Low-moisture high-purity fondaparinux sodium and preparation method thereof
A technology of fondaparinux sodium and water, which is applied in the field of chemical raw materials and its preparation, can solve the problems of infecting raw materials, inconvenient storage and transportation of raw materials, and breeding bacteria in combination with water, so as to facilitate storage and transportation, increase stability, The effect of reducing the chance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053](1) Pretreatment before freeze-drying: Dissolve 10.0 g of commercially available fondaparinux sodium (98.5% purity, 10% water) into 200 ml endotoxin-free purified water to prepare a solution with a concentration of 50 mg / ml. The solution is poured into a solvent filter and filtered. After the filtration is completed, the solution is poured into a tray and placed in a freeze dryer;
[0054] (2) Pre-freezing stage: lower the temperature of the freeze dryer partition to -45°C and keep it warm for 3 hours;
[0055] (3) Sublimation drying stage:
[0056] a) Raise the temperature of the separator from -45°C to -30°C within 1 hour, keep it warm for 1 hour, and set the vacuum degree to 0.1mbar;
[0057] b) Raise the temperature of the separator from -30°C to -25°C within 5 hours, keep it warm for 8 hours, and set the vacuum degree to 0.1mbar;
[0058] c) Raise the temperature of the separator from -25°C to 0°C within 10 hours, keep it warm for 3 hours, and set the vacuum degre...
Embodiment 2
[0065] Commercially available fondaparinux sodium is 10.0 g, with a purity of 98.2% and a water content of 15%.
[0066] The preparation method before the analysis and drying stage is the same as that in Example 1. Analytical drying stage The heating time during analytical drying was set at 3 minutes, and other conditions were the same as in Example 1.
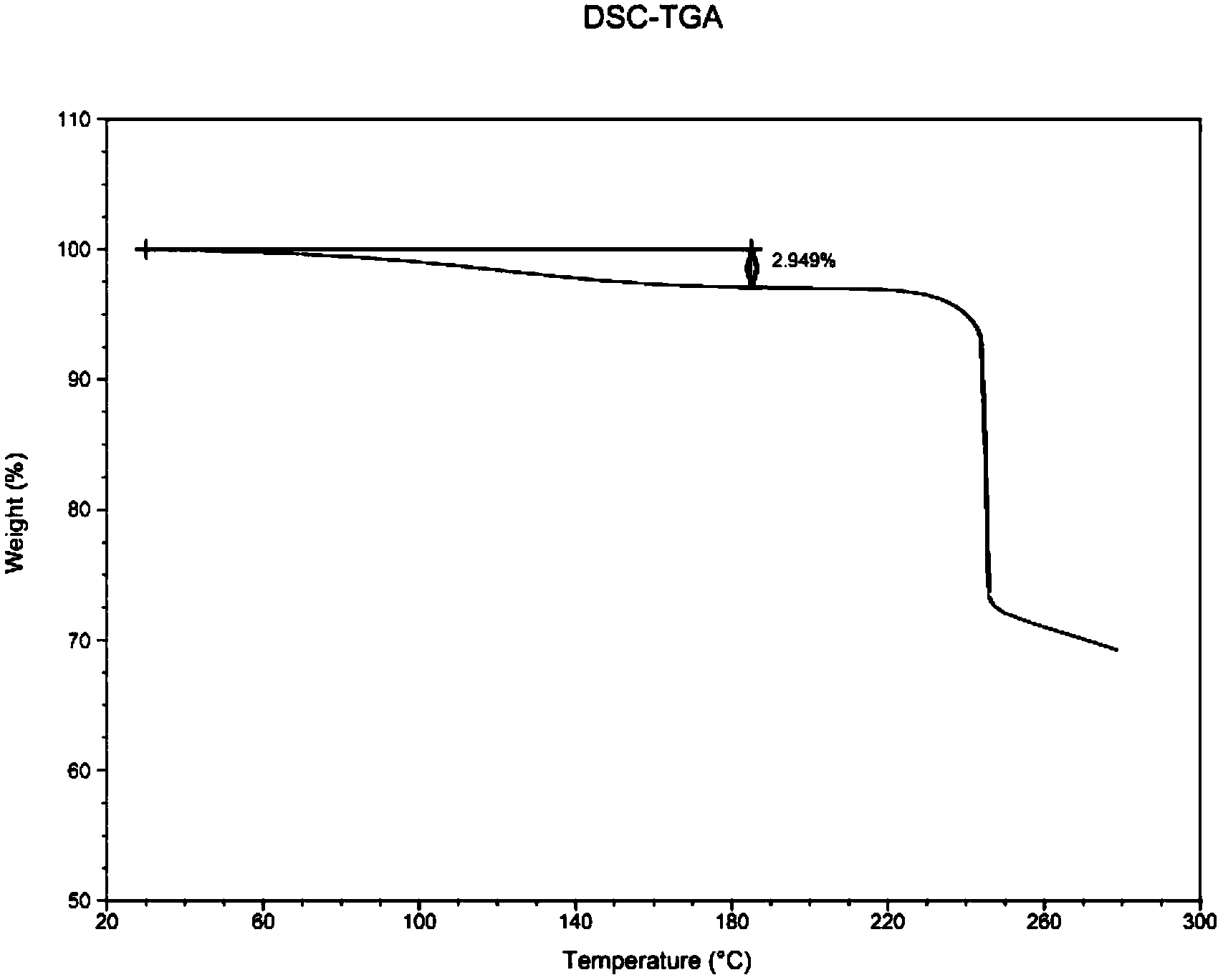
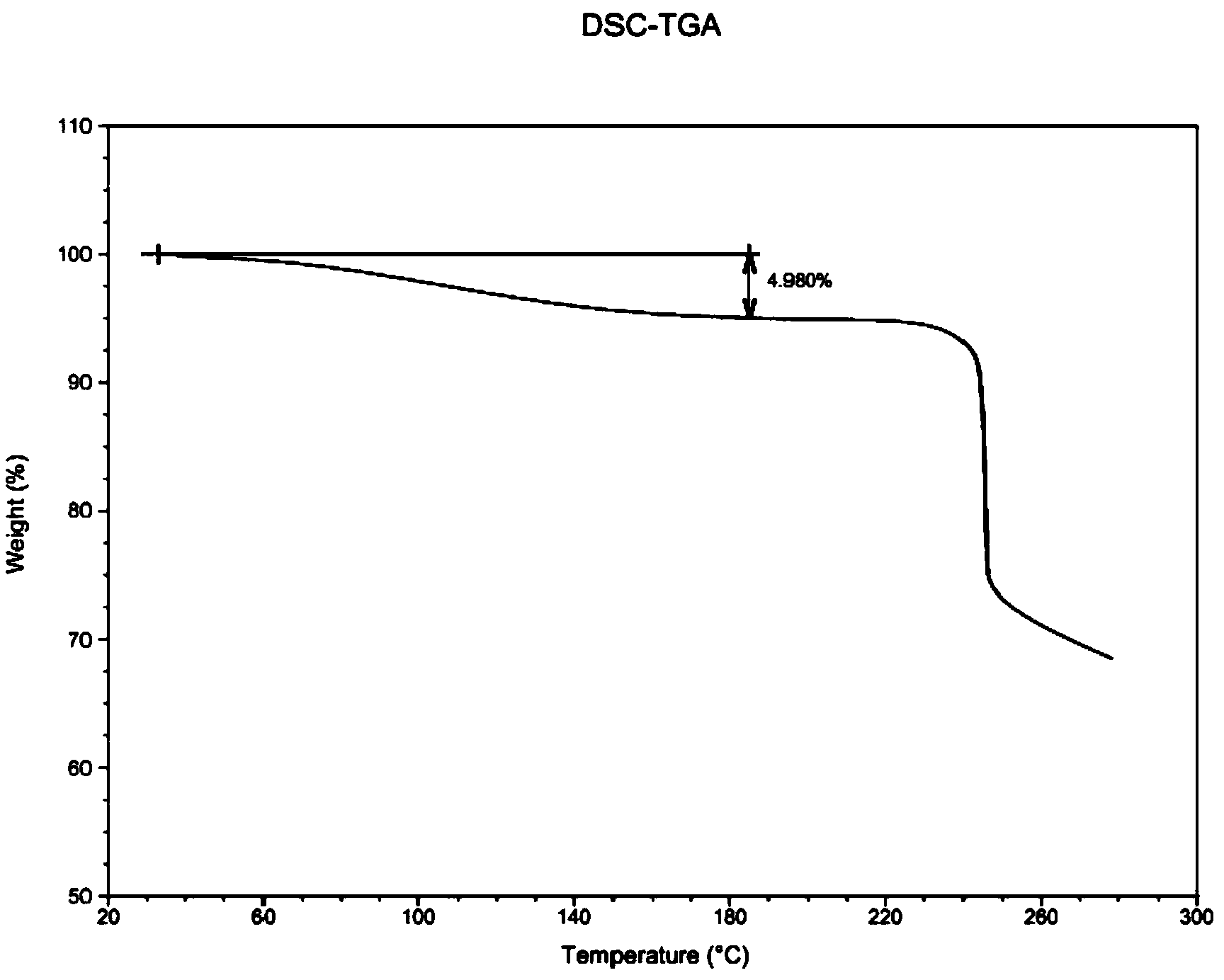
[0067] Obtain fondaparinux sodium, the purity is 99.1%, the water content is 4.980%, its DSC sees figure 2 .
