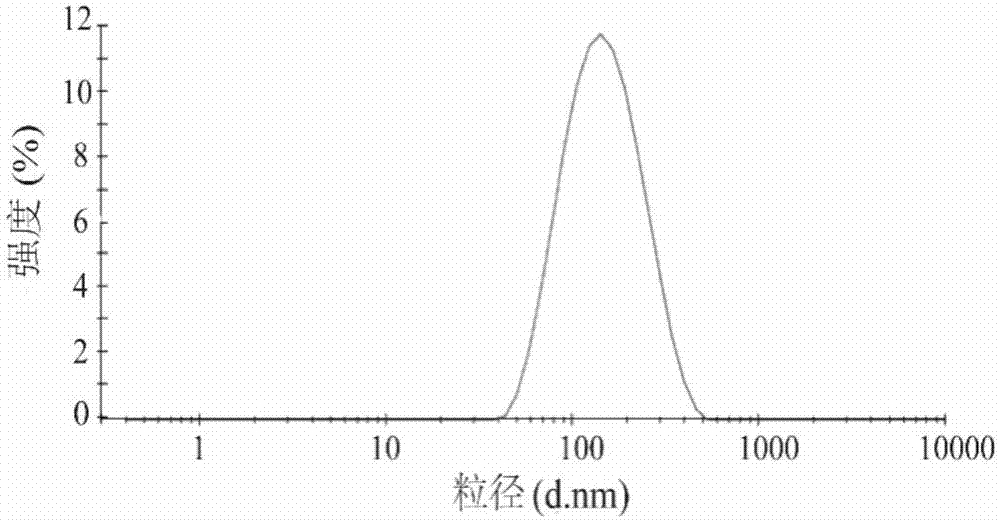
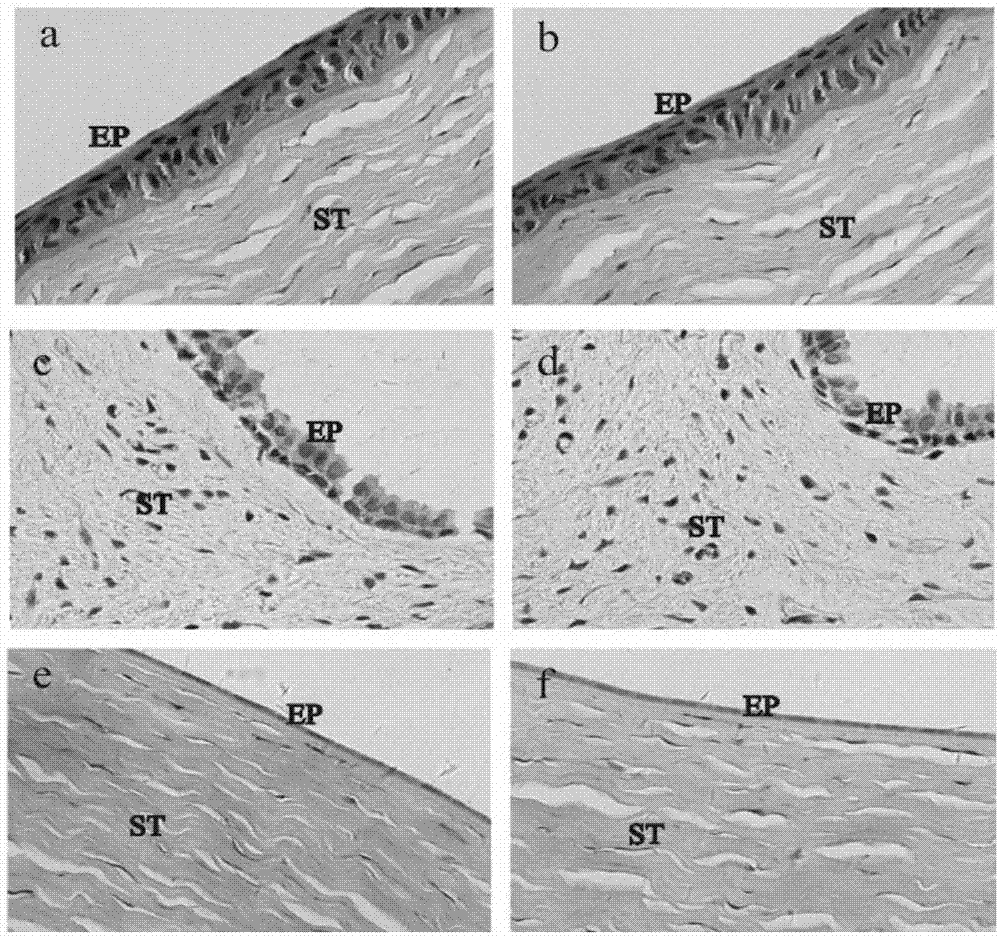
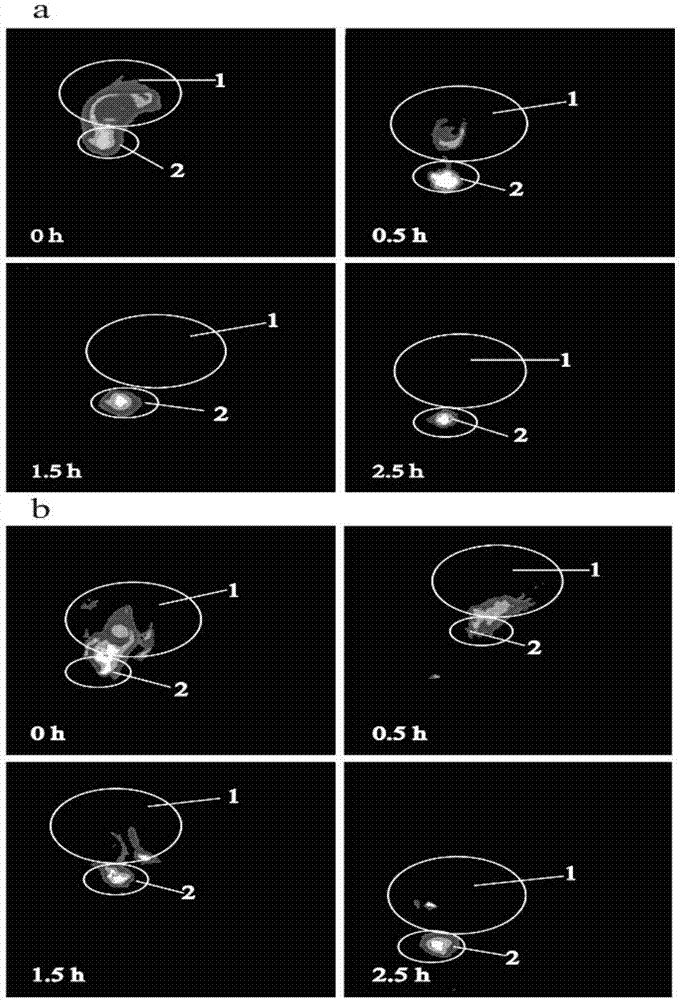
Cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and preparation method thereof
A nano-lipid and cation technology, applied in the field of medicine, can solve the problems of short eye retention time and poor corneal permeability, and achieve the effects of changing the physical and chemical properties of drugs, increasing the permeability, and reducing the elimination rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of the cation-modified pilocarpine hydrochloride flexible nano-liposome ophthalmic preparation comprises the following steps:
[0034] (1) Put 400mg of lecithin and 20mg of cholesterol in 20mL of chloroform, and sonicate for 4min to obtain a suspension; Solution 1 was obtained in methanol;
[0035] (2) Mix the suspension and the solution evenly, and remove the organic solvent by rotary evaporation at 40° C. until a uniform lipid film is formed at the bottom of the container;
[0036] (3) 25 mg sodium deoxycholate was dissolved in pure water to obtain solution 2, and the lipid film was dissolved in solution 2 to obtain a mixed primary emulsion;
[0037] (4) Under the condition of a power of 150W, the mixed primary emulsion was ultrasonicated for 60 minutes, and warmed at 60° C. for 0.5 hours to obtain a flexible nanoliposome preparation of pilocarpine hydrochloride;
[0038] (5) 20mgN-trimethyl chitosan (degree of quaternization≥60%) is dissolved...
Embodiment 2
[0041] The preparation method of the cation-modified pilocarpine hydrochloride flexible nano-liposome ophthalmic preparation comprises the following steps:
