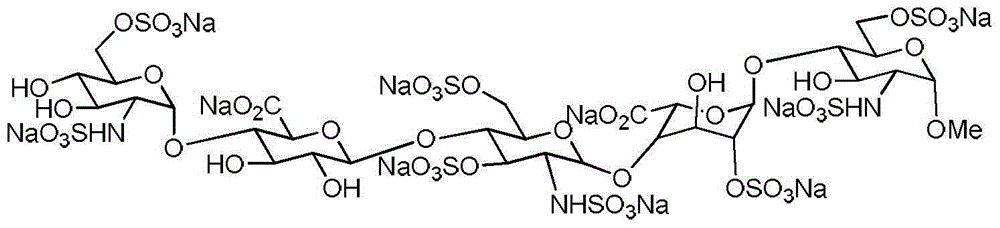
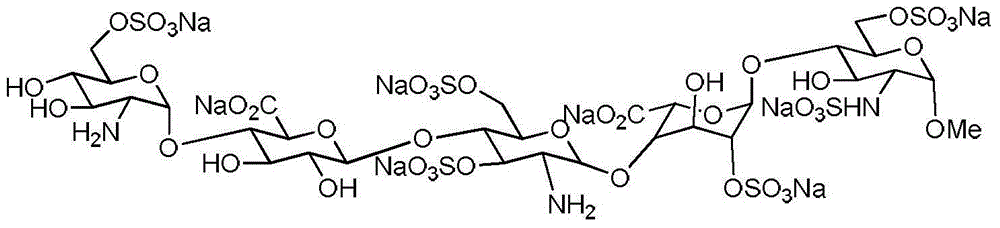
Reference compound for controlling quality of fondaparinux sodium
A reference compound, the technology of fondaparinux sodium, which is applied in the field of medicine, can solve the problems of low impurity content, large molecular weight of impurities, and difficulty in preparation and separation, and achieve the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
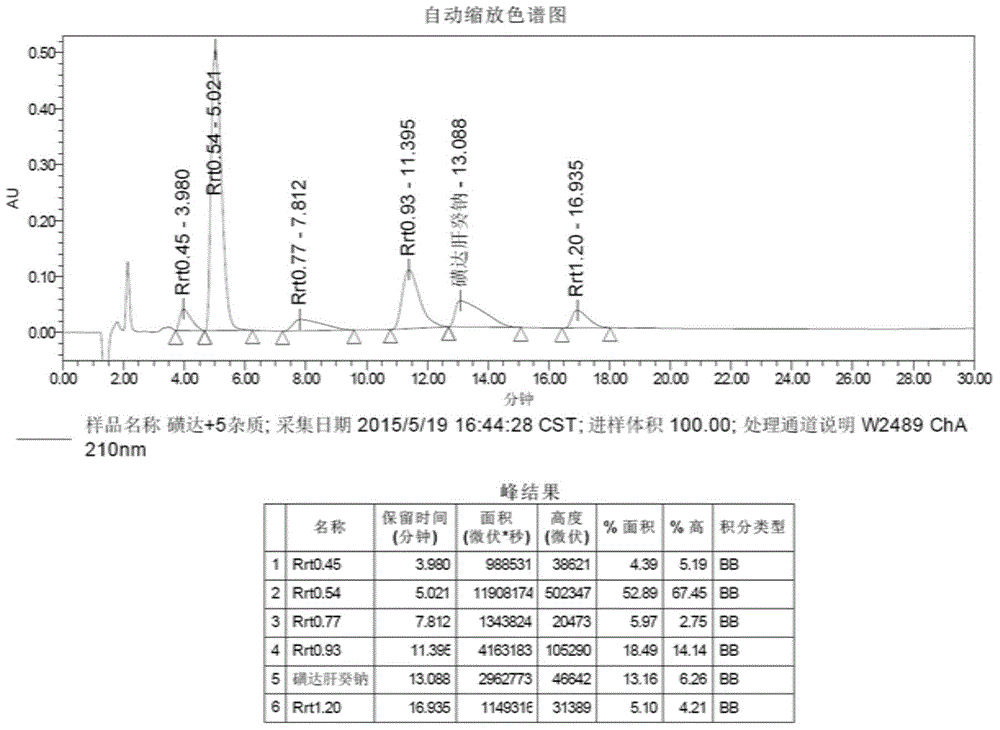
[0048] Example 1 Reference compound A (Rrt0.45):
[0049] Accurately weigh 1.5 g of pure fondaparinux sodium in a 500 ml round-bottomed flask, add 300 ml of 1 mol / L HCl aqueous solution to it, and stir in an oil bath at 40°C for 18 hours to generate acid degradation reference compound A (Rrt0. 45). Add 1 mol / L NaOH to adjust the pH to alkaline, desalt with Sephadex G-25 chromatographic column, and concentrate to dryness under reduced pressure to obtain the reference compound A crude product.
[0050] Weigh the crude product of reference compound A (Rrt0.45), dissolve it in purified water, use a high-performance preparative liquid chromatograph, select a strong base type anion exchange chromatographic column DIONEX CarboPac TMPA1 (250 × 9mm), and load a small amount of time. Separation, flow rate is 5.0ml / min, ultraviolet wavelength is 210nm, mobile phase A, B carry out gradient elution (mobile phase A: 117g / L aqueous sodium chloride solution, mobile phase B: water for injecti...
Embodiment 2
[0061] Example 2 Reference compound B (Rrt0.54):
[0062] Accurately weigh 1.5g of pure Fondaparinux sodium and 2.5g of NaCl into a 500ml soda-lime glass bottle, add 300ml of purified water to it, and seal it with an aluminum-plastic composite cover. High temperature destroyed reference compound B (Rrt 0.54). It was desalted by Sephadex G-25 chromatography, and concentrated to dryness under reduced pressure to obtain the reference compound B crude product.
[0063] The reference compound B (Rrt0.54) crude product was weighed, dissolved in purified water, and a high-efficiency preparative liquid chromatograph was used. The preparation conditions and separation steps were as shown in Example 1 to obtain a white solid reference compound B (Rrt0.54). 54).
[0064] Hydrogen and carbon spectrum data of reference compound B (Rrt0.54)
[0065]
[0066]
[0067] High-resolution mass spectrometry data of reference compound B (Rrt0.54)
[0068]
[0069] In the hydrogen spect...
Embodiment 3
[0071] Example 3 Reference compound C (Rrt0.77):
[0072] Accurately weigh 1.0g of pure fondaparinux sodium in a 500ml round-bottomed flask, add 200ml of 1 mol / L HCl aqueous solution to it, and stir at room temperature (20°C) for 65h to generate acid degradation reference compound C (Rrt0. 77). Add 1 mol / L NaOH to adjust the pH to alkaline, desalt with Sephadex G-25 chromatography column, and concentrate to dryness under reduced pressure to obtain the reference compound C crude product.
[0073] The reference compound C (Rrt0.77) crude product was weighed, dissolved in purified water, and a high-efficiency preparative liquid chromatograph was adopted. The preparation conditions and separation steps were as shown in Example 1 to obtain a white solid reference compound C (Rrt0.77). 77).
[0074] Hydrogen and carbon spectra of reference compound C (Rrt0.77)
[0075]
[0076]
[0077] High-resolution mass spectrometry data of reference compound C (Rrt0.77)
[0078]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com