 1.59kg, and the dosage of other substances should be enlarged according to the mass ratio. Refer to the operation of each step described in Example 1, to obtain 1.17kg of Fondaparinux disaccharide intermediate fragment BA, .
[0072] The invention uses a new synthesis strategy, optimizes the synthesis route, adopts mild reaction conditions, fewer synthesis steps, higher yield, convenient product purification, can effectively scale up for industrialized large-scale production, and has higher economic benefits.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com