Thiol-ene click chemistry based preparation and characterization of fatty acid modified heparin
An alkene click, chemical technology, applied in material analysis by observing the influence of chemical indicators, analysis by chemical reaction of materials, scientific instruments, etc., can solve the problem of unstable quality, poor patient compliance, and cannot replace each other. and other problems to achieve the effect of expanding the scope of applications and great attraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] In combination with the above synthetic circuit diagram, the preparation method of the fatty acidated heparin based on thiol-ene click chemistry comprises the following steps:
[0062] 1) Take the structural formula as (n=10, 12, 14, 16, 18) five different long-chain saturated fatty acids of lauric acid, myristic acid, palmitic acid, stearic acid and arachidic acid with cystamine Under alkaline conditions, an acylation reaction occurs to generate the structural formula (n=10, 12, 14) compound 2a and structural formula are Compound 2b of (n=16, 18);
[0063] 2) Reducing the disulfide bonds of compounds 2a and 2b to generate a structural formula of Compound 3 of (n=10, 12, 14, 16, 18);
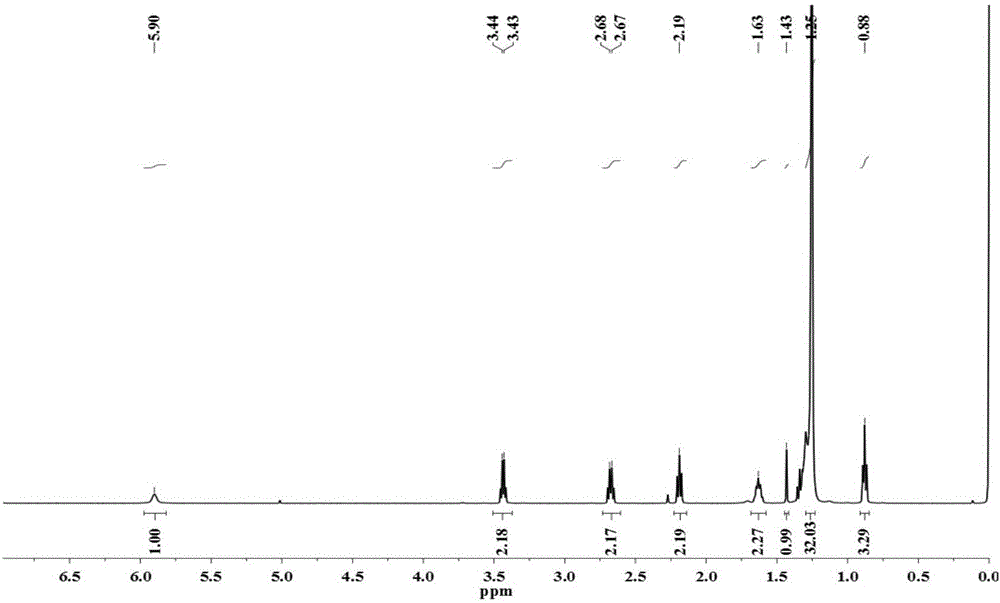
[0064] 3) the structural formula is Allyl glycidyl ether with heparin (M and N are other polysaccharide sequences in the heparin structure) Under the alkaline condition of sodium hydroxide, the ring-opening reaction of ethylene oxide occurs, and the resulting structural formu...