[0042] (1) Put 400mg of lecithin and 20mg of cholesterol in 20mL of chloroform, and sonicate for 3 minutes to obtain a suspension; dissolve 10mg of pilocarpine hydrochloride and 0.3mg of Labrasol (caprylic capric acid macrogolglyceride PEG-8) in 10mL of methanol get solution one;
[0043] (2) Mix the suspension and the solution evenly, and remove the organic solvent by rotary evaporation at 45° C. until a uniform lipid film is formed at the bottom of the container;
[0044] (3) 25mg sodium cholate was dissolved in pure water to obtain solution two, and the lipid film was dissolved in solution two to obtain a mixed primary emulsion;
[0045] (4) Under the condition of a power of 200W, the mixed primary emulsion was sonicated for 10 minutes, and warmed at 25° C. for 2 hours to obtain a flexible nanoliposome preparation of ...
Embodiment 3
[0049] The preparation method of the cation-modified pilocarpine hydrochloride flexible nano-liposome ophthalmic preparation comprises the following steps:
[0050] (1) Put 400mg of lecithin and 20mg of cholesterol in 10mL of chloroform, and sonicate for 4 minutes to obtain a suspension; dissolve 5mg of pilocarpine hydrochloride and 0.1mg of Transcutol HP (diethylene glycol monoethyl ether) in 10mL of methanol to obtain solution 1 ;
[0051] (2) Mix the suspension and the solution evenly, and remove the organic solvent by rotary evaporation at 35° C. until a uniform lipid film is formed at the bottom of the container;
[0052] (3) 12mg of 1,2 propylene glycol was dissolved in pure water to obtain solution two, and the lipid film was dissolved in solution two to obtain a mixed primary emulsion;
[0053] (4) Under the condition of a power of 100W, the mixed primary emulsion was ultrasonicated for 50 minutes, and warmed at 30° C. for 1 hour to obtain a flexible nanoliposome prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com