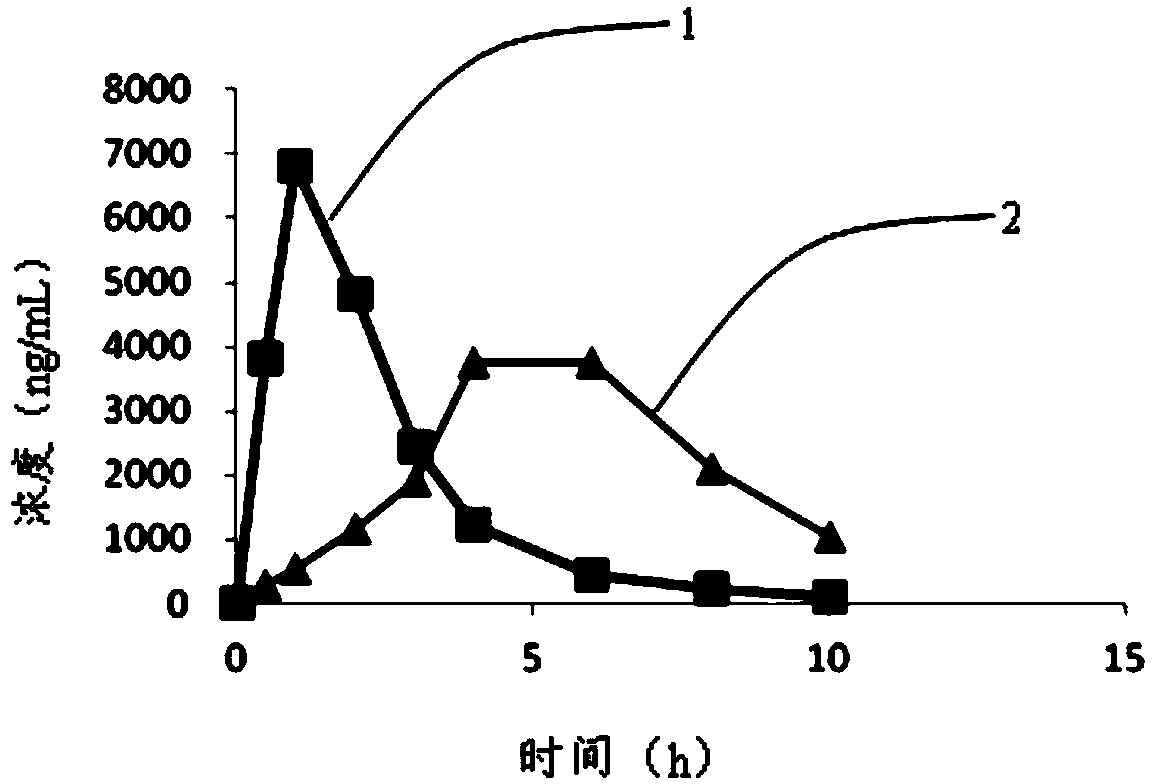
Intragastric floating sustained release tablet of praziquantel composition and preparation method thereof
A gastric floating, praziquantel technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of limited promotion and application of praziquantel, inactive metabolites, low bioavailability, etc. Odor, increased solubility and dissolution rate, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The screening of floating sustained-release tablet formula in the stomach of embodiment 1 praziquantel composition
[0104] This example screens the type, dosage and dosage of the skeleton material in the intragastric floating sustained-release tablets of the praziquantel composition provided by the present invention, wherein xanthan gum and hydroxypropyl methylcellulose consist of A mixed skeleton material is used, and stearyl alcohol is used as a bleaching aid. The experimental design is specifically: three factors and three levels L 9 (3 3 ) Orthogonal test, L 9 (3 3 ) Orthogonal test factors and level settings are shown in Table 1.
[0105] Form 1L 9 (3 3 ) Orthogonal test factor and level table
[0106]
[0107] formula:
[0108] According to the three factors and three levels in Table 1, L 9 (3 3 ) orthogonal experiment, and designed 9 different formulas of praziquantel composition gastric floating sustained-release tablets, and the composition of each ...
Embodiment 2
[0134] Preparation of floating sustained-release tablet in the stomach of embodiment 2 praziquantel composition
[0135] Take 500g of hydroxypropyl-β-cyclodextrin and dissolve it in 1166.7g of water to obtain a 30% aqueous solution of hydroxypropyl-β-cyclodextrin; take 260g of praziquantel and stir it at 50°C for 3 hours. Refrigerate at -4°C for 20 hours, dry at 60°C, and pulverize to obtain the inclusion compound of praziquantel; take the inclusion compound of praziquantel, 390 g of praziquantel, and hydroxypropyl methylcellulose (K4M, purchased at Shanghai Jingchun Biochemical Technology Co., Ltd.) 400g, xanthan gum 500g, calcium carbonate 200g, lactose 400g, talc powder 40g, mix well, use 538mL of 90% ethanol aqueous solution to make soft material, select 20 mesh sieve for granulation , dry at 40°C, granulate, add 15g of magnesium stearate, mix well, and compress with TDP single-punch tablet machine, the pressure is maintained at 4-6kg·cm -2 , compressed to obtain about 65...
Embodiment 3
[0136] Preparation of floating sustained-release tablet in the stomach of embodiment 3 praziquantel composition
[0137] Dissolve 550g of hydroxypropyl-β-cyclodextrin in 2200g of water to obtain a 20% aqueous solution of hydroxypropyl-β-cyclodextrin; take 340g of praziquantel, stir at 45°C for 10h, and place Refrigerate at 4°C for 18 hours, dry at 60°C, and pulverize to obtain the inclusion compound of praziquantel; take the inclusion compound of praziquantel, 510g of praziquantel, 500g of hydroxypropyl methylcellulose, 400g of xanthan gum, Magnesium carbonate 80g, calcium carbonate 80g, starch 200g, micropowder silica gel 100g, mix thoroughly, use 1104mL of 70% ethanol aqueous solution to make soft material, select 18 mesh sieve for granulation, dry at 60°C, granulate, add 30g of magnesium stearate, after mixing evenly, tablet with TDP single-punch tablet machine, the pressure is maintained at 4-6kg·cm -2 , compressed to obtain about 6500 tablets, that is. The weight of eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com