Embodiment 1
[0085] Example 1: Preparation of Fattylated Heparin Conjugates Modified by Lauric Acid
[0086] (1) Preparation of mercaptolated lauric acid (12 carbons):
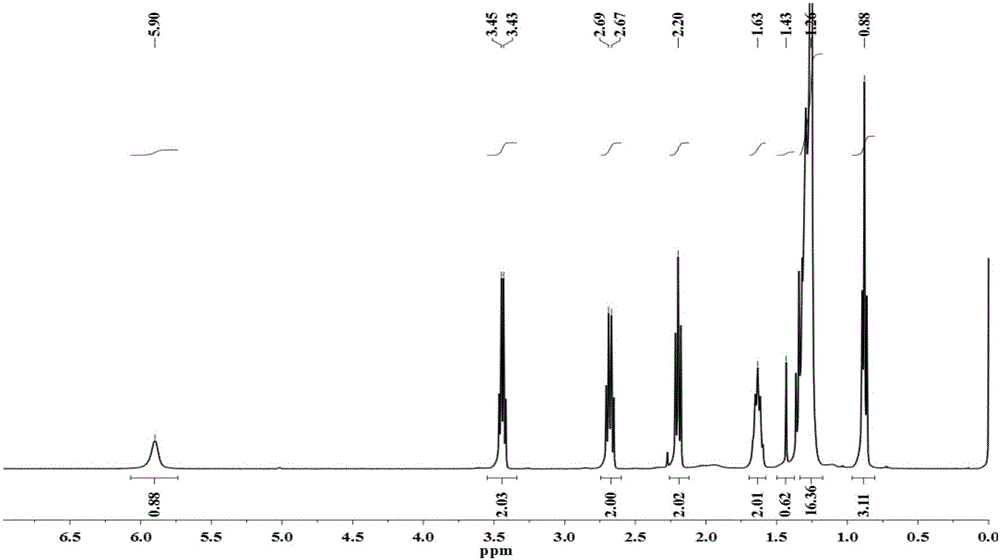
[0087] ① Preparation of intermediates containing disulfide bonds: Take 5g of lauric acid (25mmol) and 11g of Carter condensing agent (25mmol) and dissolve them with 100mL of N'N-dimethylformamide; then add 12.4mL of N'N-Dimethylformamide Isopropylethylamine (75mmol), mixed and stirred for about 10min; finally, 1.8mL of cystamine (13.75mmol) was slowly added dropwise, stirred and reacted for 8h, until the reaction was complete as detected by thin-layer chromatography. About 500 mL of water was added to the reaction solution to precipitate a solid, which was collected by filtration, heated and dissolved in ethanol at 70°C, reprecipitated, filtered again, and dried to obtain a white solid, which was an intermediate containing a disulfide bond (compound 2a, n =10), mass 5.2g, productive rate 80.5%, its mass spectrum is attach...
Embodiment 2
[0091] Example 2: Preparation of fatty acidated heparin conjugates modified with palmitic acid
[0092] (1) Preparation of mercapto-palmitic acid (16 carbons):
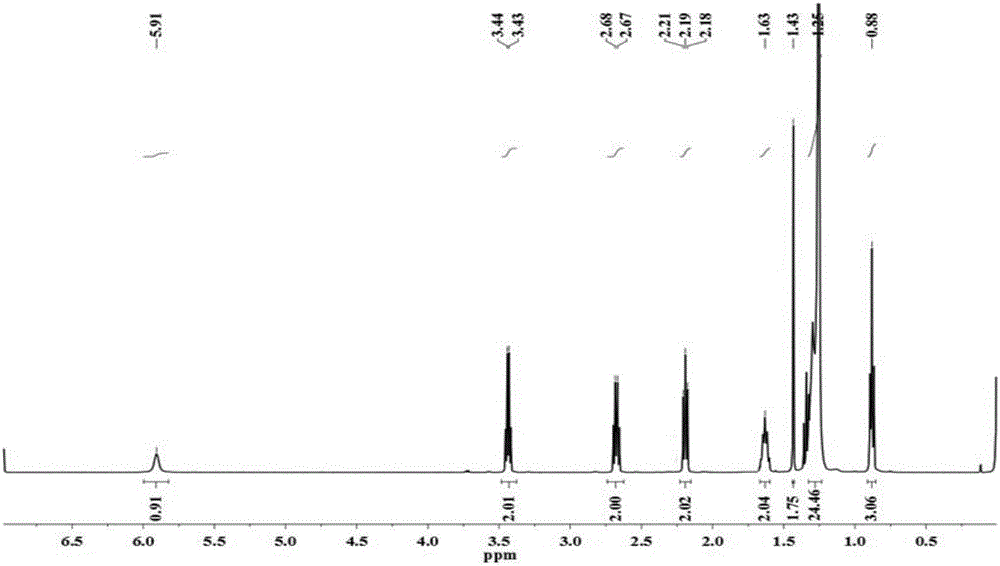
[0093] ①Preparation of intermediates containing disulfide bonds: Take 5g of palmitic acid (19.5mmol) and 8.6g of Carter condensing agent (19.5mmol) and dissolve them with 100mL of N'N-dimethylformamide; then add 9.7mL of N'N-Diisopropylethylamine (58.5mmol), mixed and stirred for about 10min; finally, 1.4mL of cystamine (10.7mmol) was slowly added dropwise, stirred for 8h, until the reaction was complete as detected by thin-layer chromatography. About 500 mL of water was added to the reaction solution to precipitate a solid, which was collected by filtration, heated and dissolved in ethanol at 70°C, reprecipitated, filtered again, and dried to obtain a white solid, which was an intermediate containing a disulfide bond (compound 2a, n =14), mass 4.4g, productive rate 71.8%, its mass spectrum is attached Figure 4 sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Replacement rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com