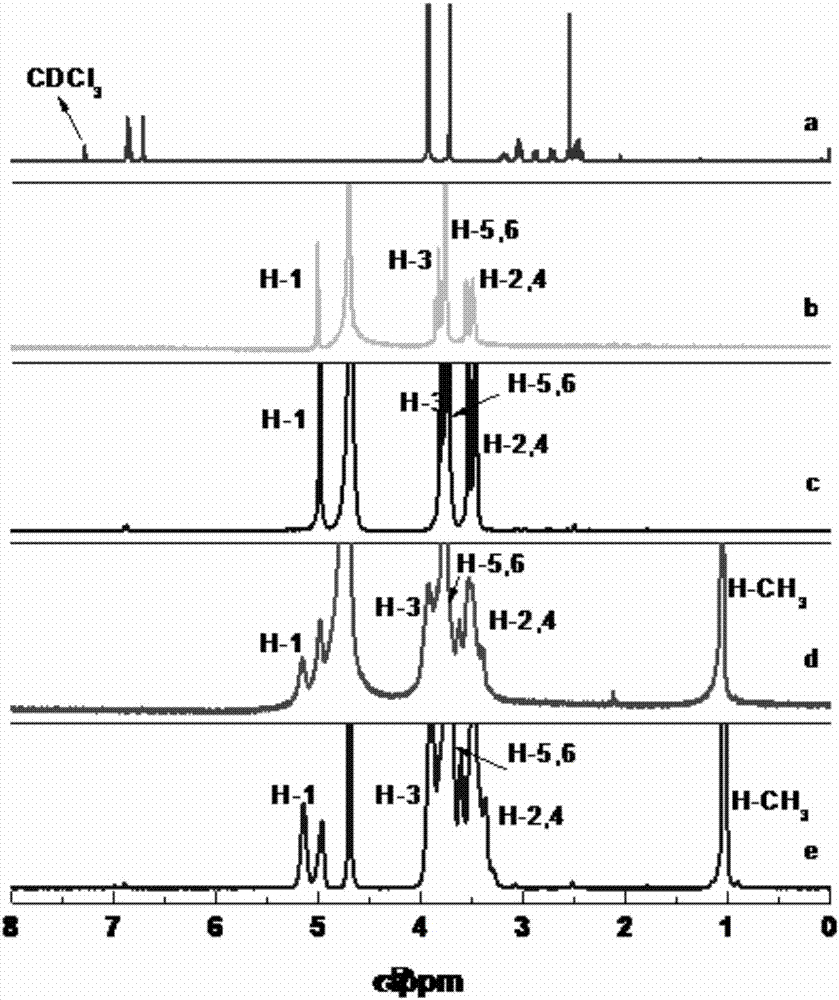
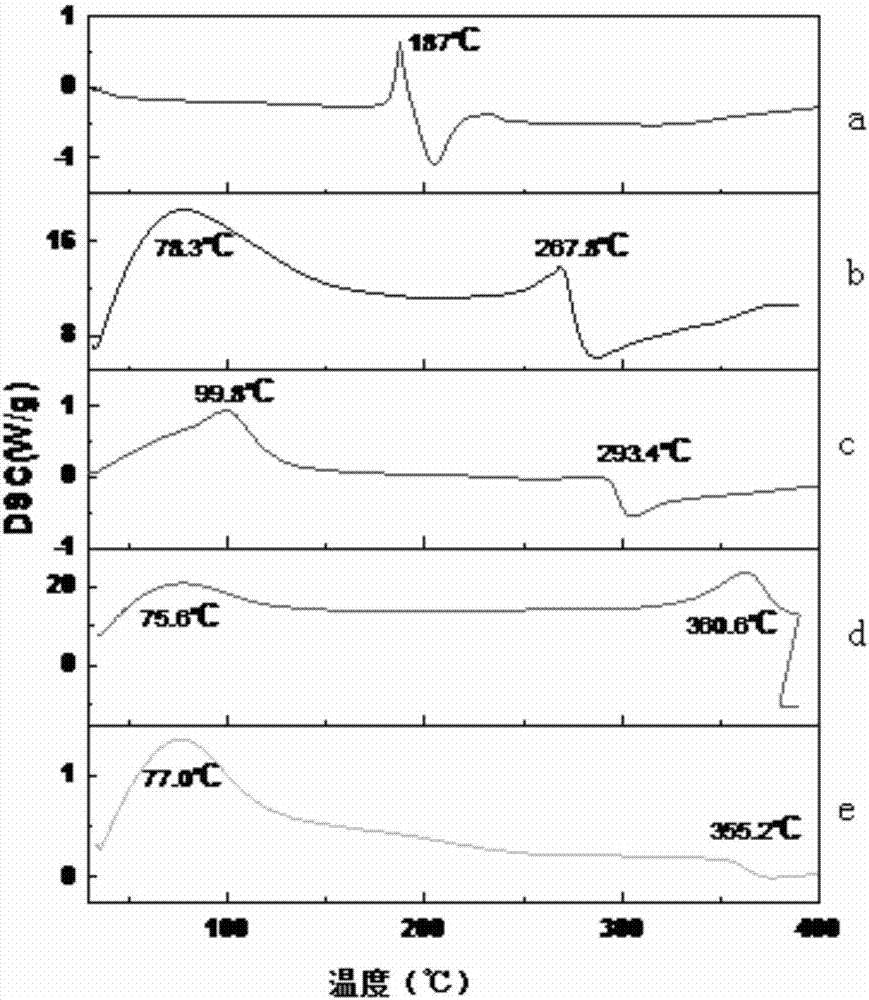
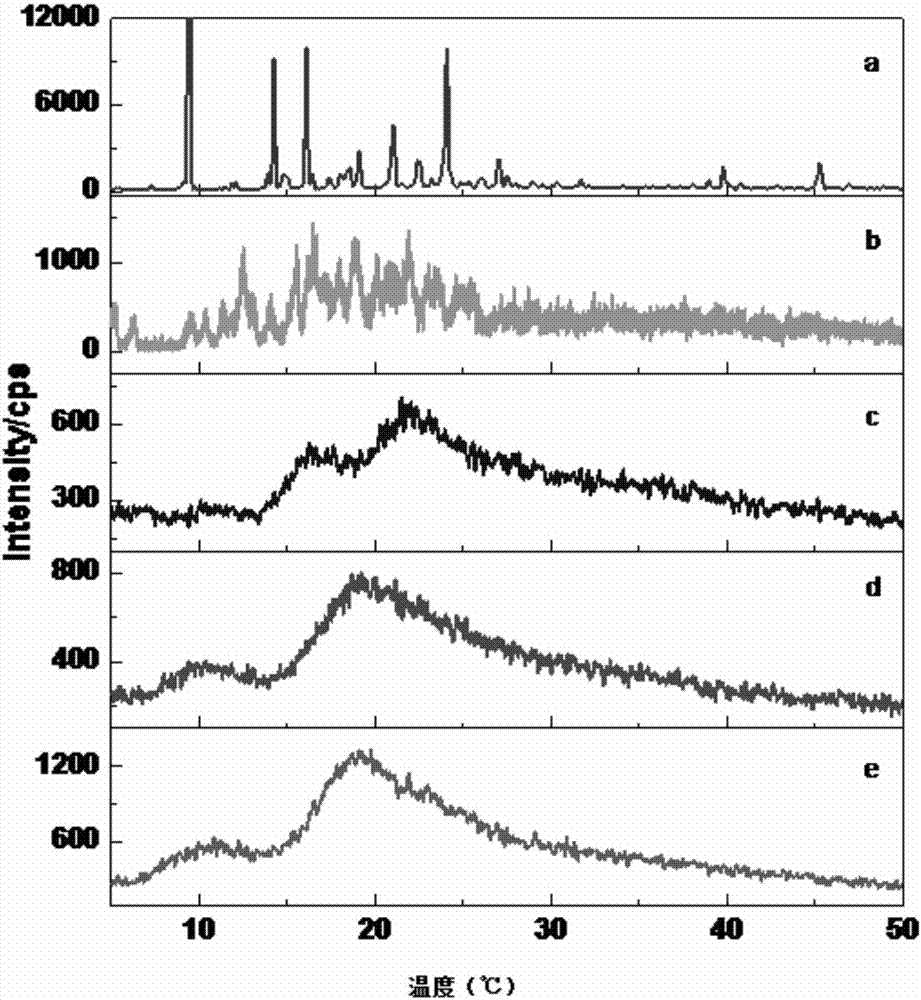
Isolorydine clathrate and preparation method thereof
A technology of isoclidine and inclusion complex, applied in the field of isoclidine inclusion complex and preparation thereof, can solve problems such as eye irritation, and achieve the effect of improving solubility and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of Isocridine Hydroxypropyl-β-cyclodextrin Inclusion Compound Solution
[0043] 1. Prescription (isocredine: hydroxypropyl-β-cyclodextrin molar ratio 1:1)
[0044] Isocridine 45mg
[0045] Hydroxypropyl-β-cyclodextrin 180mg
[0046] Second, the method
[0047] (1) Weigh 180 mg of hydroxypropyl-β-cyclodextrin, add it to 50 ml of distilled water, stir to dissolve;
[0048] (2) Weigh 45 mg of isocridine, add 50 ml of ethanol solution with a concentration of 20% by volume to dissolve and make an isocridine solution, and add the solution dropwise to the above-mentioned hydroxypropyl-β-cyclodextrin solution , the mixed solution was stirred at room temperature for 2 hours by a stirring method to obtain an isocridine hydroxypropyl-β-cyclodextrin solution;
[0049] (3) Remove the ethanol by rotary evaporation under reduced pressure, add 50 ml of distilled water to filter, remove the unincluded drug, and obtain the hydroxypropyl-β-cyclodextrin inclusion ...