Embodiment 3
[0069] (1) Pretreatment before freeze-drying: Dissolve 100.0 g of commercially available fondaparinux sodium (99.0% purity, 13% moisture) into 2000 ml endotoxin-free purified water to prepare a solution with a concentration of 50 mg / ml. The solution is poured into a solvent filter and filtered. After the filtration is completed, the solution is poured into 4 trays and put into a freeze dryer;
[0070] (2) The pre-freezing stage and (3) the sublimation drying stage are the same as in Example 1;
[0071] (4) Analytical drying stage: during analytical drying, the heating time is set to 1 minute, the set temperature is 35°C, the holding time is 10 hours, and the vacuum degree is set to 0mbar;
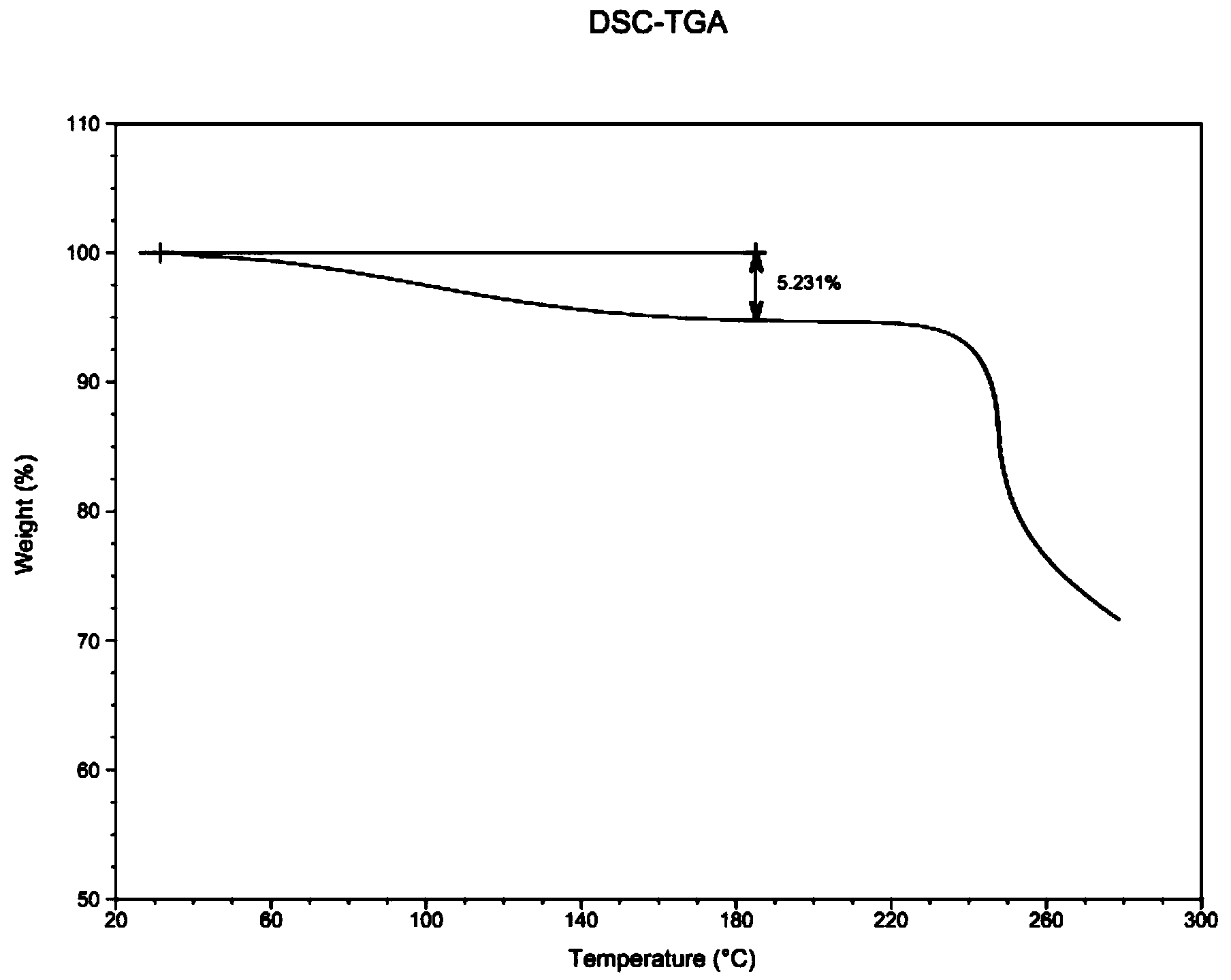
[0072] The moisture content that obtains fondaparinux sodium is 5.231%, and purity is 99.3%, and its DSC sees image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com