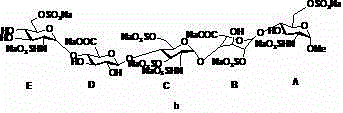
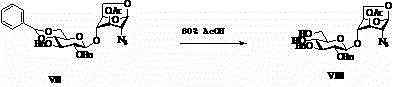Novel technology for preparing disaccharide fragment of fondaparinux sodium intermediate
A technology of fondaparinux sodium and intermediates, applied in the field of disaccharide intermediates, can solve the problems of long linear route, complicated operation steps of protecting groups, difficult separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
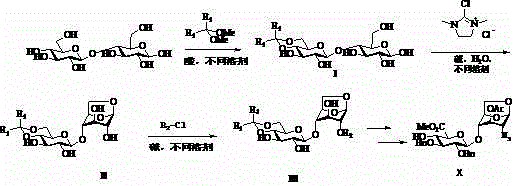
[0016] Embodiment 1: preparation compound I
[0017]
[0018] Take a 500mL straight mouth four-neck bottle, put it on the rack with mechanical stirring, and a thermometer, and start stirring with 42.80g (0.125mol, 1.0eq) of cellobiose, 300mL of DMF and 30g (0.197mol, 1.6eq) of benzaldehyde dimethyl acetal , and finally add 1.0mL methanesulfonic acid, then heat the reaction solution to 85 with an oil bath o C, react for 3 hours. 5 mL of triethylamine was added, DMF was distilled off under reduced pressure, and 300 mL of toluene was added to the mixture to precipitate a large amount of solid, which was filtered and dried to obtain 39.6 g, with a yield of 74%. The product did not need further purification and was directly used in the next reaction.
Embodiment 2
[0019] Embodiment 2: preparation compound II
[0020]
[0021] Take a 1000mL straight mouth four-neck bottle, put it on the rack with mechanical stirring, and mix 53.8g (0.125mol, 1.0eq) and 500.0ml of H 2 0 and 156.0ml of triethylamine (1.1mol, 9.0eq) began to stir, and finally 63.40g (0.379mol, 3.0eq) of DMC was added. After the addition, keep the reaction temperature at room temperature and stir the reaction for 24h. The mixture was distilled under reduced pressure, and then 300 mL of toluene was added to the mixture. A large amount of solids precipitated out. The solids were filtered, and then 500 mL of acetonitrile was added and heated to reflux. The precipitated solids were removed by thermal filtration, and the filtrate was evaporated to dryness to obtain 50.5 g of yellow solids, with a yield of 98%.
Embodiment 3
[0022] Embodiment 3: preparation compound III
[0023]
[0024] In a 250ml single-necked bottle, add 70ml of THF at room temperature, 14.4g of compound II (35mmol, 1.0eq), 7.46g of isopropylsulfonyl chloride (52.5mmol, 1.5eq) and 13g of 4-dimethylaminopyridine (10.6mmol, 3.0eq ), start stirring at room temperature, stir for 24 hours, add 100mL of ethyl acetate, wash the organic phase with 50mL of saturated ammonium chloride water, 50mL of saturated brine, then dry with sodium sulfate, and evaporate to dryness under reduced pressure to obtain the crude product . The crude product was recrystallized in methyl tert-butyl ether and heptane to give 10.7 g of white solid, yield 59.2%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com