Embodiment 2
[0050] Example 2 Preparation of solid isocridine hydroxypropyl-β-cyclodextrin inclusion compound
[0051] 1. Prescription (isocredine: hydroxypropyl-β-cyclodextrin molar ratio 1:1)
[0052] Isocridine 45mg
[0053] Hydroxypropyl-β-cyclodextrin 180mg
[0054] Second, the method
[0055] Steps (1), (2), (3) are the same as in Example 1.
[0056] Drying the isocredine hydroxypropyl-β-cyclodextrin inclusion compound solution under reduced pressure to obtain a solid isocredine hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 3
[0057] Example 3 Preparation of solid isocridine hydroxypropyl-β-cyclodextrin inclusion compound
[0058] 1. Prescription (isocredine: hydroxypropyl-β-cyclodextrin molar ratio 1:1)
[0059] Isocridine 45mg
[0060] Hydroxypropyl-β-cyclodextrin 180mg
[0061] Second, the method
[0062] (1) Weigh 180 mg of hydroxypropyl-β-cyclodextrin, put it into a mortar, add 2 ml of distilled water, and grind to make a paste.
[0063] (2) Take 45 mg of isocridine, add 15 m of ethanol solution with a concentration of 20% by volume to dissolve, add the solution to the hydroxypropyl-β-cyclodextrin solution obtained in step (1), and grind the mixed solution 2 hours, obtain homogeneous paste;
[0064] (3) Concentrate and dry under reduced pressure to obtain solid isocridine hydroxypropyl-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com